Abstract
To explore the association between DRD4 polymorphisms and schizophrenia risk, a meta-analysis was carried out with 41 case–control articles. Specifically, we included 28 articles (5,735 cases and 5,278 controls) that pertained to the 48 bp variable number tandem repeat (VNTR) polymorphism, nine articles (1,517 cases and 1,746 controls) that corresponded to the 12 bp tandem repeat (TR), six articles (1,912 cases and 1,836 controls) that addressed the 120 bp TR, 10 articles (2,927 cases and 2,938 controls) that entailed the −521 C>T polymorphism, six articles (1,735 cases and 1,724 controls) that pertained to the −616 C>G polymorphism, and four articles (1,191 cases and 1,215 controls) that involved the −376 C>T polymorphism. Pooled analysis, subgroup analysis, and sensitivity analysis were performed, and the data were visualized by means of forest and funnel plots. Results of pooled analysis indicated that the −521 CC variant (Pz=0.009, odds ratio [OR] =1.218, 95% confidence interval [CI] =1.050–1.413) and genotype L/L (ie, long allele) of the 120 bp TR were risk factors of schizophrenia (Pz=0.004, OR =1.275, 95% CI =1.081–1.504). The 48 bp VNTR, the 12 bp TR, the −616 C>G polymorphism, and the −376 C>T polymorphism were not associated with schizophrenia. Additional research is warranted to explore the association between polymorphisms of DRD4 and schizophrenia risk.
Keywords: DRD4, schizophrenia, meta-analysis, polymorphism
Introduction
Schizophrenia is a chronic, severe mental disorder with a tremendously variable clinical presentation. Results of studies in which schizophrenia occurrence was evaluated among twins or children who were adopted have shown that this disease results from an interaction of genetics and environmental factors.1 Specifically, schizophrenia is a multigene disease with a heritability of 60%–70%.2 Although the pathogenesis and etiology of schizophrenia are not understood fully,3 a large body of evidence has indicated that dopamine dysfunction is involved in the occurrence of this disease.4–6
Dopamine is an endogenous neurotransmitter that primarily functions by binding to dopamine receptors, which have five types. The D4 receptor has attracted attention in the field of schizophrenia research. In postmortem brain striatum of patients with schizophrenia, the density of D4 receptor was significantly higher than in brain tissues of unaffected patients; in contrast, the density of D2 and D3 receptors remained modest.7 This upregulation of D4 receptor has been shown to be related to the disease rather than to pharmacological effects of treatment.8 The pharmacological characteristics of D4 resemble those of D2 and D3, but the affinity of D4 for clozapine is an order of magnitude higher.9 Hence, DRD4 (chromosome 11p15.5) is a potential susceptibility gene for schizophrenia.10
The SZGene database is a viable resource for ascertaining the risk of schizophrenia.11,12 Other investigators have determined that the −521 C>T and 120 bp tandem repeat (TR) polymorphisms in DRD4 are associated with nominally significant summary odds ratios (ORs) as risk factors for schizophrenia (P=0.003 and 0.005, respectively).11 However, despite a great deal of research, an association between DRD4 polymorphisms and schizophrenia risk remains debatable.
TRs in DRD4 include a 48 bp variable number TR (VNTR), a 12 bp TR, and a 120 bp TR. The 48 bp VNTR is located in the third exon of DRD4 and encodes a sequence of 16 amino acids in the region of the third cytoplasmic loop. Polymorphisms in the 48 bp VNTR were found to differ in the recruitment of cellular cAMP.13 The 12 bp TR (rs4646983) is located in the first exon of DRD4, which corresponds to the N terminus of the gene product. Variants of the 12 bp TR modify an N-terminal glycosylation site, which affects expression levels of the membrane protein.14 The 120 bp TR is located 1.2 kb upstream from the initiation codon, and polymorphisms at this site affect transcriptional efficiency.15 Some researchers noted that the 120 bp TR was associated with attention-deficit hyperactivity disorder (ADHD)16,17 and schizophrenia.18 However, Tsutsumi et al19 demonstrated that the 120 bp TR was not related to the risk of schizophrenia.
The −521 C>T polymorphism (rs1800955), located in the promoter region of DRD4, has been shown to be associated with novelty seeking20,21 and schizophrenia.22 Mitsuyasu et al suggested that the −521C variant could be a risk factor for schizophrenia among female patients.23 However, other investigators found no relationship between −521 C>T and schizophrenia.24 The −616 C>G (rs747302) and the −376 C>T (rs916455) polymorphisms are located in the promoter region of DRD4; these variants have not been associated conclusively with schizophrenia risk. A pooled analysis of data regarding polymorphisms in DRD4 and schizophrenia risk is warranted.
Meta-analyses are proven tools for ascertaining associations of gene polymorphisms with disease.25–27 Several meta-analyses previously have addressed the potential associations between DRD4 polymorphisms and schizophrenia risk.28–31 However, the authors of these studies examined just one polymorphic locus28 or did not include the latest data.31 Herein, we describe the results of our meta-analysis of the association between DRD4 and schizophrenia risk.
Materials and methods
Literature searches
The SZGene, PubMed, and China National Knowledge Infrastructure (CNKI) databases were searched with the keywords “schizophrenia” and “DRD4”. Reference lists from relevant articles also were screened to identify additional studies.
Inclusion criteria and exclusion criteria
Studies with the following features were included in the meta-analysis: 1) case–control design; 2) involved patients with schizophrenia; 3) presented relevant data for case and control groups (eg, allele/genotype frequencies, sample size, ethnicity, schizophrenia diagnostic criteria, and control group source); 4) removed duplicate sample data; and 5) published before September 1, 2017. Studies were excluded for the following reasons: 1) no control group; 2) no usable genotype frequency data (attempts were made to contact authors via email for these data); and 3) duplicate reported sample data.
Statistical analysis
A meta-analysis was carried out using Stata Version 10.0 (StataCorp LP, College Station, TX, USA). The P-value of Hardy–Weinberg equilibrium (PHWE) was calculated for the control groups. ORs and 95% confidence intervals (CIs) were calculated to evaluate the strength of the associations. Under a random model,32,33 associations between DRD4 and the risk of schizophrenia were analyzed. A random model took into account population differences and heterogeneity among studies.25,34 Pairwise differences between genotypes (AA vs aa, Aa vs aa, and AA vs Aa [A being the risk factor]) were used to determine a suitable genetic model.35
The heterogeneity of the studies was determined by Cochran’s chi-square-based Q-statistic test.36 The degree of heterogeneity was expressed as I2 and was divided into low (I2<25%), medium (I2~50%), and high (I2>75%) heterogeneity groups.37 I2>50% was regarded as indicating substantial heterogeneity.38 Publication bias was calculated using Egger’s test and was represented as a funnel plot in which the standard error of log(OR) of each study was plotted against its log(OR). A sensitivity analysis was conducted to test the impact of removing each single study on the pooled result. Statistical power was calculated by means of the PS program, as described previously.39,40 P-values corresponding to association, heterogeneity, and publication bias tests were represented as Pz, Ph, and Pe, respectively. Statistical significance was defined as P<0.05 for all analyses.41
Results
Description of studies
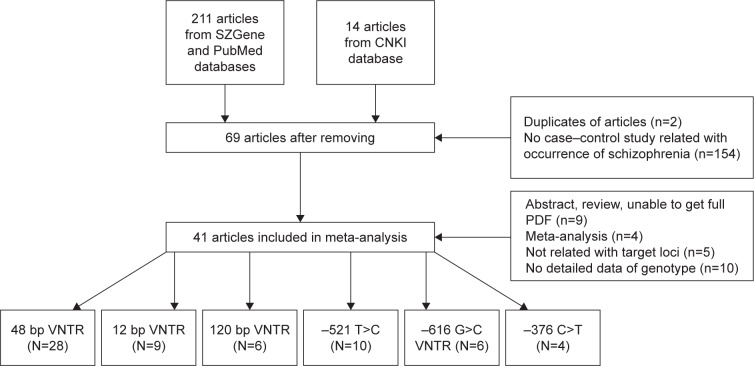
A total of 211 English-language articles were obtained from SZGene and PubMed, and 14 Chinese-language articles were obtained from CNKI. After removing duplicate studies and those that did not meet our inclusion criteria, 41 articles were used in the meta-analysis (Figure 1). Specifically, 28 articles addressed the 48 bp VNTR,23,42–68 nine articles involved the 12 bp TR,23,43,48–50,69–72 six articles pertained to the 120 bp TR,18,19,65,72–74 10 articles addressed the −521 C>T polymorphism,18,22–24,58,72–76 six articles referred to −616 C>G,18,23,72,74–76 and four articles entailed −376 C>T.18,23,72,75 Details of these studies are listed in Table 1. We omitted loci from our meta-analysis that were not represented in at least four articles.
Figure 1.
Study selection process in this meta-analysis.
Abbreviations: CNKI, China National Knowledge Infrastructure; VNTR, variable number tandem repeat.
Table 1.
Characteristics of studies that qualified to be included in the meta-analysis
| Author | Year | Country | Ethnicity | Controls source | Mean age of control group | Gender index (case) | Gender index (control) |
|---|---|---|---|---|---|---|---|
| Kaiser et al42 | 2000 | German | Caucasian | Hospital based | 43.53 | 0.83 | 0.34 |
| Kohn et al43,a | 1997 | Israel | Israeli | Hospital based | – | – | – |
| Kohn et al43,b | 1997 | Israel | Israeli | Hospital based | – | – | – |
| Serretti et al44,69 | 1999, 2001 | Italy | Caucasian | Hospital based | 47.45 | – | 1.27 |
| Hattori et al45 | 2009 | Japan | East Asian | Population based | 46.70 | 1.00 | 1.00 |
| Tanaka et al46 | 1995 | Japan | East Asian | Population based | 45.80 | 0.84 | 0.56 |
| Nanko et al47 | 1993 | Japan | East Asian | Population based | – | – | – |
| Petronis et al48 | 1995 | USA, Canada | Caucasian | Hospital based | – | – | – |
| Ohara et al49 | 1996 | Japan | East Asian | Population based | 34.40 | 0.99 | 1.37 |
| Aguirre et al50 | 2007 | Mexico | Indian | Population based | 40.00 | 0.97 | |
| Mitsuyasu et al23,c | 2007 | Japan | East Asian | Hospital based | 50.20 | 0.81 | 0.76 |
| Daniels et al51 | 1994 | UK | Caucasian | Hospital based | 49.60 | 0.80 | 0.68 |
| Sommer et al52 | 1993 | Minnesota | Caucasian | Population based | 65.00 | 0.44 | 1.61 |
| Rao et al53 | 1994 | USA | Caucasian | Population based | – | – | – |
| Hong et al54 | 1997 | Taiwan | East Asian | Hospital based | 28.70 | 0.68 | 0.62 |
| Jonsson et al55 | 1996 | Sweden | Caucasian | Population based | 38.70 | 0.573 | 0.73 |
| Rinetti et al56 | 2001 | Mexico | Mestizos | Hospital based | – | – | – |
| Fujiwara et al57 | 1997 | Japan | East Asian | Population based | – | – | – |
| Lung et al58 | 2006 | Taiwan | East Asian | Population based | 45.37 | – | – |
| Nakamura et al59 | 1995 | Japan | East Asian | Population based | – | – | – |
| Lung et al60 | 2009 | Taiwan | East Asian | Population based | – | – | – |
| Fresan et al61 | 2007 | Mexico | Caucasian | Population based | 34.60 | 0.45 | 94.23 |
| Zhang et al62 | 2003 | China | East Asian | Population based | 42.00 | 0.43 | – |
| Tang et al68 | 2001 | China | East Asian | Population based | 33.00 | 0.44 | 0.40 |
| Liang67 | 2005 | China | East Asian | Population based | 26.00 | 0.98 | 0.98 |
| Zhao et al63 | 2005 | China | East Asian | Population based | 34.00 | 0.88 | 0.88 |
| Zhao et al64 | 2006 | China | East Asian | Population based | 29.40 | 0.84 | 1.00 |
| Chen et al65 | 2016 | China | East Asian | Hospital based | 39.19 | 0.81 | 0.89 |
| Lu et al66 | 2003 | China | East Asian | Population based | 65.00 | 0.74 | 1.22 |
| Serretti et al69 | 1999 | Italy | Caucasian | Population based | – | – | – |
| Hong et al70 | 1998 | Taiwan | East Asian | Hospital based | 30.20 | 28.70 | 0.62 |
| Catalano et al71 | 1993 | Italy | Caucasian | Hospital based | 46.90 | 30.00 | 1.34 |
| Nakajima et al72 | 2007 | Japan | East Asian | Population based | 47.00 | 46.70 | 1.00 |
| Okuyama et al22 | 1999 | Japan | East Asian | Population based | 47.10 | 47.90 | 0.63 |
| Mitsuyasu et al75,d | 2001 | Japan | East Asian | Hospital based | 51.50 | 50.50 | 0.75 |
| Jonsson et al24 | 2001 | Sweden | Caucasian | Population based | 44.80 | 42.60 | 0.80 |
| Pai et al73 | 2015 | India | Indian | Population based | – | – | – |
| Xing et al18 | 2003 | China | East Asian | Hospital based | 41.20 | 41.80 | – |
| Lai et al74 | 2010 | China | East Asian | Hospital based | 40.60 | 43.20 | 1.00 |
| Zhong et al76 | 2010 | China | East Asian | Population based | 39.20 | 37.50 | 1.00 |
| Tsutsumi et al19 | 2011 | Japan | East Asian | Hospital based | 47.20 | 42.10 | 0.50 |
Notes: Gender index = female/male.
Ethnicity is Ashkenazi which included Jews whose origin (or whose parents’ origin), was in European countries, apart from the Balkans;
ethnicity is non-Ashkenazi which included Jews whose origin was in North Africa or Asia.
Included 48 bp VNTR, 12 bp TR, and 120 bp TR;
did not include 48 bp VNTR, 12 bp TR, and 120 bp TR.
Results of data analysis
No association between the 48 bp VNTR and schizophrenia risk
Allele frequencies of the 48 bp VNTR are listed in Table 2. Results of pooled analyses are summarized in Table 3, and data from subgroup analyses are depicted in Table 4. We were unable to obtain specific data regarding the number of 7-repeat (7R) alleles in the 48 bp VNTR,44 despite multiple attempts to contact the corresponding author. Thus, this study was omitted from our analysis of an association between 7R and schizophrenia risk. When we conducted a pooled analysis of the remaining 5,316 cases and 4,677 controls, we found that 7R was not associated with schizophrenia risk (Pz=0.349, OR =1.071, 95% CI =0.928–1.236) under a random effects model with a power of 0.271 (Table 3 and Figure S1).35 No association was found in subgroup analysis by ethnicity (ie, Caucasian [Pz=0.238, OR =1.127, 95% CI =0.924–1.375], East Asian [Pz=0.901, OR =0.966, 95% CI =0.560–1.667], Indian [Pz=0.211, OR =0.772, 95% CI =0.514–1.158], Mestizos [Pz=0.310, OR =1.413, 95% CI =0.725–2.754], and Israeli [Pz=0.512, OR =1.164, 95% CI =0.739–1.835]). Moreover, no association of 7R with the risk of schizophrenia was ascertained in subgroup analysis by source of controls. No significant heterogeneity was found in the pooled or subgroup analyses.
Table 2.
Allele frequency of 48 bp VNTR polymorphism
| Author | Allele distribution
|
Allele frequency
|
||||||
|---|---|---|---|---|---|---|---|---|
| Cases (n)
|
Controls (n)
|
Cases (n)
|
Controls (n)
|
|||||
| Short (≤4) | Long (≥5) | Short (≤4) | Long (≥5) | 7R | Others | 7R | Others | |
| Kaiser et al42 | 1,006 | 270 | 1,182 | 322 | 232 | 1,044 | 282 | 1,222 |
| Kohn et al43,a | – | – | – | – | 22 | 76 | 52 | 238 |
| Kohn et al43,b | – | – | – | – | 13 | 85 | 17 | 101 |
| Serretti et al44,69 | 709 | 129 | 990 | 212 | – | – | – | – |
| Hattori et al45 | 1,066 | 54 | 1,076 | 60 | 2 | 1,118 | 6 | 1,130 |
| Tanaka et al46 | 134 | 6 | 133 | 7 | 0 | 140 | 2 | 138 |
| Nanko et al47 | 148 | 12 | 152 | 10 | 2 | 158 | 0 | 162 |
| Petronis et al48 | 77 | 23 | 154 | 46 | 21 | 79 | 32 | 168 |
| Ohara et al49 | 286 | 20 | 227 | 15 | 18 | 288 | 10 | 232 |
| Aguirre et al50 | 120 | 72 | 224 | 114 | 46 | 146 | 98 | 240 |
| Mitsuyasu et al23,c | 406 | 18 | 447 | 27 | 1 | 423 | 3 | 471 |
| Daniels et al51 | 159 | 53 | 193 | 45 | 52 | 160 | 45 | 193 |
| Sommer et al52 | 182 | 48 | 171 | 59 | 43 | 187 | 54 | 176 |
| Rao et al53 | 39 | 17 | 31 | 9 | 17 | 39 | 8 | 32 |
| Hong et al54 | 172 | 6 | 78 | 6 | 0 | 178 | 0 | 84 |
| Jonsson et al55 | 187 | 49 | 124 | 28 | 42 | 194 | 23 | 129 |
| Rinetti et al56 | 36 | 38 | 48 | 26 | 31 | 43 | 25 | 49 |
| Fujiwara et al57 | 34 | 0 | 22 | 0 | 0 | 34 | 0 | 22 |
| Lung et al58 | 1,216 | 44 | 846 | 10 | 3 | 1,257 | 0 | 856 |
| Nakamura et al59 | 189 | 13 | 98 | 6 | 0 | 202 | 0 | 104 |
| Lung et al60 | 1,774 | 54 | 838 | 8 | 0 | 1,828 | 0 | 846 |
| Fresan et al61 | 88 | 54 | 285 | 119 | 47 | 95 | 104 | 300 |
| Zhang et al62 | 131 | 3 | 145 | 7 | 0 | 134 | 0 | 152 |
| Tang et al68 | 980 | 40 | 332 | 10 | 1 | 1,019 | 0 | 342 |
| Liang67 | 176 | 26 | 185 | 25 | 4 | 198 | 2 | 208 |
| Zhao et al63 | 78 | 246 | 75 | 249 | 3 | 321 | 6 | 318 |
| Zhao et al64 | 41 | 121 | 40 | 136 | 0 | 162 | 3 | 173 |
| Chen et al65 | 219 | 49 | 299 | 37 | 0 | 268 | 0 | 336 |
| Lu et al66 | 155 | 5 | 157 | 3 | 1 | 159 | 0 | 160 |
Notes:
Ethnicity is Ashkenazi which included Jews whose origin (or whose parents’ origin), was in European countries, apart from the Balkans;
ethnicity is non-Ashkenazi which included Jews whose origin was in North Africa or Asia.
Included 48 bp VNTR, 12 bp TR, and 120 bp TR.
Abbreviations: 7R, 7 repeat; VNTR, variable number tandem repeat.
Table 3.
Pooled associations of DRD4 polymorphisms and schizophrenia
| Loci | Genetic model | Studies (n) |
Statistical model | OR | 95% CI | Pz | I2 | Ph | Pe |
|---|---|---|---|---|---|---|---|---|---|
| 48 bp VNTR | Allele contrast (7R and others) | 27 | Random | 1.071 | 0.928–1.236 | 0.349 | 8.1 | 0.352 | 0.727 |
| Allele contrast (S and L) | 27 | Random | 1.135 | 0.988–1.303 | 0.073 | 44.0 | 0.009 | 0.151 | |
| 12 bp TR | Allele contrast | 9 | Random | 1.037 | 0.885–1.215 | 0.659 | 0.0 | 0.931 | 0.584 |
| Homozygous codominant | 9 | Random | 0.756 | 0.434–1.317 | 0.323 | 0.0 | 0.729 | 0.214 | |
| Heterozygous codominant | 9 | Random | 1.117 | 0.930–1.341 | 0.236 | 0.0 | 0.644 | 0.077 | |
| Dominant | 9 | Random | 1.083 | 0.907–1.293 | 0.377 | 0.0 | 0.834 | 0.192 | |
| Recessive | 9 | Random | 0.724 | 0.417–1.259 | 0.253 | 0.0 | 0.681 | 0.180 | |
| 120 bp TR | Allele contrast | 6 | Random | 1.189 | 1.040–1.358 | 0.011 | 37.1 | 0.159 | 0.701 |
| Homozygous codominant | 6 | Random | 1.291 | 0.892–1.868 | 0.176 | 47.2 | 0.092 | 0.213 | |
| Heterozygous codominant | 6 | Random | 1.010 | 0.744–1.372 | 0.949 | 22.9 | 0.262 | 0.223 | |
| Dominant | 6 | Random | 1.152 | 0.837–1.584 | 0.386 | 34.3 | 0.179 | 0.176 | |
| Recessive | 6 | Random | 1.275 | 1.081–1.504 | 0.004 | 33.1 | 0.187 | 0.756 | |
| −521 T>C | Allele contrast | 10 | Random | 1.113 | 1.024–1.209 | 0.011 | 16.2 | 0.294 | 0.628 |
| Homozygous codominant | 10 | Random | 1.240 | 1.041–1.477 | 0.016 | 18.7 | 0.271 | 0.765 | |
| Heterozygous codominant | 10 | Random | 1.105 | 0.971–1.256 | 0.129 | 13.8 | 0.316 | 0.751 | |
| Dominant | 10 | Random | 1.136 | 1.004–1.289 | 0.043 | 19.2 | 0.266 | 0.620 | |
| Recessive | 10 | Random | 1.177 | 1.024–1.353 | 0.021 | 0.0 | 0.467 | 0.812 | |
| −616 G>C | Allele contrast | 6 | Random | 1.103 | 0.991–1.226 | 0.071 | 6.7 | 0.373 | 0.604 |
| Homozygous codominant | 6 | Random | 0.637 | 0.469–0.866 | 0.004 | 46.4 | 0.096 | 0.488 | |
| Heterozygous codominant | 6 | Random | 1.123 | 0.974–1.296 | 0.110 | 0.0 | 0.986 | 0.169 | |
| Dominant | 6 | Random | 1.133 | 0.991–1.295 | 0.068 | 0.0 | 0.889 | 0.965 | |
| Recessive | 6 | Random | 1.140 | 0.840–1.548 | 0.400 | 48.3 | 0.085 | 0.338 | |
| −376 C>T | Allele contrast | 4 | Random | 1.124 | 0.940–1.344 | 0.198 | 0.0 | 0.707 | 0.200 |
| Homozygous codominant | 4 | Random | 0.854 | 0.416–1.749 | 0.665 | 0.0 | 0.996 | 0.456 | |
| Heterozygous codominant | 4 | Random | 0.730 | 0.351–1.520 | 0.401 | 0.0 | 0.993 | 0.911 | |
| Dominant | 4 | Random | 0.820 | 0.401–1.676 | 0.586 | 0.0 | 0.994 | 0.583 | |
| Recessive | 4 | Random | 1.171 | 0.962–1.425 | 0.117 | 0.0 | 0.707 | 0.214 |
Notes: L, long allele; S, short allele.
Abbreviations: CI, confidence interval; OR, odds ratio; 7R, 7 repeat; TR, tandem repeat; VNTR, variable number TR.
Table 4.
Subgroup associations of DRD4 polymorphisms with schizophrenia
| Polymorphism | Subgroup analysis | Studies (n) | OR | 95% CI | Pz | Ph | I2 |
|---|---|---|---|---|---|---|---|
| 48 bp VNTR (7R and others) | Overall | 22 | 1.095 | 0.953–1.259 | 0.349 | 0.352 | 8.1 |
| Ethnicity | |||||||
| Caucasian | 7 | 1.127 | 0.924–1.375 | 0.238 | 0.195 | 30.6 | |
| East Asian | 11 | 0.966 | 0.560–1.667 | 0.901 | 0.413 | 3.1 | |
| Indian | 1 | 0.772 | 0.514–1.158 | 0.211 | – | – | |
| Mestizos | 1 | 1.413 | 0.725–2.754 | 0.310 | – | – | |
| Israeli | 2 | 1.164 | 0.739–1.835 | 0.512 | 0.441 | 0.0 | |
| Source of controls | |||||||
| Population based | 15 | 1.031 | 0.796–1.336 | 0.816 | 0.242 | 18.9 | |
| Hospital based | 7 | 1.070 | 0.917–1.248 | 0.393 | 0.490 | 0.0 | |
| 48 bp VNTR (S and L) | Overall | 26 | 1.147 | 1.003–1.312 | 0.073 | 0.009 | 44.0 |
| Ethnicity | |||||||
| Caucasian | 8 | 1.037 | 0.882–1.219 | 0.662 | 0.173 | 32.0 | |
| East Asian | 16 | 1.165 | 0.919–1.475 | 0.206 | 0.014 | 49.1 | |
| Indian | 1 | 1.179 | 0.815–1.705 | 0.382 | – | – | |
| Mestizos | 1 | 1.949 | 1.007–3.770 | 0.048 | – | – | |
| Israeli | |||||||
| Source of controls | |||||||
| Population based | 18 | 1.165 | 0.977–1.390 | 0.089 | 0.069 | 35.4 | |
| Hospital based | 8 | 1.091 | 0.862–1.381 | 0.468 | 0.016 | 59.2 | |
| 12 bp TR | Overall | 10 | 1.083 | 0.907–1.293 | 0.377 | 0.834 | 0.0 |
| Ethnicity | |||||||
| Indian | 1 | 0.927 | 0.533–1.612 | 0.788 | – | – | |
| Caucasian | 3 | 0.787 | 0.509–1.218 | 0.283 | 0.611 | 0.0 | |
| East Asian | 4 | 1.178 | 0.949–1.462 | 0.137 | 0.910 | 0.0 | |
| Israeli | 2 | 1.312 | 0.642–2.679 | 0.456 | 0.610 | 0.0 | |
| Source of controls | |||||||
| Population based | 4 | 1.133 | 0.908–1.413 | 0.270 | 0.710 | 0.0 | |
| Hospital based | 6 | 1.012 | 0.706–1.450 | 0.949 | 0.272 | 21.5 | |
| 120 bp TR | Overall | 6 | 1.275 | 1.081–1.504 | 0.004 | 0.187 | 33.1 |
| Ethnicity | |||||||
| East Asian | 5 | 1.317 | 1.108–1.565 | 0.002 | 0.196 | 33.9 | |
| Indian | 1 | 0.979 | 0.629–1.524 | 0.924 | – | – | |
| Source of controls | |||||||
| Population based | 2 | 1.102 | 0.892–1.360 | 0.368 | 0.551 | 0.0 | |
| Hospital based | 4 | 1.319 | 1.134–1.708 | 0.002 | 0.225 | 31.2 | |
| −521 T>C | Overall | 10 | 1.177 | 1.024–1.353 | 0.021 | 0.467 | 0.0 |
| Ethnicity | |||||||
| Caucasian | 1 | 1.136 | 0.670–1.925 | 0.636 | – | – | |
| East Asian | 8 | 1.218 | 1.050–1.413 | 0.009 | 0.571 | 0.0 | |
| Indian | 1 | 0.715 | 0.395–1.293 | 0.267 | |||
| Source of controls | |||||||
| Population based | 6 | 1.188 | 0.972–1.451 | 0.092 | 0.258 | 23.5 | |
| Hospital based | 4 | 1.143 | 0.901–1.450 | 0.270 | 0.561 | 0.0 | |
| −616 G>C | Overall | 6 | 1.133 | 0.991–1.295 | 0.068 | 0.889 | 0.0 |
| Source of controls | |||||||
| Population based | 2 | 1.117 | 0.915–1.364 | 0.275 | 0.966 | 0.0 | |
| Hospital based | 4 | 1.146 | 0.956–1.317 | 0.140 | 0.645 | 0.0 | |
| −376 C>T | Overall | 4 | 1.171 | 0.962–1.425 | 0.117 | 0.707 | 0.0 |
| Source of controls | |||||||
| Population based | 1 | 1.079 | 0.805–1.447 | 0.611 | – | – | |
| Hospital based | 3 | 1.252 | 0.960–1.632 | 0.117 | 0.653 | 0.0 |
Notes: L, long allele; S, short allele.
Abbreviations: CI, confidence interval; OR, odds ratio; 7R, 7 repeat; TR, tandem repeat; VNTR, variable number TR.
To incorporate data from the study of Serretti et al,44 the 48 bp VNTR was classified into S (short allele, ≤4 TRs) and L (long allele, ≥5 TRs) groups. In the study by Kohn et al,43 the 48 bp VNTR data could not be categorized into S and L groups, so this study was omitted from the analysis. The remaining data comprised 5,637 cases and 5,074 controls (Table 3 and Figure S2). Results of a pooled analysis indicated no relationship between this polymorphism and schizophrenia risk (Pz=0.073, OR =1.135, 95% CI =0.988–1.303) with a power of 0.909. No association was found in the subgroup analysis by source of control or by ethnicity, except for Mestizos (Pz=0.048, OR =1.949, 95% CI =1.007–3.77). Significant heterogeneity was found in the pooled analysis (Pe=0.009, I2=44%) and in the subgroup analysis by ethnicity in the East Asian subgroup (Pe=0.014, I2=49.1%) and by source of control in the hospital-based subgroup (Pe=0.016, I2 =59.2%).
No association between the 12 bp TR and schizophrenia risk
To evaluate the relationship between the 12 bp TR and the risk of schizophrenia, 1,517 cases and 1,746 controls were included (Table 5 and Figures S3–S7). Allele groups were defined as in (ie, inserted) and de (ie, deleted). In the dominant model,34,35 the pooled OR using a random effects model was 1.083 (Pz=0.377, 95% CI =0.907–1.293) with a power of 0.154 (Table 3). No association was found in subgroup analysis by ethnicity or by source of controls (Table 4). No significant heterogeneity was observed in the pooled or subgroup analyses.
Table 5.
Genotype distribution and allele frequency of 12 bp TR
| Author | Genotype distribution
|
PHWE | Allele frequency
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases, n
|
Controls, n
|
Case (%)
|
Controls (%)
|
||||||||
| in/in | in/de | de/de | in/in | in/de | de/de | in | de | in | de | ||
| Petronis et al48 | 43 | 6 | 1 | 80 | 20 | 0 | 0.267 | 92.00 | 8.00 | 90.00 | 10.00 |
| Ohara et al49 | 144 | 9 | 0 | 116 | 5 | 0 | 0.816 | 97.06 | 2.94 | 97.93 | 2.07 |
| Serretti et al44,69 | 184 | 28 | 0 | 225 | 37 | 1 | 0.689 | 0.93 | 0.07 | 0.93 | 0.07 |
| Aguirre et al50 | 48 | 34 | 1 | 75 | 55 | 4 | 0.102 | 78.31 | 21.69 | 76.49 | 23.51 |
| Mitsuyasu et al23,a | 136 | 56 | 5 | 176 | 53 | 10 | 0.027 | 83.20 | 16.70 | 84.70 | 15.30 |
| Kohn et al43,b | 40 | 9 | 0 | 126 | 19 | 0 | 0.311 | 94.00 | 6.00 | 91.00 | 9.00 |
| Kohn et al43,c | 44 | 4 | 1 | 53 | 6 | 0 | 0.416 | 94.00 | 6.00 | 94.00 | 6.00 |
| Hong et al54 | 68 | 10 | 2 | 35 | 7 | 0 | 0.556 | 91.25 | 8.75 | 91.70 | 8.30 |
| Catalano et al71 | 76 | 3 | 0 | 69 | 6 | 0 | 0.718 | 98.10 | 1.90 | 96.00 | 4.00 |
| Nakajima et al72 | 413 | 140 | 12 | 431 | 119 | 18 | 0.008 | 85.50 | 14.50 | 86.50 | 13.50 |
Notes: PHWE, P-value of Hardy–Weinberg equilibrium.
Included 48 bp VNTR, 12 bp TR, and 120 bp TR.
Ethnicity is Ashkenazi which included Jews whose origin (or whose parents’ origin), was in European countries, apart from the Balkans;
ethnicity is non-Ashkenazi which included Jews whose origin was in North Africa or Asia.
Abbreviations: de, deleted; in, inserted; TR, tandem repeat.
Genotype L/L of the 120 bp TR might be a risk factor for schizophrenia
In a random model, a pooled analysis was conducted (1,912 cases and 1,836 controls) to evaluate the relationship between genotype L/L of the 120 bp TR and schizophrenia risk (Table 6 and Figures S8–S12). In the recessive model,34,35 genotype L/L was found to be a potential risk factor for schizophrenia (Pz=0.004, OR =1.275, 95% CI =1.081–1.504) with a power of 0.959 (Table 3). Findings from subgroup analysis indicated significant associations in East Asian (Pz=0.002, OR =1.317, 95% CI =1.108–1.565) and hospital-based subgroups (Pz=0.002, OR =1.319, 95% CI =1.134–1.708) (Table 4). No association was found for the other subgroups, and no significant heterogeneity was ascertained in the pooled or subgroup analyses.
Table 6.
Genotype distribution and allele frequency of 120 bp TR
| Author | Genotype distribution
|
PHWE | Allele frequency
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases, n
|
Controls, n
|
Cases (%)
|
Controls (%)
|
||||||||
| S/S | S/L | L/L | S/S | S/L | L/L | S | L | S | L | ||
| Mitsuyasu et al23,a | 10 | 75 | 129 | 13 | 87 | 139 | 0.898 | 77.80 | 22.20 | 76.40 | 23.60 |
| Pai et al73 | 23 | 77 | 87 | 11 | 61 | 64 | 0.501 | 32.90 | 67.10 | 30.50 | 69.50 |
| Xing et al18 | 20 | 77 | 113 | 28 | 98 | 80 | 0.816 | 27.90 | 72.10 | 37.40 | 62.60 |
| Nakajima et al72 | 24 | 183 | 362 | 33 | 192 | 345 | 0.363 | 20.00 | 80.00 | 23.00 | 78.00 |
| Tsutsumi et al19 | 24 | 138 | 248 | 16 | 158 | 211 | 0.041 | 22.68 | 77.32 | 24.68 | 75.32 |
| Lai et al74 | 28 | 161 | 133 | 40 | 166 | 94 | 0.013 | 33.70 | 66.30 | 41.00 | 59.00 |
Notes: L, long allele; PHWE, P-value of Hardy–Weinberg equilibrium; S, short allele.
Included 48 bp VNTR, 12 bp TR, and 120 bp TR.
Abbreviation: TR, tandem repeat.
The −521 CC variant might be a risk factor for schizophrenia
Pooled and subgroup analyses were performed in a random model with 2,927 cases and 2,938 controls (Table 7 and Figures S13–S17). In the recessive model, −521 CC was found to be a potential risk factor for schizophrenia in the pooled analysis (Pz=0.021, OR =1.177, 95% CI =1.024–1.353) with a power of 0.656 (Table 3). In subgroup analyses by ethnicity and source of controls, the association was only detected in the East Asian subgroup (Pz=0.009, OR =1.218, 95% CI =1.050–1.413) (Table 4). No significant heterogeneity was noted in the pooled or subgroup analyses.
Table 7.
Genotype distribution and allele frequency of −521 C>T
| Author | Genotype distribution
|
PHWE | Allele frequency
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases, n
|
Controls, n
|
Cases (%)
|
Controls (%)
|
||||||||
| CC | CT | TT | CC | CT | TT | C | T | C | T | ||
| Okuyama et al22 | 58 | 125 | 69 | 38 | 142 | 89 | 0.119 | 48.00 | 52.00 | 41.00 | 59.00 |
| Mitsuyasu et al23,a | 33 | 106 | 67 | 31 | 115 | 93 | 0.623 | 41.75 | 58.25 | 37.05 | 62.95 |
| Mitsuyasu et al75,b | 25 | 122 | 61 | 25 | 110 | 75 | 0.109 | 41.30 | 58.70 | 38.10 | 61.90 |
| Lung et al58 | 80 | 320 | 230 | 48 | 204 | 173 | 0.294 | 38.10 | 61.90 | 35.30 | 64.70 |
| Jonsson et al24 | 23 | 74 | 35 | 60 | 205 | 118 | 0.061 | 45.50 | 54.50 | 42.00 | 58.00 |
| Pai et al73 | 27 | 92 | 62 | 26 | 77 | 29 | 0.055 | 40.30 | 59.70 | 48.90 | 51.10 |
| Xing et al18 | 37 | 103 | 70 | 25 | 111 | 70 | 0.059 | 42.10 | 57.90 | 39.10 | 60.90 |
| Nakajima et al72 | 106 | 270 | 190 | 89 | 285 | 195 | 0.368 | 43.00 | 58.00 | 41.00 | 59.00 |
| Lai et al74 | 87 | 115 | 120 | 81 | 95 | 124 | – | 44.88 | 55.12 | 42.83 | 57.17 |
| Zhong et al76 | 62 | 78 | 60 | 53 | 64 | 83 | – | 45.91 | 54.09 | 42.50 | 57.50 |
Notes: PHWE, P-value of Hardy–Weinberg equilibrium.
Included 48 bp VNTR, 12 bp TR, and 120 bp TR;
did not include 48 bp VNTR, 12 bp TR, and 120 bp TR.
Abbreviation: TR, tandem repeat.
No association between −616 C>G and the risk of schizophrenia
In a random model, pooled (Table 3) and subgroup (Table 4) analyses were performed with 1,735 cases and 1,724 controls (Table 8 and Figures S18–S22). Using the dominant model, results of the pooled analysis indicated a lack of an association between −616 C>G and schizophrenia risk (Pz=0.068, OR =1.133, 95% CI =0.991–1.295) with a power of 0.45. All cases and controls in this analysis corresponded to the East Asian subgroup. Findings from a subgroup analysis of source of controls showed no association. There was no significant heterogeneity in the pooled or subgroup analyses.
Table 8.
Genotype distribution and allele frequency of −616 C>G
| Author | Genotype distribution
|
PHWE | Allele frequency
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases, n
|
Controls, n
|
Cases (%)
|
Controls (%)
|
||||||||
| GG | GC | CC | GG | GC | CC | G | C | G | C | ||
| Mitsuyasu et al23,a | 102 | 89 | 19 | 112 | 98 | 30 | 0.243 | 69.80 | 30.20 | 67.10 | 32.90 |
| Mitsuyasu et al75,b | 89 | 89 | 30 | 100 | 85 | 25 | 0.296 | 64.20 | 35.80 | 67.90 | 32.10 |
| Xing et al18 | 83 | 100 | 27 | 91 | 102 | 13 | 0.025 | 63.30 | 36.70 | 68.90 | 31.10 |
| Nakajima et al72 | 267 | 248 | 49 | 285 | 224 | 59 | 0.134 | 69.00 | 31.00 | 69.50 | 29.50 |
| Lai et al74 | 161 | 113 | 48 | 166 | 102 | 32 | 0.009 | 67.55 | 32.45 | 72.33 | 27.67 |
| Zhong et al76 | 112 | 77 | 31 | 107 | 68 | 25 | 0.010 | 68.41 | 31.59 | 70.50 | 29.50 |
Notes: PHWE, P-value of Hardy–Weinberg equilibrium.
Included 48 bp VNTR, 12 bp TR, and 120 bp TR;
did not include 48 bp VNTR, 12 bp TR, and 120 bp TR.
Abbreviation: TR, tandem repeat.
No association of the −376 C>T variant with schizophrenia
We assessed the relationship between the −376 C>T polymorphism and schizophrenia risk in pooled and subgroup analyses of 1,191 cases and 1,215 controls in a random model (Tables 3, 4, and 9 and Figures S23–S27). In the recessive model, −376 C>T was not associated with the risk of schizophrenia in a pooled analysis (Pz=0.117, OR =1.171, 95% CI =0.962–1.425) with a power of 0.357 (Table 3). No association was detected in subgroup analyses by ethnicity or source of controls (Table 4). No significant heterogeneity was ascertained in the pooled or subgroup analyses.
Table 9.
Genotype distribution and allele frequency of −376 C>T
| Author | Genotype distribution
|
PHWE | Allele frequency
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases, n
|
Controls, n
|
Cases (%)
|
Controls (%)
|
||||||||
| CC | CT | TT | CC | CT | TT | C | T | C | T | ||
| Mitsuyasu et al23,a | 177 | 34 | 1 | 193 | 43 | 1 | 0.39 | 91.50 | 8.50 | 90.45 | 9.45 |
| Mitsuyasu et al75,b | 179 | 28 | 1 | 168 | 41 | 1 | 0.367 | 92.80 | 0.72 | 89.80 | 1.02 |
| Xing et al74 | 137 | 66 | 7 | 127 | 74 | 5 | 0.126 | 81.00 | 19.00 | 79.60 | 20.40 |
| Nakajima et al72 | 453 | 100 | 8 | 447 | 108 | 7 | 0.869 | 90.00 | 10.00 | 89.50 | 10.50 |
Notes: PHWE, P-value of Hardy–Weinberg equilibrium.
Included 48 bp VNTR, 12 bp TR, and 120 bp TR;
did not include 48 bp VNTR, 12 bp TR, and 120 bp TR.
Abbreviation: TR, tandem repeat.
Sensitivity analysis
The results of sensitivity analyses showed that the combined ORs did not change significantly for meta-analyses in which each study was omitted singly. Thus, the results were considered stable and reasonable.
Publication bias
Potential publication bias was found in funnel plots in which the standard error of log(OR) of each study was plotted against its log(OR). No evidence of publication bias was found in pooled analyses (Figures 2–8).
Figure 2.
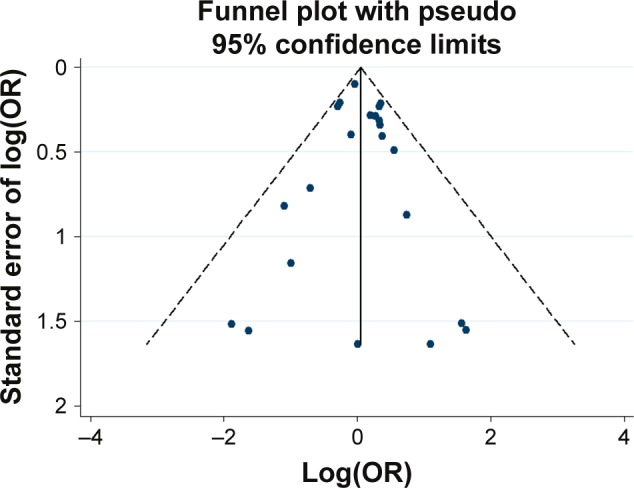
Funnel plot analysis for the detection of publication bias in the association between the 48 bp VNTR (7R vs others) and schizophrenia.
Abbreviations: OR, odds ratio; 7R, 7 repeat; VNTR, variable number tandem repeat.
Figure 3.
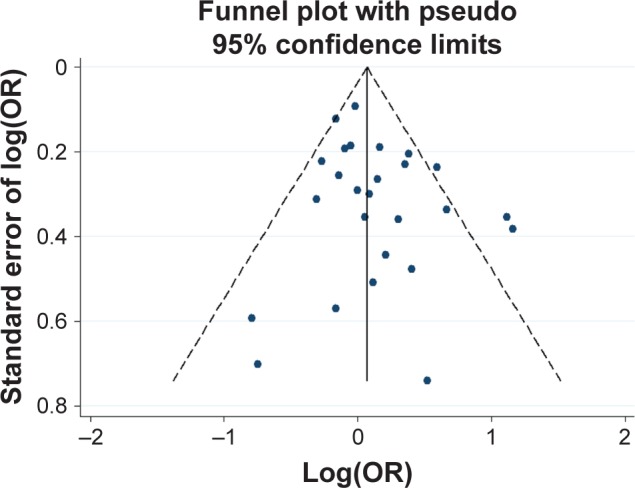
Funnel plot analysis for the detection of publication bias in the association between 48 bp VNTR (L vs S) and schizophrenia.
Notes: L, long allele; S, short allele.
Abbreviations: OR, odds ratio; VNTR, variable number tandem repeat.
Figure 4.
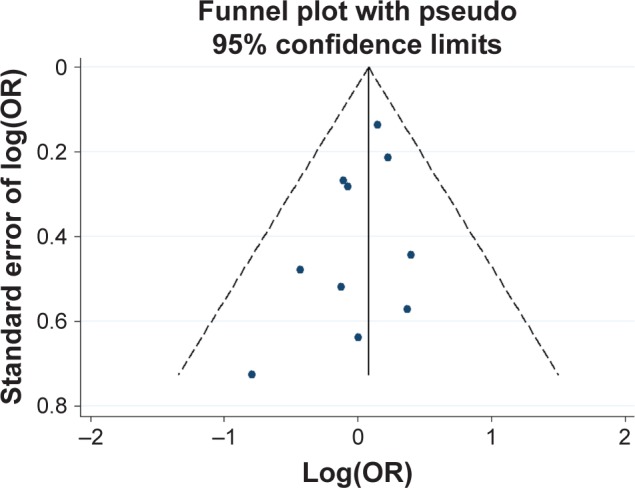
Funnel plot analysis for the detection of publication bias in the association between 12 bp TR and schizophrenia.
Abbreviations: OR, odds ratio; TR, tandem repeat.
Figure 5.
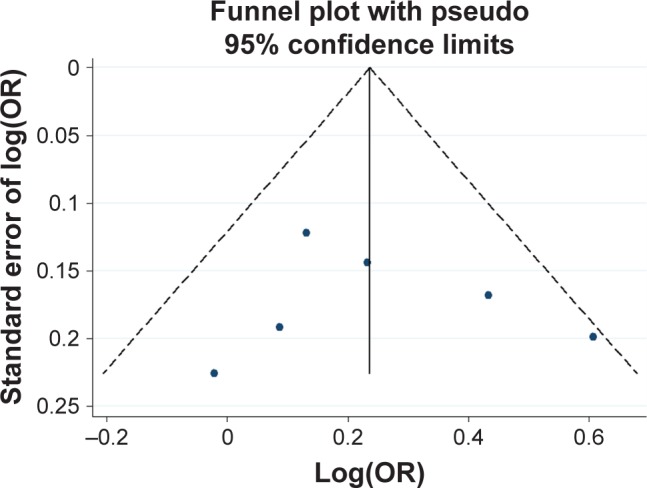
Funnel plot analysis for the detection of publication bias in the association between 120 bp TR and schizophrenia.
Abbreviations: OR, odds ratio; TR, tandem repeat.
Figure 6.
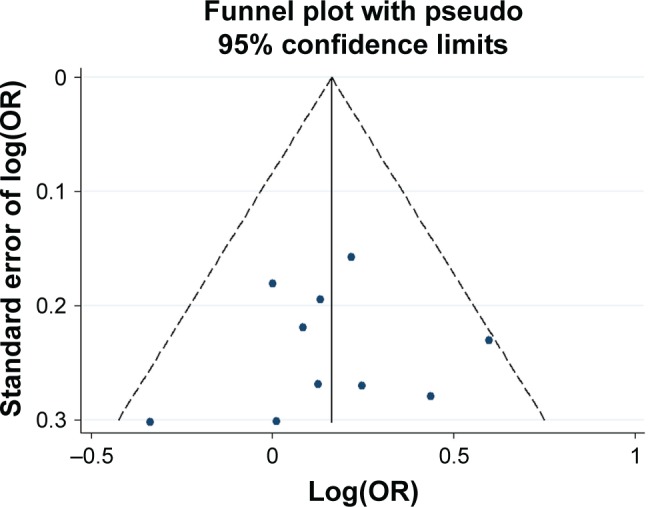
Funnel plot analysis for the detection of publication bias in the association between −521 C>T and schizophrenia.
Abbreviation: OR, odds ratio.
Figure 7.
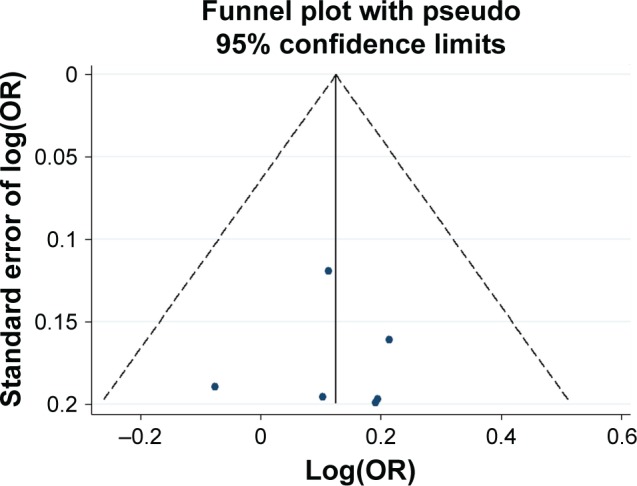
Funnel plot analysis for the detection of publication bias in the association between −616 C>G and schizophrenia.
Abbreviation: OR, odds ratio.
Figure 8.
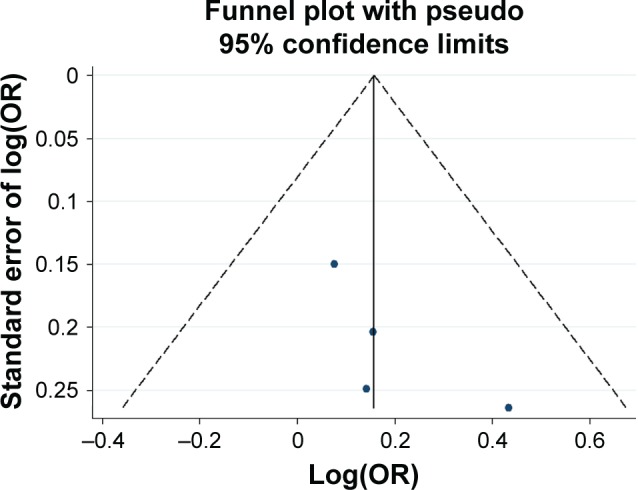
Funnel plot analysis for the detection of publication bias in the association between −376 C>T and schizophrenia.
Abbreviation: OR, odds ratio.
Discussion
Results of other studies have associated the 7R polymorphism with ADHD in a meta-analysis77 and with increased brain activity to unpleasant stimuli.78 We sought to determine whether 7R was also associated with schizophrenia risk. Findings of our pooled and subgroup analyses indicated that 7R was not associated with the risk of schizophrenia. Similarly, we found that the 48 bp VNTR (classified into L and S groups) was not associated with schizophrenia risk in most of our pooled and subgroup analyses, which is consistent with previously published meta-analyses.29,30 Only in the Mestizos subgroup, an association was detected. Our literature search yielded one article addressing Mestizos patients, and this article had an insufficient sample size to verify this association. Hence, the utility of the 48 bp VNTR as a means to assess schizophrenia risk in the Mestizo population warrants additional investigation. Lung et al28 demonstrated an association between the 48-bp VNTR and schizophrenia risk but noted that sample bias might have led to a false-positive result.29
We determined that the L/L genotype of the 120 bp TR and the −521 CC variant might be risk factors for schizophrenia among East Asians; this relationship was not found in other populations. This discrepancy between East Asians and other populations might have resulted from the small sample sizes of the other ethnicity subgroups, the distinct genetic backgrounds, or different demographic or lifestyle factors within the subgroups. The statistical power for the pooled analysis of the 12 bp TR, the −616 C>G polymorphism, and the −376 C>T variant was low. Therefore, these results will need to be validated further. In a study of linkage disequilibrium (LD) of DRD4 that included 17 polymorphisms,23 the authors found no LD between −521 T>C and 120 bp TR (r2=0.00). For all pairs of −616 C>G, −376 C>T, 12 bp TR, and 48 bp VNTR, no significant LD was observed.
Multiple meta-analyses have been conducted to date on the association between DRD4 and schizophrenia. The current meta-analysis included some new studies, involved a large sample size, and had high statistical power. We addressed six loci in DRD4; no other meta-analysis involving four of these loci (12 bp TR, 120 bp TR, −616 C>G, and −376 C>T) has been carried out. Moreover, we conducted subgroup analysis by ethnicity and by source of controls and included data both from SZGene and the CNKI databases.
The results described herein should be interpreted with caution. The present study was limited by a lack of exact allele/genotype frequencies for some of the included articles, despite our efforts to acquire this information from the corresponding authors. Therefore, these articles were omitted from the meta-analysis. Second, controls in some of the articles did not conform to Hardy–Weinberg equilibrium because of sample bias. Third, case–control studies were included in this meta-analysis, but family-based studies were not.77 The ability to exploit cosegregation of variants with disease within families helps distinguish causal from noncausal factors. Family-based studies are more powerful to detect risk factors of diseases.79 Moreover, we did not address possible interactions between the six loci and epigenetic factors. An association between DRD4 and schizophrenia risk was detected based on case–control studies rather than on functional ones. Our results will need to be validated on a functional level.
Conclusion
The −521 CC variant and the L/L genotype of the 120-bp TR might be risk factors for schizophrenia. No association with schizophrenia was detected for the 48 bp VNTR, the 12 bp TR, −616 C>G, or −376 C>T. Our results may provide an informative reference for subsequent genome-wide association studies.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (No 81601653) and the Doctoral Research Start Foundation of Liaoning Province (201601115) (both to Dr Jun Yao). The article was edited by native English-speaking experts of BioMed Proofreading, LLC.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Sullivan PF. The genetics of schizophrenia. PLoS Med. 2005;2(7):e212. doi: 10.1371/journal.pmed.0020212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tsuang MT, Stone WS, Faraone SV. Schizophrenia: a review of genetic studies. Harv Rev Psychiatry. 1999;7(4):185–207. [PubMed] [Google Scholar]
- 3.Wray NR, Visscher PM. Narrowing the boundaries of the genetic architecture of schizophrenia. Schizophr Bull. 2010;36(1):14–23. doi: 10.1093/schbul/sbp137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carlsson A. The current status of the dopamine hypothesis of schizophrenia. Neuropsychopharmacology. 1988;1(3):179–186. doi: 10.1016/0893-133x(88)90012-7. [DOI] [PubMed] [Google Scholar]
- 5.Sit SY. Dopamine agonists in the treatment of Parkinson’s disease past, present and future. Curr Pharm Des. 2000;6(12):1211–1248. doi: 10.2174/1381612003399581. [DOI] [PubMed] [Google Scholar]
- 6.Howes O, McCutcheon R, Stone J. Glutamate and dopamine in schizophrenia: an update for the 21st century. J Psychopharmacol. 2015;29(2):97–115. doi: 10.1177/0269881114563634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seeman P, Guan HC, Van Tol HH. Dopamine D4 receptors elevated in schizophrenia. Nature. 1993;365(6445):441–445. doi: 10.1038/365441a0. [DOI] [PubMed] [Google Scholar]
- 8.Sumiyoshi T, Stockmeier CA, Overholser JC, Thompson PA, Meltzer HY. Dopamine D4 receptors and effects of guanine nucleotides on [3H]raclopride binding in postmortem caudate nucleus of subjects with schizophrenia or major depression. Brain Res. 1995;681(1–2):109–116. doi: 10.1016/0006-8993(95)00301-6. [DOI] [PubMed] [Google Scholar]
- 9.Van Tol HH, Bunzow JR, Guan HC, et al. Cloning of the gene for a human dopamine D4 receptor with high affinity for the antipsychotic clozapine. Nature. 1991;350(6319):610–614. doi: 10.1038/350610a0. [DOI] [PubMed] [Google Scholar]
- 10.Macciardi F, Petronis A, Van Tol HH, et al. Analysis of the D4 dopamine receptor gene variant in an Italian schizophrenia kindred. Arch Gen Psychiatry. 1994;51(4):288–293. doi: 10.1001/archpsyc.1994.03950040032004. [DOI] [PubMed] [Google Scholar]
- 11.Allen NC, Bagade S, McQueen MB, et al. Systematic meta-analyses and field synopsis of genetic association studies in schizophrenia: the SzGene database. Nat Genet. 2008;40(7):827–834. doi: 10.1038/ng.171. [DOI] [PubMed] [Google Scholar]
- 12.Bertram L. Genetic research in schizophrenia: new tools and future perspectives. Schizophr Bull. 2008;34(5):806–812. doi: 10.1093/schbul/sbn079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asghari V, Sanyal S, Buchwaldt S, Paterson A, Jovanovic V, Van Tol HH. Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. J Neurochem. 1995;65(3):1157–1165. doi: 10.1046/j.1471-4159.1995.65031157.x. [DOI] [PubMed] [Google Scholar]
- 14.Kornfeld R, Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- 15.D’Souza UM, Russ C, Tahir E, et al. Functional effects of a tandem duplication polymorphism in the 5′flanking region of the DRD4 gene. Biol Psychiatry. 2004;56(9):691–697. doi: 10.1016/j.biopsych.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 16.McCracken JT, Smalley SL, McGough JJ, et al. Evidence for linkage of a tandem duplication polymorphism upstream of the dopamine D4 receptor gene (DRD4) with attention deficit hyperactivity disorder (ADHD) Mol Psychiatry. 2000;5(5):531–536. doi: 10.1038/sj.mp.4000770. [DOI] [PubMed] [Google Scholar]
- 17.Mill J, Fisher N, Curran S, Richards S, Taylor E, Asherson P. Polymorphisms in the dopamine D4 receptor gene and attention-deficit hyperactivity disorder. Neuroreport. 2003;14(11):1463–1466. doi: 10.1097/00001756-200308060-00011. [DOI] [PubMed] [Google Scholar]
- 18.Xing QH, Wu SN, Lin ZG, et al. Association analysis of polymorphisms in the upstream region of the human dopamine D4 receptor gene in schizophrenia. Schizophr Res. 2003;65(1):9–14. doi: 10.1016/s0920-9964(03)00064-1. [DOI] [PubMed] [Google Scholar]
- 19.Tsutsumi A, Glatt SJ, Kanazawa T, et al. The genetic validation of heterogeneity in schizophrenia. Behav Brain Funct. 2011;7:43. doi: 10.1186/1744-9081-7-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okuyama Y, Ishiguro H, Nankai M, Shibuya H, Watanabe A, Arinami T. Identification of a polymorphism in the promoter region of DRD4 associated with the human novelty seeking personality trait. Mol Psychiatry. 2000;5(1):64–69. doi: 10.1038/sj.mp.4000563. [DOI] [PubMed] [Google Scholar]
- 21.Ronai Z, Szekely A, Nemoda Z, et al. Association between Novelty Seeking and the −521 C/T polymorphism in the promoter region of the DRD4 gene. Mol Psychiatry. 2001;6(1):35–38. doi: 10.1038/sj.mp.4000832. [DOI] [PubMed] [Google Scholar]
- 22.Okuyama Y, Ishiguro H, Toru M, Arinami T. A genetic polymorphism in the promoter region of DRD4 associated with expression and schizophrenia. Biochem Biophys Res Commun. 1999;258(2):292–295. doi: 10.1006/bbrc.1999.0630. [DOI] [PubMed] [Google Scholar]
- 23.Mitsuyasu H, Kawasaki H, Ninomiya H, et al. Genetic structure of the dopamine receptor D4 gene (DRD4) and lack of association with schizophrenia in Japanese patients. J Psychiatr Res. 2007;41(9):763–775. doi: 10.1016/j.jpsychires.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 24.Jonsson EG, Ivo R, Forslund K, et al. No association between a promoter dopamine D(4) receptor gene variant and schizophrenia. Am J Med Genet. 2001;105(6):525–528. doi: 10.1002/ajmg.1478. [DOI] [PubMed] [Google Scholar]
- 25.Munafo MR, Flint J. Meta-analysis of genetic association studies. Trends Genet. 2004;20(9):439–444. doi: 10.1016/j.tig.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 26.Barendregt JJ, Doi SA, Lee YY, Norman RE, Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67(11):974–978. doi: 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- 27.Lanara Z, Giannopoulou E, Fullen M, et al. Comparative study and meta-analysis of meta-analysis studies for the correlation of genomic markers with early cancer detection. Hum Genomics. 2013;7(1):14. doi: 10.1186/1479-7364-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lung FW, Tzeng DS, Shu BC. Ethnic heterogeneity in allele variation in the DRD4 gene in schizophrenia. Schizophr Res. 2002;57(2–3):239–245. doi: 10.1016/s0920-9964(01)00313-9. [DOI] [PubMed] [Google Scholar]
- 29.Glatt SJ, Faraone SV, Tsuang MT. Schizophrenia is not associated with DRD4 48-base-pair-repeat length or individual alleles: results of a meta-analysis. Biol Psychiatry. 2003;54(6):629–635. doi: 10.1016/s0006-3223(03)00180-x. [DOI] [PubMed] [Google Scholar]
- 30.Jonsson EG, Sedvall GC, Nothen MM, Cichon S. Dopamine D4 receptor gene (DRD4) variants and schizophrenia: meta-analyses. Schizophr Res. 2003;61(1):111–119. doi: 10.1016/s0920-9964(02)00287-6. [DOI] [PubMed] [Google Scholar]
- 31.Shi J, Gershon ES, Liu C. Genetic associations with schizophrenia: meta-analyses of 12 candidate genes. Schizophr Res. 2008;104(1–3):96–107. doi: 10.1016/j.schres.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terracciano A, Sutin AR, An Y, et al. Personality and risk of Alzheimer’s disease: new data and meta-analysis. Alzheimers Dement. 2014;10(2):179–186. doi: 10.1016/j.jalz.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Palmer SC, Gardner S, Tonelli M, et al. Phosphate-binding agents in adults with CKD: a network meta-analysis of randomized trials. Am J Kidney Dis. 2016;68(5):691–702. doi: 10.1053/j.ajkd.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 34.Pan Y, Yao J, Wang B. Association of dopamine D1 receptor gene polymorphism with schizophrenia: a meta-analysis. Neuropsychiatr Dis Treat. 2014;10:1133–1139. doi: 10.2147/NDT.S63776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Thakkinstian A, McElduff P, D’Este C, Duffy D, Attia J. A method for meta-analysis of molecular association studies. Stat Med. 2005;24(9):1291–1306. doi: 10.1002/sim.2010. [DOI] [PubMed] [Google Scholar]
- 36.Zintzaras E, Ioannidis JP. Heterogeneity testing in meta-analysis of genome searches. Genet Epidemiol. 2005;28(2):123–137. doi: 10.1002/gepi.20048. [DOI] [PubMed] [Google Scholar]
- 37.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Naing C, Reid SA, Aung K. Comparing antibiotic treatment for leptospirosis using network meta-analysis: a tutorial. BMC Infect Dis. 2017;17(1):29. doi: 10.1186/s12879-016-2145-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dupont WD, Plummer WD., Jr Power and sample size calculations for studies involving linear regression. Control Clin Trials. 1998;19(6):589–601. doi: 10.1016/s0197-2456(98)00037-3. [DOI] [PubMed] [Google Scholar]
- 40.Xu FL, Ding M, Yao J, et al. Association between mitochondrial DNA variations and schizophrenia in the northern Chinese Han population. PLoS One. 2017;12(8):e0182769. doi: 10.1371/journal.pone.0182769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sedgwick P, Marston L. How to read a funnel plot in a meta-analysis. BMJ. 2015;351:h4718. doi: 10.1136/bmj.h4718. [DOI] [PubMed] [Google Scholar]
- 42.Kaiser R, Konneker M, Henneken M, et al. Dopamine D4 receptor 48-bp repeat polymorphism: no association with response to antipsychotic treatment, but association with catatonic schizophrenia. Mol Psychiatry. 2000;5(4):418–424. doi: 10.1038/sj.mp.4000729. [DOI] [PubMed] [Google Scholar]
- 43.Kohn Y, Ebstein RP, Heresco-Levy U, et al. Dopamine D4 receptor gene polymorphisms: relation to ethnicity, no association with schizophrenia and response to clozapine in Israeli subjects. Eur Neuropsychopharmacol. 1997;7(1):39–43. doi: 10.1016/s0924-977x(96)00380-x. [DOI] [PubMed] [Google Scholar]
- 44.Serretti A, Lilli R, Lorenzi C, Lattuada E, Smeraldi E. DRD4 exon 3 variants associated with delusional symptomatology in major psychoses: a study on 2,011 affected subjects. Am J Med Genet. 2001;105(3):283–290. doi: 10.1002/ajmg.1321. [DOI] [PubMed] [Google Scholar]
- 45.Hattori E, Nakajima M, Yamada K, et al. Variable number of tandem repeat polymorphisms of DRD4: re-evaluation of selection hypothesis and analysis of association with schizophrenia. Eur J Hum Genet. 2009;17(6):793–801. doi: 10.1038/ejhg.2008.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanaka T, Igarashi S, Onodera O, et al. Lack of association between dopamine D4 receptor gene and schizophrenia. Am J Med Genet. 1995;60(6):580–582. doi: 10.1002/ajmg.1320600620. [DOI] [PubMed] [Google Scholar]
- 47.Nanko S, Hattori M, Ikeda K, Sasaki T, Kazamatsuri H, Kuwata S. Dopamine D4 receptor polymorphism and schizophrenia. Lancet. 1993;341(8846):689–690. doi: 10.1016/0140-6736(93)90456-q. [DOI] [PubMed] [Google Scholar]
- 48.Petronis A, Macciardi F, Athanassiades A, et al. Association study between the dopamine D4 receptor gene and schizophrenia. Am J Med Genet. 1995;60(5):452–455. doi: 10.1002/ajmg.1320600518. [DOI] [PubMed] [Google Scholar]
- 49.Ohara K, Nakamura Y, Xie DW, et al. Polymorphisms of dopamine D2-like (D2, D3, and D4) receptors in schizophrenia. Biol Psychiatry. 1996;40(12):1209–1217. doi: 10.1016/0006-3223(95)00673-7. [DOI] [PubMed] [Google Scholar]
- 50.Aguirre AJ, Apiquian R, Fresan A, Cruz-Fuentes C. Association analysis of exon III and exon I polymorphisms of the dopamine D4 receptor locus in Mexican psychotic patients. Psychiatry Res. 2007;153(3):209–215. doi: 10.1016/j.psychres.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 51.Daniels J, Williams J, Mant R, Asherson P, McGuffin P, Owen MJ. Repeat length variation in the dopamine D4 receptor gene shows no evidence of association with schizophrenia. Am J Med Genet. 1994;54(3):256–258. doi: 10.1002/ajmg.1320540313. [DOI] [PubMed] [Google Scholar]
- 52.Sommer SS, Lind TJ, Heston LL, Sobell JL. Dopamine D4 receptor variants in unrelated schizophrenic cases and controls. Am J Med Genet. 1993;48(2):90–93. doi: 10.1002/ajmg.1320480207. [DOI] [PubMed] [Google Scholar]
- 53.Rao PA, Pickar D, Gejman PV, Ram A, Gershon ES, Gelernter J. Allelic variation in the D4 dopamine receptor (DRD4) gene does not predict response to clozapine. Arch Gen Psychiatry. 1994;51(11):912–917. doi: 10.1001/archpsyc.1994.03950110072009. [DOI] [PubMed] [Google Scholar]
- 54.Hong CJ, Lee YL, Sim CB, Hwu HG. Dopamine D4 receptor variants in Chinese sporadic and familial schizophrenics. Am J Med Genet. 1997;74(4):412–415. doi: 10.1002/(sici)1096-8628(19970725)74:4<412::aid-ajmg12>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 55.Jonsson E, Brene S, Geijer T, et al. A search for association between schizophrenia and dopamine-related alleles. Eur Arch Psychiatry Clin Neurosci. 1996;246(6):297–304. doi: 10.1007/BF02189022. [DOI] [PubMed] [Google Scholar]
- 56.Rinetti G, Camarena B, Cruz C, et al. Dopamine D4 receptor (DRD4) gene polymorphism in the first psychotic episode. Arch Med Res. 2001;32(1):35–38. doi: 10.1016/s0188-4409(00)00257-5. [DOI] [PubMed] [Google Scholar]
- 57.Fujiwara Y, Yamaguchi K, Tanaka Y, et al. Polymorphism of dopamine receptors and transporter genes in neuropsychiatric diseases. Eur Neurol. 1997;38(Suppl 1):6–10. doi: 10.1159/000113436. [DOI] [PubMed] [Google Scholar]
- 58.Lung FW, Chen N, Shu BC. Dopamine D4 receptor gene and the −521C>T polymorphism of the upstream region of the dopamine D4 receptor gene in schizophrenia. Psychiatr Genet. 2006;16(4):139–143. doi: 10.1097/01.ypg.0000199446.54420.ff. [DOI] [PubMed] [Google Scholar]
- 59.Nakamura M, Inoue A, Hemmi H, Suzuki J. Positive associations between dopamine D4 receptor polymorphism and schizophrenia. Eur Neuropsychopharmacol. 2006;6(Suppl 3):136–136. [Google Scholar]
- 60.Lung FW, Shu BC, Kao WT, Chen CN, Ku YC, Tzeng DS. Association of DRD4 uVNTR and TP53 codon 72 polymorphisms with schizophrenia: a case-control study. BMC Med Genet. 2009;10:147. doi: 10.1186/1471-2350-10-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fresan A, Camarena B, Apiquian R, Aguilar A, Urraca N, Nicolini H. Association study of MAO-A and DRD4 genes in schizophrenic patients with aggressive behavior. Neuropsychobiology. 2007;55(3–4):171–175. doi: 10.1159/000106477. [DOI] [PubMed] [Google Scholar]
- 62.Zhang M, Jianjun Y, Yongchao Q, Xia Z, Yinping H, et al. Association of schizophrenia with six candidate functional genes. Zhonghua Yi Xue Yi Chuan Xue Za Zhi. 2003;20(1):69–71. [PubMed] [Google Scholar]
- 63.Zhao A, Zhimin X, Jindong C, Xiaogang C, Zhening L, et al. Association analysis of dopamine D4 receptor gene with quality traits and quantitative traits of schizophrenia. Chin J Psychiatry. 2005;38(1):3–6. [Google Scholar]
- 64.Zhao A, Jinuo Z, Jia F, Yuhu Z. Association analysis of dopamine D4 gene and serotonin 2A receptor gene in schizophrenia. Chin J Behav Med Brain Sci. 2006;15(10):865–867. [Google Scholar]
- 65.Chen X, Jianhua C, Weidong J. Association of DRD4 exon III gene polymorphism with attack behavior and psychiatric symptoms in patients with schizophrenia. Chin J Clin Med. 2016;10(18):2692–2695. [Google Scholar]
- 66.Lu Z, Dongxiang W, Yiping Q, Fei L, Ye Z, et al. Association analysis of dopamine DR4 receptor gene and apolipoprotein E gene in schizophrenia. Chin J Psychiatry. 2003;36(1):17–20. [Google Scholar]
- 67.Liang KW. doctor’s thesis. Shenyang: China Medical University; 2005. The association of DA2, DA3, and DA4 receptor gene polymorphology with schizophrenia also in medical jurisprudence and human genetics studies. Chinese. [Google Scholar]
- 68.Tang Y, Zhuoji C, Rulun Z, Chaofeng Z. Association analysis of schizophrenia and dopamine D4 receptor gene polymorphism. Chin J Med Sci. 2001;81(16):995–998. [PubMed] [Google Scholar]
- 69.Serretti A, Lilli R, Di Bella D, et al. Dopamine receptor D4 gene is not associated with major psychoses. Am J Med Genet. 1999;88(5):486–491. [PubMed] [Google Scholar]
- 70.Hong CJ, Chiu HJ, Chang YS, Sim CB. Twelve-nucleotide repeat polymorphism of D4 dopamine receptor gene in Chinese familial schizophrenic patients. Biol Psychiatry. 1998;43(6):432–435. doi: 10.1016/s0006-3223(97)00207-2. [DOI] [PubMed] [Google Scholar]
- 71.Catalano M, Nobile M, Novelli E, Nothen MM, Smeraldi E. Distribution of a novel mutation in the first exon of the human dopamine D4 receptor gene in psychotic patients. Biol Psychiatry. 1993;34(7):459–464. doi: 10.1016/0006-3223(93)90236-7. [DOI] [PubMed] [Google Scholar]
- 72.Nakajima M, Hattori E, Yamada K, et al. Association and synergistic interaction between promoter variants of the DRD4 gene in Japanese schizophrenics. J Hum Genet. 2007;52(1):86–91. doi: 10.1007/s10038-006-0084-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pai P, Arathil P, Kotambail A, et al. Association of GRIN1, ABCB1, and DRD4 genes and response to antipsychotic drug treatment in schizophrenia. Psychiatr Genet. 2015;25(3):135–136. doi: 10.1097/YPG.0000000000000079. [DOI] [PubMed] [Google Scholar]
- 74.Lai JH, Zhu YS, Huo ZH, et al. Association study of polymorphisms in the promoter region of DRD4 with schizophrenia, depression, and heroin addiction. Brain Res. 2010;1359:227–232. doi: 10.1016/j.brainres.2010.08.064. [DOI] [PubMed] [Google Scholar]
- 75.Mitsuyasu H, Hirata N, Sakai Y, et al. Association analysis of polymorphisms in the upstream region of the human dopamine D4 receptor gene (DRD4) with schizophrenia and personality traits. J Hum Genet. 2001;46(1):26–31. doi: 10.1007/s100380170120. [DOI] [PubMed] [Google Scholar]
- 76.Zhong H, Liang P, Yongsheng Z, Jie P, Zhenghao H. Association of dopamine D4 receptor gene promoter region polymorphism with schizophrenia. Adv Mod Biomed. 2010;10(11):3231–3234. [Google Scholar]
- 77.Smith TF. Meta-analysis of the heterogeneity in association of DRD4 7-repeat allele and AD/HD: stronger association with AD/HD combined type. Am J Med Genet B Neuropsychiatr Genet. 2010;153B(6):1189–1199. doi: 10.1002/ajmg.b.31090. [DOI] [PubMed] [Google Scholar]
- 78.Gehricke JG, Swanson JM, Duong S, et al. Increased brain activity to unpleasant stimuli in individuals with the 7R allele of the DRD4 gene. Psychiatry Res. 2015;231(1):58–63. doi: 10.1016/j.pscychresns.2014.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang Z, Thomas DC. Two-stage family-based designs for sequencing studies. BMC Proc. 2014;8(Suppl 1):S32. doi: 10.1186/1753-6561-8-S1-S32. [DOI] [PMC free article] [PubMed] [Google Scholar]