Abstract
Background
There is a knowledge gap on the effect of early childhood deworming on development in low- and middle-income countries. This evidence is important in the critical window of growth and development before two years of age.
Methods
A randomized controlled trial of the benefit, and optimal timing and frequency, of deworming on development was conducted in Iquitos, Peru. Children were enrolled during routine 12-month growth and development visits and randomly allocated to: (1) deworming at the 12-month visit and placebo at the 18-month visit; (2) placebo at the 12-month visit and deworming at the 18-month visit; (3) deworming at the 12 and 18-month visits; or (4) placebo at the 12 and 18-month visits. The Bayley Scales of Infant Development III was used to assess cognitive, language and motor skills at the 12 and 24-month visits. One-way ANOVA analyses used an intention-to-treat approach.
Results
Between September 2011 and June 2012, 1760 children were enrolled. Attendance at the 24-month visit was 88.8% (n=1563). Raw scores on all subtests increased over 12 months; however, cognitive and expressive language scaled scores decreased. There was no statistically significant benefit of deworming, or effect of timing or frequency, on any of the development scores. Baseline height and weight and maternal education were associated with development scores at 24 months.
Conclusions
After 12 months of follow-up, an overall benefit of deworming on cognition, language or fine motor development was not detected. Additional integrated child and maternal interventions should be considered to prevent developmental deficits in this critical period.
Keywords: Deworming, Preschool-age children, Cognition, Language, Fine motor skills, Bayley Scales of Infant Development, Low- and middle-income countries, Randomized controlled trial
Highlights
-
•
This is the first and largest deworming trial exclusively in 1-year-old children.
-
•
We examined the effect of deworming on early childhood development.
-
•
Deworming did not impact cognition, language or motor skills.
-
•
Concomitant poverty and malnutrition may obscure the true effect of deworming.
Introduction
Evidence from low- and middle-income countries (LMICs) has highlighted the importance of ensuring optimal conditions in early childhood, and in particular, the first two years of life, for healthy development in the short and long-term (Grantham-McGregor et al., 2007, Martorell et al., 2010, Victora et al., 2008, Victora et al., 2010). Poverty is a major underlying cause of developmental deficits, through increased nutritional deficiencies and infection, an inadequate home environment and stimulation, and low parental education (Grantham-McGregor et al., 2007). These risk factors can impact brain development and thus cognitive functioning in early life, and later school achievement and productivity in adulthood (Martorell et al., 2010, Victora et al., 2008). Thus, appropriate and integrated interventions must be provided to improve early child development, reduce health inequities, and provide those most vulnerable populations an opportunity to escape the vicious cycle of poverty (Grantham-McGregor et al., 2007).
Interventions to improve child development include micronutrient supplementation and breastfeeding, and targeting the social components linked to poverty, such as mother–child interactions and child stimulation (Walker et al., 2011, Engle et al., 2011, Grantham-McGregor et al., 2014). There has been less evidence on the potential benefits of interventions for infections in early childhood on short or long-term development. The soil-transmitted helminth (STH) disease cluster (i.e. Ascaris, Trichuris and hookworm) is common in the most vulnerable populations in LMICs. STHs persist in contaminated environments with poor sanitation and limited access to improved water sources. The impact of providing large-scale single-dose anthelminthic treatment (i.e. deworming) on cognition has been studied almost exclusively in school-age children. STH infection is thought to primarily impact child development indirectly by affecting host nutrition, mainly through competition for nutrients and energy; however, direct pathways between infection and cognition have also been hypothesized (Kvalsvig and Albonico,, Hall et al., 2008). Some observational studies and randomized controlled trials (RCTs) have shown a benefit of deworming (mainly through a reduction in hookworm or Trichuris infection) on cognition, measured directly through psychometric tests, or indirectly through school indicators such as school performance and attendance (Sakti et al., 1999, Nokes and Bundy, 1993, Nokes et al., 1992, Ezeamama et al., 2012). However, the combined evidence is mixed, and a recent Cochrane review was unable to detect an overall significant benefit of deworming on cognition in school-age children (Taylor-Robinson, Maayan, Soares-Weiser, Donegan, & Garner, 2015). In addition, the validity of previous research which had demonstrated a positive effect of deworming on school indicators, such as attendance, has been called into question (Hicks et al., 2015, Aiken et al., 2015).
The evidence base in preschool-age children is even more limited. One cross-sectional study found some evidence for a link between intestinal parasite infections (not limited to STH) and deficient scores on the Denver Developmental Screening Test II in children living in rural Nicaragua (Oberhelman, Guerrero, & Fernandez, 1998). This relationship did not persist in multivariable analysis nor in a subgroup analysis of children under 24 months of age. Stoltzfus, Kvalsvig, and Chwaya (2001) conducted an RCT on deworming and development in preschool-age children. Although not statistically significant, there was a trend towards a benefit of deworming on language and gross motor development.
With little research attention and challenges in measuring developmental outcomes in younger children, a large research gap exists as to the potential benefits of deworming in early preschool-age children. We therefore report on the results of a randomized controlled trial on the effects of a deworming intervention provided at 12 months of age on the secondary outcome of child development at 24 months of age, measured by cognitive, language and fine motor skills.
Methods
Details on baseline associations and the primary outcome of the trial have been described elsewhere (Joseph et al., 2014, Joseph et al., 2015). Briefly:
-
1)
Study design and enrollment procedures: We conducted a randomized, parallel, double-blind, placebo-controlled trial of deworming at 12 and/or 18 months of age in children living in Iquitos, a soil-transmitted helminth (STH)-endemic area of the Peruvian Amazon (ClinicalTrials.gov: NCT01314937). Children were enrolled during their routine 12-month growth and development visits in participating health centers and followed-up to their 24-month visit. Children were eligible to participate in the trial if they were: (1) living in the study area; and (2) attending one of the 12 participating study health centers for their 12-month growth and development visit. Children were not eligible to participate if they: (1) were attending the clinic for suspected STH infection; (2) had received deworming in the six months prior to enrollment in the trial; (3) had plans to move outside of the study area in the next year; (4) were younger than 12 months of age or 14 months of age or older; or (5) suffered from serious congenital or chronic medical conditions. Parents (or guardians) provided a signed informed consent form to confirm participation of their child in the study.
-
2)
Outcome measurements and follow-up visits: The primary outcome of the trial was weight gain over 12 months of follow-up. Additional growth outcomes included length gain and derived indices (i.e. weight-for-age and length-for-age z scores). These results have been published elsewhere (Joseph et al., 2015). A pre-specified secondary outcome was the effect of deworming on child development (as defined below). A socio-demographic and epidemiological questionnaire was administered to the primary caregiver of the child at the 12-month visit. Baseline outcome measurements, including weight, length and STH infection, were ascertained in a subsequent visit in the health center. These measurements were repeated at the 18 and 24-month visits. All measurements were assessed by trained research assistants (RAs).
Development was assessed at the 12 and 24-month visits using the Bayley Scales of Infant and Toddler Development, Third Edition (Bayley-III). The Bayley-III is a rigorous instrument that is used to assess developmental functioning in children under 42 months of age (Bayley, 2006). It has been adapted for use in international settings (Aboud et al., 2013, Manji et al., 2014, Yousafzai et al., 2014), and previous versions have been used in Peruvian populations (Colombo, Zavaleta, & Kannass, 2014). Subtests that were included in the current trial were cognitive, receptive language, expressive language, and fine motor. The gross motor subtest was not included as these skills were thought to be less variable in the age group of children studied; however, the World Health Organization (WHO) gross motor milestones were used to assess the age at which the child began to walk without support (WHO Multicentre Growth, 2006). Each subtest consisted of items which were administered by trained RAs in the presence of one or two caregivers. RAs were all healthcare personnel (e.g. nurse or nurse–midwife) with a minimum Bachelor’s degree education. All attempts were made to complete the assessment in one visit, including taking breaks for feedings. To ensure the child was performing under optimal conditions, a second visit was scheduled if needed. The test was administered as recommended during the health center visit (Bayley, 2006). Some modifications of items and test administration were required:
-
a)
If a child did not answer all first three items correctly, the RA would reverse in blocks of three items at a time (i.e. rather than to the previous age start point) until three correct responses were achieved (i.e. the basal). The RA would then continue in a forward manner from the first unadministered item until the stopping point was reached (i.e. incorrect responses to five sequential items).
-
b)
For items where verbal instructions were not specified, we developed specific instructions and a maximum number of times that they could be repeated to standardize practices among RAs.
-
c)
Adaptation of words and images in some items, including all pictures in the Picture Book and some pictures in the Stimulus Book, was required. The age-appropriateness of both the image and the accompanying word were considered when adapting the items. All modifications were pre-tested in children of the same target age of the trial and in older children.
Extensive training of RAs and pretesting of the adapted instrument took place for two months prior to the start of the 12 and 24-month visits. Adaptation and training of the Bayley-III was performed by FL and SAJ. On-site supervision, video recordings, and re-training were used to ensure consistency of administration and scoring throughout the trial. All data collection activities were regularly supervised by SAJ and LP.
-
3)
Intervention groups: After the completion of all baseline outcome measurements, participating children were randomly allocated to:
Group 1 (MBD/PBO): Deworming (i.e. 500 mg single-dose mebendazole) at the 12-month visit and placebo at the 18-month visit.
Group 2 (PBO/MBD): Placebo at the 12-month visit and deworming at the 18-month visit.
Group 3 (MDB/MBD): Deworming at both the 12 and 18-month visits.
Group 4 (PBO/PBO): Placebo at both the 12 and 18-month visits.
Usual care interventions and services (e.g. vaccinations) were provided by health center personnel, according to Peruvian Ministry of Health guidelines (MINSA, 2011). The deworming tablet was manufactured by Janssen Pharmaceuticals Inc. and donated by INMED Peru. The identical placebo tablet was manufactured and purchased from Laboratorios Hersil, Peru. Tablets were crushed and mixed with juice, and administered by RAs upon completion of all other visit procedures.
-
4)
Sample size: A total sample size of 1760 was estimated (i.e. 440 children per group), based on detecting a minimum difference of 0.20 kg in the primary outcome of weight gain over one year among the different deworming intervention groups. The sample size took into account 80% power, a common standard deviation of 0.8, estimated loss-to-follow-up of 20% over 12 months, and the Tukey correction for multiple comparisons (MC4G Software©, GP Brooks, Ohio University, 2008).
-
5)
Randomization and masking: Intervention assignment was determined using a computer-generated random sequence and permuted block sizes of eight and twelve. Envelopes containing the intervention were prepared and numbered between 1 and 1760, corresponding to the computer-generated sequence. These were stored in the pharmacy of the local research office and handed out in sequential order to RAs (by SAJ or LP). All research personnel involved in trial design, outcome measurements, and/or analysis, as well as parents/guardians of participants were blinded to intervention status.
-
6)
Analyses: Development scores were calculated separately for each subtest. The raw score was calculated as the number of correct responses between the basal and the stopping point, added to the total number of unadministered items prior to the basal. Raw scores were converted to scaled scores between 1 and 19, derived from age-standardization tables (based on a developed country population) (Bayley, 2006). Scaled scores were analyzed to make comparisons within the trial (i.e. among groups and different time points) and not as an indication of development delays or deficits compared to other populations.
The effect of deworming on development was examined for each subtest in unadjusted intention-to-treat analysis using one-way ANOVA. Developmental outcomes included absolute raw scores and scaled scores at the 24-month visit, and the change in raw and scaled scores from baseline to the 24-month visit. Analyses were also adjusted for baseline anthropometry, baseline development score (in the case of absolute score outcomes), age, sex, breastfeeding to 12 months of age and socioeconomic status (SES) (based on a proxy asset-based indicator) (Joseph et al., 2014). For children who were missing their 24-month visit, multiple imputation using a Markov Chain Monte Carlo model was used to impute development scores at follow-up (e.g. based on baseline values of age, sex, anthropometry and SES). These analyses were all specified a priori.
Additional multivariable linear regression analyses were specified a posteriori to examine the relationship between other baseline child, maternal and household factors and development scores at the 24-month visit. Variables with a p value <0.20 in univariable analyses were included in further multivariable model building. The final model included all significant variables at p<0.05, as well as adjustment for age, intervention group, and the RA who performed the assessment.
All statistical analyses were performed using the Statistical Analysis Systems statistical software package version 9.3 (SAS Institute, Cary, NC, USA).
Role of the funding source
The funding agencies had no role in study design, data collection, data analysis, data interpretation, manuscript writing, or the decision to submit the manuscript for publication. The corresponding author had full access to the data and final responsibility for the decision to submit the manuscript for publication.
Results
Participant flow and baseline characteristics of the study population
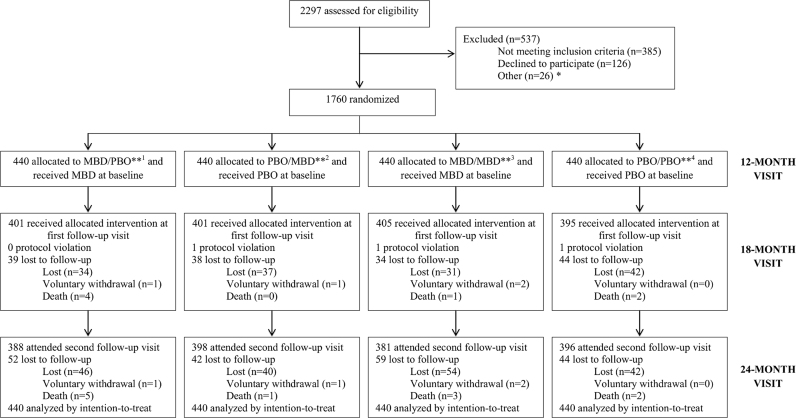
Details on participant enrollment, baseline characteristics and follow-up are described elsewhere (Joseph et al., 2014, Joseph et al., 2015). Briefly, children were enrolled in the trial between September 2011 and June 2012 to reach the total required sample size of 1760. All children received their randomly assigned intervention at baseline. A total of 1606 children attended the 18-month visit: 1603 receiving the allocated intervention and three parents refused the receipt of the allocated intervention by their children. Eighty-eight percent (n=1563) of children attended their final follow-up visit between September 2012 and July 2013 (Fig. 1). Characteristics of children at baseline were similar by intervention group (Table 1). Baseline prevalence of any STH infection was 14.5%, which increased to 42.6% by 24 months of age (Joseph et al., 2015). Prevalences of stunting and underweight also increased from 24.2% to 46.8% and 8.6% to 10.2%, respectively, from 12 months of age to 24 months of age (Joseph et al., 2015).
Fig. 1.
Flow of trial participants (Joseph et al., 2015).
Table 1.
Baseline characteristics of the study population (N=1760) by intervention group, Iquitos, Loreto, Peru (September 2011–July 2013) (Joseph et al., 2015).
| MBD/PBOa1 |
PBO/MBD a2 |
MBD/MBD a3 |
PBO/PBOa4 |
|
|---|---|---|---|---|
| (n=440) | (n=440) | (n=440) | (n=440) | |
| Child characteristics | ||||
| Weight [mean kg (SDb)] | 8.6 (1.0) | 8.8 (1.0) | 8.7 (1.0) | 8.7 (0.9) |
| Length [mean cm (SD)] | 71.9 (2.4) | 72.3 (2.4) | 72.1 (2.5) | 72.2 (2.5) |
| Age [mean months (SD)] | 12.5 (0.4) | 12.5 (0.5) | 12.5 (0.4) | 12.5 (0.5) |
| Birth weight [mean kg (SD)] | 3.1 (0.5) | 3.2 (0.5) | 3.2 (0.5) | 3.2 (0.5) |
| Birth length [mean cm (SD)] | 49.2 (2.5) | 49.5 (2.5) | 49.4 (2.3) | 49.5 (2.7) |
| Sex [n (%) female] | 215 (48.9) | 222 (50.5) | 203 (46.1) | 200 (45.5) |
| Continued breastfeeding at 12 months [n (%)] | 394 (89.6) | 395 (89.8) | 394 (89.6) | 392 (89.1) |
| Up-to-date vaccinationsc [n (%)] | 346 (78.8) | 351 (79.8) | 358 (81.6) | 355 (80.9) |
| Received vitamin A in previous year [n (%)] | 213 (48.4) | 241 (54.8) | 251 (57.1) | 216 (49.1) |
| Hospitalizations since birth [n (%)] | 402 (91.4) | 397 (90.2) | 402 (91.4) | 396 (90.0) |
| Walking without support [n (%)] | 111 (25.2) | 104 (23.7) | 117 (26.6) | 101 (23.1) |
| Maternal characteristics | ||||
| Married or common-law [n (%)] | 358 (81.4) | 351 (79.8) | 357 (81.1) | 357 (81.1) |
| Secondary education completed [n (%)] | 142 (32.4) | 140 (31.8) | 133 (30.2) | 139 (31.6) |
| Employment outside the home [n (%)] | 47 (10.7) | 45 (10.2) | 50 (11.4) | 37 (8.4) |
| Household characteristics | ||||
| Peri-urban or rural residence [n (%)] | 382 (86.8) | 391 (88.9) | 388 (88.2) | 399 (90.7) |
| Potable water in home [n (%)] | 230 (52.3) | 218 (49.6) | 230 (52.3) | 220 (50.0) |
| Earth or wood house material [n (%)] | 342 (77.7) | 342 (77.7) | 338 (76.8) | 332 (75.5) |
1Group 1 (MBD/PBO)=mebendazole at the 12-month visit and placebo at the 18-month visit; 2Group 2 (PBO/MBD)=placebo at the 12-month visit and mebendazole at the 18-month visit; 3Group 3 (MBD/MBD)=mebendazole at the 12 and 18-month visit; 4Group 4 (PBO/PBO)=placebo at the 12 and 18-month visit.
SD=standard deviation.
Up-to-date vaccinations include those scheduled between birth and 11 months of age (i.e. one dose of Bacille Calmette-Guérin (BCG), one dose of hepatitis B, three doses of polio, three doses of pentavalent, two doses of rotavirus, and two doses of pneumococcal).
At baseline, only 17 children (1.0%) required two visits to complete the developmental assessment. At the 24-month visit, there were 38 children out of 1563 (2.4%) who required two visits to complete the test.
Development scores by intervention group
Raw and scaled scores on each of the four subtests were similar for all intervention groups at baseline (Table 2). Raw scores increased between the 12 and 24-month visits as is expected with increasing age, and were similar among intervention groups. Scaled scores increased over the 12-month period in receptive language and fine motor skills; however, over this same period of time, both cognitive and expressive language scaled scores decreased in all four groups, suggesting increasing developmental deficits.
Table 2.
Absolute raw and scaled development scores and change in scores at baseline (12-month visit) and follow-up (24-month visit) by intervention group (n=1760), Iquitos, Loreto, Peru (September 2011–July 2013).
| Group 1a1 (n=440) |
Group 2a2 (n=440) |
Group 3a3 (n=440) |
Group 4a4 (n=440) |
|||||
|---|---|---|---|---|---|---|---|---|
| Raw scores | Scaled scoreb | Raw score | Scaled score | Raw score | Scaled score | Raw score | Scaled score | |
| Baseline (12-month visit) | ||||||||
| Cognitive | 42.5 | 10.3 | 42.5 | 10.2 | 42.4 | 10.4 | 42.4 | 10.2 |
| (42.2, 42.7) | (10.0, 10.3) | (42.2, 42.8) | (10.0, 10.4) | (42.2, 42.7) | (10.3, 10.6) | (42.2, 42.7) | (10.0, 10.4) | |
| Receptive language | 12.9 | 7.4 | 12.8 | 7.3 | 13.0 | 7.5 | 13.0 | 7.4 |
| (12.8, 13.1) | (7.2, 7.6) | (12.7, 13.0) | (7.1, 7.4) | (12.8, 13.1) | (7.3, 7.7) | (12.8, 13.1) | (7.2, 7.6) | |
| Expressive language | 13.4 | 8.2 | 13.5 | 8.3 | 13.5 | 8.4 | 13.5 | 8.4 |
| (13.2, 13.6) | (8.1, 8.4) | (13.3, 13.6) | (8.1, 8.4) | (13.3, 13.7) | (8.2, 8.5) | (13.3, 13.7) | (8.2, 8.5) | |
| Fine motor | 29.2 | 9.4 | 29.2 | 9.4 | 29.2 | 9.4 | 29.2 | 9.4 |
| (29.0, 29.3) | (9.2, 9.5) | (29.1, 29.4) | (9.3, 9.6) | (29.1, 29.3) | (9.3, 9.6) | (29.1, 29.3) | (9.3, 9.6) | |
| Follow-up (24-month visit) | ||||||||
| Cognitive | 58.8 | 7.7 | 59.3 | 7.9 | 59.1 | 7.9 | 59.1 | 7.8 |
| (58.5, 59.1) | (7.6, 7.8) | (59.0, 59.6) | (7.8, 8.0) | (58.8, 59.4) | (7.7, 8.0) | (58.8, 59.4) | (7.6, 7.9) | |
| Receptive language | 23.9 | 8.1 | 23.9 | 8.2 | 23.9 | 8.1 | 23.9 | 8.1 |
| (23.6, 24.1) | (8.0, 8.2) | (23.7, 24.2) | (8.0, 8.3) | (23.7, 24.1) | (8.0, 8.2) | (23.7, 24.1) | (8.0, 8.2) | |
| Expressive language | 24.6 | 7.1 | 24.5 | 7.2 | 24.6 | 7.1 | 24.6 | 7.2 |
| (24.3, 24.8) | (7.0, 7.3) | (24.2, 24.9) | (7.0, 7.3) | (24.2, 24.9) | (7.0, 7.2) | (24.2, 24.9) | (7.0, 7.3) | |
| Fine motor | 39.4 | 9.9 | 39.5 | 10.0 | 39.4 | 9.9 | 39.4 | 9.9 |
| (39.2, 39.6) | (9.7, 10.1) | (39.3, 39.7) | (9.9, 10.2) | (39.1, 39.6) | (9.7, 10.0) | (39.1, 39.6) | (9.7, 10.1) | |
| Change from baseline to follow-up | ||||||||
| Cognitive | 16.4 | −2.5 | 16.9 | −2.3 | 16.6 | −2.6 | 16.6 | −2.4 |
| (16.0, 16.7) | (−2.7, −2.3) | (16.5, 17.3) | (−2.5, −2.1) | (16.2, 17.0) | (−2.8, −2.4) | (16.2, 17.0) | (−2.6, −2.2) | |
| Receptive language | 10.9 | 0.7 | 11.1 | 0.9 | 11.0 | 0.6 | 11.0 | 0.7 |
| (10.7, 11.2) | (0.5, 0.9) | (10.9, 11.4) | (0.7, 1.1) | (10.7, 11.2) | (0.4, 0.8) | (10.7, 11.2) | (0.5, 0.9) | |
| Expressive language | 11.1 | −1.1 | 11.1 | −1.1 | 11.1 | −1.3 | 11.1 | −1.2 |
| (10.8, 11.5) | (−1.3, −0.9) | (10.7, 11.4) | (−1.3, −0.9) | (10.7, 11.4) | (−1.5, −1.1) | (10.7, 11.4) | (−1.4, −1.0) | |
| Fine motor | 10.2 | 0.5 | 10.2 | 0.6 | 10.2 | 0.5 | 10.2 | 0.5 |
| (9.9, 10.4) | (0.3, 0.7) | (10.0, 10.5) | (0.4, 0.8) | (9.9, 10.4) | (0.3, 0.7) | (9.9, 10.4) | (0.3, 0.7) | |
Results are expressed as mean (95% confidence interval).
1Group 1 (MBD/PBO)=mebendazole at the 12-month visit and placebo at the 18-month visit; 2Group 2 (PBO/MBD)=placebo at the 12-month visit and mebendazole at the 18-month visit; 3Group 3 (MBD/MBD=mebendazole at the 12 and 18-month visit; 4Group 4 (PBO/PBO)=placebo at the 12 and 18-month visit.
Scaled scores are the raw scores scaled between 1 and 19 based on age of child in months and days and the specific subtest.
Benefit, timing and frequency of deworming on development outcomes
When comparing the scaled score at the 24-month visit in each of the deworming intervention groups to the control group, no statistically significant benefit of deworming on any of the development subtests was detected in unadjusted or adjusted intention-to-treat analysis (Table 3). Results remained consistent when using the outcomes of absolute raw scores, and change in raw and scaled scores (results not shown). There was some evidence for improved cognitive outcomes in terms of timing, with greater scores in Group 2 compared to Group 1 at follow-up (Table 4). The effect size decreased with adjustment of baseline cognitive scores and nutritional status, and no effect was seen on any of the other subtests. There was no statistically significant effect of deworming frequency on any of the developmental outcomes (Table 5). No benefits of deworming on any cognitive outcomes were apparent in additional sensitivity analyses, including complete case analysis, per-protocol analysis, and subgroup analysis by malnutrition status (e.g. stunted and/or underweight) at baseline.
Table 3.
Overall benefit of deworming on absolute scaled development scores at 24 months, using unadjusted and adjusted ANOVA analysis, n=1760a, Iquitos, Loreto, Peru (September 2011–July 2013).
| Group 1 |
Group 2 |
Group 3 |
Group 4 |
|
|---|---|---|---|---|
| MBD/PBOb1 |
PBO/MBDb2 |
MBD/MBDb3 |
PBO/PBOb4 |
|
| (n=440) | (n=440) | (n=440) | (n=440) | |
| Cognition | ||||
| Unadjusted difference | −0.07 | 0.14 | 0.10 | Reference |
| (95% CI) | (−0.24, 0.10) | (−0.04, 0.32) | (−0.08, 0.27) | |
| p Value | 0.431 | 0.122 | 0.291 | |
| Adjustedc difference | −0.05 | 0.12 | 0.07 | Reference |
| (95% CI) | (−0.22, 0.12) | (−0.05, 0.29) | (−0.10, 0.24) | |
| p Value | 0.542 | 0.158 | 0.440 | |
| Receptive language | ||||
| Unadjusted difference | −0.02 | 0.03 | −0.05 | Reference |
| (95% CI) | (−0.19, 0.14) | (−0.14, 0.20) | (−0.23, 0.12) | |
| p value | 0.780 | 0.730 | 0.543 | |
| Adjusted difference | 0.00 | 0.03 | −0.06 | Reference |
| (95% CI) | (−0.16, 0.16) | (−0.13, 0.19) | (−0.23, 0.11) | |
| p value | 0.990 | 0.710 | 0.502 | |
| Expressive language | ||||
| Unadjusted difference | −0.01 | 0.00 | −0.06 | Reference |
| (95% CI) | (−0.22, 0.19) | (−0.21, 0.21) | (−0.26, 0.15) | |
| p Value | 0.907 | 0.997 | 0.582 | |
| Adjusted difference | 0.03 | −0.01 | −0.05 | Reference |
| (95% CI) | (−0.16, 0.23) | (−0.21, 0.19) | (−0.24, 0.15) | |
| p Value | 0.755 | 0.914 | 0.640 | |
| Fine motor skills | ||||
| Unadjusted difference | −0.02 | 0.12 | −0.04 | Reference |
| (95% CI) | (−0.27, 0.24) | (−0.14, 0.37) | (−0.29, 0.21) | |
| p Value | 0.906 | 0.364 | 0.746 | |
| Adjusted difference | 0.00 | 0.10 | −0.06 | Reference |
| (95% CI) | (−0.24, 0.24) | (−0.15, 0.35) | (−0.30, 0.19) | |
| p Value | 0.995 | 0.418 | 0.654 |
Results are expressed as mean (95% confidence interval).
Intention-to-treat analysis includes data from 1563 children for whom final outcome information was available, and 197 children who were lost to follow-up and whose outcome information was estimated using multiple imputation.
1Group 1 (MBD/PBO)=mebendazole at the 12-month visit and placebo at the 18-month visit; 2Group 2 (PBO/MBD)=placebo at the 12-month visit and mebendazole at the 18-month visit; 3Group 3 (MBD/MBD=mebendazole at the 12 and 18-month visit; 4Group 4 (PBO/PBO)=placebo at the 12 and 18-month visit.
Adjusted models include age, sex, socioeconomic status, continued breastfeeding at 12 months of age, baseline height and weight, and baseline development score.
Table 4.
The effect of the timing of deworming on absolute scaled development scores at 24 months, using unadjusted and adjusted ANOVA analysis, n=880a, Iquitos, Loreto, Peru (September 2011–July 2013).
| Group 1 |
Group 2 |
|
|---|---|---|
| MBD/PBOb1 |
PBO/MBDb2 |
|
| (n=440) | (n=440) | |
| Cognition | ||
| Unadjusted difference | −0.21 | Reference |
| (95% CI) | (−0.38, −0.03) | |
| p Value | 0.019 | |
| Adjustedc difference | −0.18 | Reference |
| (95% CI) | (−0.34, −0.01) | |
| p Value | 0.040 | |
| Receptive language | ||
| Unadjusted difference | −0.05 | Reference |
| (95% CI) | (−0.21, 0.11) | |
| p Value | 0.514 | |
| Adjusted difference | −0.03 | Reference |
| (95% CI) | (−0.18, 0.12) | |
| p Value | 0.708 | |
| Expressive language | ||
| Unadjusted difference | −0.01 | Reference |
| (95% CI) | (−0.22, 0.19) | |
| p Value | 0.904 | |
| Adjusted difference | 0.04 | Reference |
| (95% CI) | (−0.15, 0.24) | |
| p Value | 0.672 | |
| Fine motor skills | ||
| Unadjusted difference | −0.13 | Reference |
| (95% CI) | (−0.36, 0.10) | |
| p value | 0.262 | |
| Adjusted difference | −0.10 | Reference |
| (95% CI) | (−0.33, 0.12) | |
| p Value | 0.382 |
Results are expressed as mean (95% confidence interval).
Intention-to-treat analysis includes data from 786 children for whom final outcome information was available, and 94 children who were lost to follow-up and whose outcome information was estimated using multiple imputation.
1Group 1 (MBD/PBO)=mebendazole at the 12-month visit and placebo at the 18-month visit; 2Group 2 (PBO/MBD)=placebo at the 12-month visit and mebendazole at the 18-month visit.
Adjusted models include age, sex, socioeconomic status, continued breastfeeding at 12 months of age, baseline height and weight, and baseline development score.
Table 5.
The effect of the frequency of deworming on absolute scaled development scores at 24 months, using unadjusted and adjusted ANOVA analysis, n=1320a, Iquitos, Loreto, Peru (September 2011–July 2013).
| MBD/PBOb1 |
PBO/MBDb2 |
MBD/MBDb3 |
|
|---|---|---|---|
| (n=440) | (n=440) | (n=440) | |
| Cognition | |||
| Unadjusted difference | −0.17 | 0.04 | Reference |
| (95% CI) | (−0.34, 0.01) | (−0.13, 0.22) | |
| p Value | 0.063 | 0.630 | |
| Adjustedc difference | −0.12 | 0.06 | Reference |
| (95% CI) | (−0.29, 0.05) | (−0.12, 0.23) | |
| p Value | 0.168 | 0.529 | |
| Receptive language | |||
| Unadjusted difference | 0.03 | 0.08 | Reference |
| (95% CI) | (−0.14, 0.20) | (−0.09, 0.26) | |
| p value | 0.713 | 0.343 | |
| Adjusted difference | 0.06 | 0.09 | Reference |
| (95% CI) | (−0.11, 0.23) | (−0.08, 0.26) | |
| p Value | 0.480 | 0.305 | |
| Expressive language | |||
| Unadjusted difference | 0.05 | 0.06 | reference |
| (95% CI) | (−0.16, 0.25) | (−0.15, 0.27) | |
| p value | 0.657 | 0.587 | |
| Adjusted difference | 0.08 | 0.04 | Reference |
| (95% CI) | (−0.11, 0.27) | (−0.17, 0.24) | |
| p value | 0.425 | 0.730 | |
| Fine motor skills | |||
| Unadjusted difference | 0.03 | 0.16 | Reference |
| (95% CI) | (−0.20, 0.25) | (−0.07, 0.38) | |
| p value | 0.827 | 0.173 | |
| Adjusted difference | 0.06 | 0.16 | reference |
| (95% CI) | (−0.17, 0.28) | (−0.07, 0.38) | |
| p Value | 0.624 | 0.169 |
Results are expressed as mean (95% confidence interval).
Intention-to-treat analysis includes data from 1167 children for whom final outcome information was available, and 153 children who were lost to follow-up and whose outcome information was estimated using multiple imputation.
1Group 1 (MBD/PBO)=mebendazole at the 12-month visit and placebo at the 18-month visit; 2Group 2 (PBO/MBD)=placebo at the 12-month visit and mebendazole at the 18-month visit; 3Group 3 (MBD/MBD)=mebendazole at the 12 and 18-month visit.
Adjusted models include age, sex, socioeconomic status, continued breastfeeding at 12 months of age, baseline height and weight, and baseline development score.
Predictors of development at 24 months
Variables that were examined but were not found to be statistically significantly related to developmental outcomes at the 24-month visit included continued breastfeeding to 12 months of age, up-to-date vaccinations at 12-months of age, marital status, iron supplementation received during the study, timing of introduction of liquids and foods, and place of delivery. Other child, maternal and household variables were found to be significantly related to development scores at 24-months (Table 6). Predictors of cognitive and language score were similar in multivariable analyses and included baseline length (cm), female sex, maternal education, age at which child began to walk without support, and vitamin A supplementation. Predictors of fine motor score included baseline weight, sex, maternal education, and age at which child began to walk without support. SES was not found to be a significant predictor for any of the developmental outcomes.
Table 6.
Child, maternal and household factors associated with raw development score at 24 months in unadjusted and adjusted linear regression (n=1563), Iquitos, Loreto, Peru (September 2011–July 2013).
| Cognitive score |
Language score |
Fine motor score |
|||||
|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusteda | Unadjusted | Adjusted | Unadjusted | Adjusted | ||
| Baseline weight (per kg increase) | 0.3 (0.1, 0.4) | NSb | 0.6 (0.4, 0.9) | NS | 0.2 (0.1, 0.3) | 0.2 (0.1, 0.3) | |
| Baseline length (per cm increase) | 0.2 (0.1, 0.2) | 0.2 (0.1, 0.2) | 0.3 (0.2, 0.4) | 0.3 (0.2, 0.4) | 0.1 (0.0, 0.1) | NS | |
| Sex (female vs. male) | 0.5 (0.2, 0.9) | 0.7 (0.4, 1.0) | 1.3 (0.8, 1.7) | 1.5 (1.1, 2.0) | 0.2 (0.0, 0.4) | 0.3 (0.1, 0.5) | |
| Maternal education (secondary incomplete vs. complete) | −0.7 (−1.0, −0.3) | −0.7 (−1.0, −0.4) | −1.7 (−2.2, −1.2) | −1.1 (−1.6, −0.7) | −0.3 (−0.5, −0.1) | −0.3 (−0.5, −0.1) | |
| SESc (vs. first quartile) | |||||||
| Second quartile | 0.0 (−0.5, 0.4) | NS | 0.3 (−0.3, 1.0) | NS | NS | NS | |
| Third quartile | −0.2 (−0.6, 0.3) | NS | 0.4 (−0.3, 1.0) | NS | NS | NS | |
| Fourth quartile | 0.3 (−0.1, 0.8) | NS | 1.7 (1.0, 2.3) | NS | NS | NS | |
| Periurban/rural residence vs. urban | −0.5 (−1.0, 0.0) | NS | −0.7 (−1.4, 0.0) | NS | −0.3 (−0.6, 0.1) | NS | |
| Maternal employment outside home (yes vs. no) | 0.5 (0.0, 1.0) | NS | NS | NS | NS | NS | |
| Hospitalizations in first year of life (no vs. yes) | 0.5 (0.0, 1.1) | NS | 0.7 (−0.1, 1.5) | NS | NS | NS | |
| Antenatal care attendance (no vs. yes) | −0.6 (−1.3, 0.1) | NS | −1.0 (−2.1, 0.0) | NS | NS | NS | |
| Walking without support (per increasing year) | −0.2 (−0.3, −0.1) | −0.2 (−0.2, −0.1) | −0.4 (−0.5, −0.3) | −0.2 (−0.3, −0.1) | −0.1 (−0.2, −0.1) | −0.1 (−0.2, −0.1) | |
| Number of children in home | NS | NS | −0.4 (−0.5, −0.2) | NS | NS | NS | |
| Vitamin A received in past year (no vs. yes) | 1.0 (0.6, 1.4) | 0.5 (0.0, 0.9) | −0.9 (−1.5, −0.3) | −0.8 (−1.4, −0.1) | 0.2 (0.0, 0.5) | NS | |
Adjusted linear regression models control for age, intervention group, evaluator, and all other statistically significant variables in the multivariable model.
NS=not significant.
SES=socioeconomic status, where the first quartile corresponds to the poorest SES and the fourth quartile corresponds to the highest SES.
Discussion
This is the first randomized controlled trial to examine the effect of deworming on cognitive, language and fine motor skills in early preschool-age children using a rigorous developmental assessment instrument. Overall, this trial was unable to demonstrate a benefit of any of the deworming schedules on development with a follow-up of 12 months (i.e. between one and two years of age). Only one other trial has examined the potential benefit of deworming on development in early childhood, measured by cognitive and gross motor skills, but it was limited by a small sample size, the use of parental report for assessment of developmental outcomes, and a lack of a rigorous developmental test (Stoltzfus et al., 2001). All other trials looking at the link between deworming and developmental outcomes have been conducted in school-age populations. Although results in older age groups are not necessarily comparable to early preschool-age children in the critical window of development, our results are consistent with the majority of evidence which has not yet demonstrated a clear association between deworming and improvements in cognition (Taylor-Robinson et al., 2015).
If there is a true benefit to deworming, it may have been difficult to detect this after only one-year of follow-up. The low prevalence of STH infection at baseline may also explain our findings. In addition, one of the mechanisms through which STH infection is thought to impact cognition is by causing malnutrition (Kvalsvig & Albonico, 2013). As we were unable to demonstrate a benefit of deworming on the primary outcome of growth, and prevalences of malnutrition, particularly stunting, increased dramatically over one year of follow-up, this may explain why there was no effect of deworming on development outcomes (Joseph et al., 2015).
We were able to demonstrate improved developmental outcomes at the 24-month visit in children with greater height and weight measures at baseline, indicating the importance of targeting the high prevalence of malnutrition in this population. Not surprisingly, maternal education was found to be associated with development scores; however, SES was not. This population was specifically chosen to be homogeneous in terms of endemicity for STH, high prevalence of malnutrition, and lower SES; therefore, there is likely low variability in SES to detect differences in risk. The association of lower development scores with vitamin A supplementation is also somewhat surprising, but likely due to the fact that vitamin A is distributed strategically in the study area (i.e. to high risk individuals and by certain health centers) according to Ministry of Health guidelines (MINSA, 2011).
The overall results are relevant to children in the second year of life in other STH-endemic areas. The developmental instrument was adapted for our specific population in Peru, so there are limitations in generalizing the scores to other populations, even within Peru. The scaled scores are useful in making comparisons within the study, including detecting the increasing deficits over time; however, they should not be used to compare to other populations.
Overall strengths of this study include the RCT design, a large sample size, a high follow-up rate and the inclusion of children in a very specific and narrow age range in a rapid phase of growth and development. This study also benefited from the use of the Bayley-III. We were able to demonstrate the feasibility of employing a rigorous assessment instrument in a research and LMIC context.
The trial is limited by the short follow-up time between assessments. We are also limited by the lack of detailed information on potential social confounders such as characteristics related to the home environment (e.g. mother–child interactions and child stimulation).
Overall, the deworming interventions were not sufficient to improve development scores over one year, or to prevent the increasing developmental deficits in cognition and language skills. Due to the multifactorial nature of development, future research should evaluate the effect of integrated interventions, including micronutrient supplementation and child stimulation, to combat malnutrition and developmental deficits in vulnerable early childhood populations (Grantham-McGregor et al., 2014). Participants of the trial are currently being followed-up in an observational cohort with repeated yearly measurements on cognitive, language and motor functioning. This will allow us to evaluate any effect of the early deworming interventions on development in the longer-term.
Acknowledgments
We acknowledge financial support from the Thrasher Research Fund (USA), the Canadian Institutes of Health Research (MOP-110969; Vanier Canada Graduate Scholarship; Michael Smith Foreign Study Supplement; Planning and Dissemination Grant) and the Fonds de Recherche du Québec – Santé (Canada). We are grateful for the support and collaboration of the local Ministry of Health (Dirección Regional de Salud Loreto), and in particular, the director, Dr. Hugo Rodriguez and all personnel at the participating health centres. We thank our Peruvian-based team, including the research assistants and laboratory personnel who were involved in data collection activities in the field and laboratory, as well as Hugo Razuri and Patrick Belisle for their support in Canada. We acknowledge Drs. Grace Marquis, Antonio Montresor and Brian Ward for their contribution to the original study design, and Dr. Frances Aboud for her input into the Bayley-III adaptation and for her helpful discussions on the manuscript. We are also grateful to Mr. Luis Enrique Caycho Gutierrez for his illustrations for the adapted Bayley-III Picture and Stimulus books. We express gratitude to all participating parents and children for their willingness to participate in this research project.
References
- Aboud F.E., Singla D.R., Nahil M.I., Borisova I. Effectiveness of a parenting program in Bangladesh to address early childhood health, growth and development. Social Science Medicine. 2013;97:250–258. doi: 10.1016/j.socscimed.2013.06.020. [DOI] [PubMed] [Google Scholar]
- Aiken A.M., Davey C., Hargreaves J.R., Hayes R.J. Re-analysis of health and educational impacts of a school-based deworming programme in western Kenya: A pure replication. International Journal of Epidemiology. 2015;22 doi: 10.1093/ije/dyv127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayley N. 3rd Ed. Pearson Education, Inc.; San Antonio, TX: 2006. Bayley scales of infant and toddler development: Administration manual. [Google Scholar]
- Colombo J., Zavaleta N., Kannass K.N., Lazarte F., Albornoz C., Kapa L.L. Zinc supplementation sustained normative neurodevelopment in a randomized, controlled trial of Peruvian infants aged 6–18 months. Journal of Nutrition. 2014;144(8):1298–1305. doi: 10.3945/jn.113.189365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engle P.L., Fernald L.C., Alderman H., Behrman J., O'Gara C., Yousafzai A. Strategies for reducing inequalities and improving developmental outcomes for young children in low-income and middle-income countries. The Lancet. 2011;378(9799):1339–1353. doi: 10.1016/S0140-6736(11)60889-1. [DOI] [PubMed] [Google Scholar]
- Ezeamama A.E., McGarvey S.T., Hogan J., Lapane K.L., Bellinger D.C., Acosta L.P. Treatment for Schistosoma japonicum, reduction of intestinal parasite load, and cognitive test score improvements in school-aged children. PLOS Neglected Tropical Diseases. 2012;6(5):e1634. doi: 10.1371/journal.pntd.0001634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham-McGregor S., Cheung Y.B., Cueto S., Glewwe P., Richter L., Strupp B. Developmental potential in the first 5 years for children in developing countries. The Lancet. 2007;369(9555):60–70. doi: 10.1016/S0140-6736(07)60032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham-McGregor S.M., Fernald L.C., Kagawa R.M., Walker S. Effects of integrated child development and nutrition interventions on child development and nutritional status. Annals of the New York Academy of Sciences. 2014;1308:11–32. doi: 10.1111/nyas.12284. [DOI] [PubMed] [Google Scholar]
- Hall A., Hewitt G., Tuffrey V., de Silva N. A review and meta-analysis of the impact of intestinal worms on child growth and nutrition. Maternal and Child Nutrition. 2008;4(Suppl. 1):118–236. doi: 10.1111/j.1740-8709.2007.00127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks J.H., Kremer M., Miguel E. Commentary: Deworming externalities and schooling impacts in Kenya: A comment on Aiken et al. (2015) and Davey et al. (2015) International Journal of Epidemiology. 2015 doi: 10.1093/ije/dyv129. [DOI] [PubMed] [Google Scholar]
- Joseph S.A., Casapia M., Blouin B., Maheu-Giroux M., Rahme E., Gyorkos T.W. Risk factors associated with malnutrition in one-year-old children living in the Peruvian Amazon. PLOS Neglected Tropical Diseases. 2014;8:e3369. doi: 10.1371/journal.pntd.0003369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph S.A., Casapia M., Montresor A., Rahme E., Ward B.J., Marquis G.S. The effect of deworming on growth in one-year-old children living in a soil-transmitted helminth-endemic area of Peru: A randomized controlled trial. PLOS Neglected Tropical Diseases. 2015;9:e0004020. doi: 10.1371/journal.pntd.0004020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvalsvig, J., Albonico, M. (2013). Effects of geohelminth infections on neurological development. In H.H. Garcia, H.B. Tanowitz, O.H. Del Brutto (Eds.), Handbook of clinical veurology (Vol. 114, pp. 369–379). [DOI] [PubMed]
- Manji K.P., McDonald C.M., Kupka R., Bosch R.J., Kisenge R., Aboud S. Effect of multivitamin supplementation on the neurodevelopment of HIV-exposed Tanzanian infants: A randomized, double-blind, placebo-controlled clinical trial. Journal of Tropical Pediatrics. 2014;60(4):279–286. doi: 10.1093/tropej/fmu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martorell R., Horta B.L., Adair L.S., Stein A.D., Richter L., Fall C.H. Weight gain in the first two years of life is an important predictor of schooling outcomes in pooled analyses from five birth cohorts from low- and middle-income countries. Journal of Nutrition. 2010;140(2):348–354. doi: 10.3945/jn.109.112300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MINSA (2011). Norma técnica de salud para el control del crecimiento y desarrollo de la niña y el niño menor de cinco años. Lima, Peru: Ministerio de Salud. Dirección General de Salud de las Personas.
- Nokes C., Bundy D.A.P. Compliance and absenteeism in school children: Implications for helminth control. Transactions of the Royal Society of Tropical Medicine and Hygiene. 1993;87(2):148–152. doi: 10.1016/0035-9203(93)90464-2. [DOI] [PubMed] [Google Scholar]
- Nokes C., Grantham-McGregor S.M., Sawyer A.W., Cooper E.S., Robinson B.A., Bundy D.A. Moderate to heavy infections of Trichuris trichiura affect cognitive function in Jamaican school children. Parasitology. 1992;104:539–547. doi: 10.1017/s0031182000063800. [DOI] [PubMed] [Google Scholar]
- Oberhelman R.A., Guerrero E.S., Fernandez M.L., Silio M., Mercado D., Comiskey N. Correlations between intestinal parasitosis, physical growth, and psychomotor development among infants and children from rural Nicaragua. American Journal of Tropical Medicine and Hygiene. 1998;58(4):470–475. doi: 10.4269/ajtmh.1998.58.470. [DOI] [PubMed] [Google Scholar]
- Sakti H., Nokes C., Hertanto W.S., Hendratno S., Hall A., Bundy D.A.P. Evidence for an association between hookworm infection and cognitive function in Indonesian school children. Tropical Medicine and International Health. 1999;4(5):322–334. doi: 10.1046/j.1365-3156.1999.00410.x. [DOI] [PubMed] [Google Scholar]
- Stoltzfus R.J., Kvalsvig J.D., Chwaya H.M., Montresor A., Albonico M., Tielsch J.M. Effects of iron supplementation and anthelmintic treatment on motor and language development of preschool children in Zanzibar: Double blind, placebo controlled study. British Medical Journal. 2001;323(7326):1389–1393. doi: 10.1136/bmj.323.7326.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson D.C., Maayan N., Soares-Weiser K., Donegan S., Garner P. Deworming drugs for soil-transmitted intestinal worms in children: Effects on nutritional indicators, haemoglobin, and school performance. Cochrane Database of Systematic Reviews. 2015;7:CD000371. doi: 10.1002/14651858.CD000371.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora C.G., Adair L., Fall C., Hallal P.C., Martorell R., Richter L. Maternal and child undernutrition: Consequences for adult health and human capital. The Lancet. 2008;371(9609):340–357. doi: 10.1016/S0140-6736(07)61692-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victora C.G., de Onis M., Hallal P.C., Blössner M., Shrimpton R. Worldwide timing of growth faltering: Revisiting implications for interventions. Pediatrics. 2010;125(3):473–480. doi: 10.1542/peds.2009-1519. [DOI] [PubMed] [Google Scholar]
- Walker S.P., Wachs T.D., Grantham-McGregor S., Black M.M., Nelson C.A., Huffman S.L. Inequality in early childhood: Risk and protective factors for early child development. The Lancet. 2011;378(9799):1325–1338. doi: 10.1016/S0140-6736(11)60555-2. [DOI] [PubMed] [Google Scholar]
- WHO Multicentre Growth Reference Study Group. (2006). WHO Motor Development Study: Windows of achievement for six gross motor development milestones. Acta Paediatrica Supplement, 450, 86–95. [DOI] [PubMed]
- Yousafzai A.K., Rasheed M.A., Rizvi A., Armstrong R., Bhutta Z.A. Effect of integrated responsive stimulation and nutrition interventions in the Lady Health Worker programme in Pakistan on child development, growth, and health outcomes: A cluster-randomised factorial effectiveness trial. The Lancet. 2014;384(9950):1282–1293. doi: 10.1016/S0140-6736(14)60455-4. [DOI] [PubMed] [Google Scholar]