Abstract
Purpose
Primary ductal adenocarcinoma arising in the structures of the lacrimal apparatus is extremely rare, and the entity is considered a lacrimal counterpart of salivary duct carcinoma, of which the majority are known to express androgen receptor (AR). Less than 10 cases of AR-positive carcinomas of lacrimal gland or lacrimal sac have been described.
Observations
We present a primary ductal adenocarcinoma with AR expression involving the nasolacrimal duct of a middle-aged patient who had suffered from right eyelid swelling, diplopia and epiphora for 4 months. Although the tumor histologically resembled oncocytic carcinoma, electron microscopic examination did not show cytoplasmic accumulation of mitochondria, which excluded the diagnosis of oncocytic carcinoma with AR positivity.
Conclusions and importance
We concluded that this is the first case of AR-positive ductal adenocarcinoma arising from nasolacrimal duct. It is possible that some of the previously documented oncocytic carcinomas of the lacrimal drainage system may include ductal adenocarcinomas with oncocytic features.
Keywords: Nasolacrimal duct, Ductal carcinoma, Androgen receptor, Oxyphilic cells
1. Introduction
Tumors of the lacrimal apparatus are rare. The lacrimal gland, which is histologically similar to the salivary gland, can be involved by a variety of salivary type neoplasms including pleomorphic adenoma, adenoid cystic carcinoma, and lacrimal duct carcinoma. The lacrimal sac and nasolacrimal duct are normally covered by a stratified columnar epithelium containing goblet cells without glands, and squamous cell carcinoma and transitional carcinoma are the most common malignancies.1 Primary ductal adenocarcinoma of the lacrimal apparatus is a counterpart of salivary duct carcinoma (SDC). In the salivary glands, 70–100% of SDCs are known to express AR, but AR expression is not restricted to SDC. Strong nuclear reactivity for AR has been shown in virtually all types of salivary carcinomas.
Here, we present the first case of AR-positive primary ductal adenocarcinoma arising from the nasolacrimal duct, which histologically resembled a case of oncocytic carcinoma rather than ductal adenocarcinoma.
2. Case report
A middle-aged patient visited the Ophthalmology Department of the Asan Medical Center for re-evaluation of an orbital mass. He had suffered from right eyelid swelling, diplopia, and epiphora for 4 months after receiving cataract surgery in a local clinic. An orbital magnetic resonance imaging (MRI) performed in a different hospital showed a mass involving the right orbital floor and nasal cavity; a punch biopsy from the right inferior meatus confirmed the diagnosis of malignancy. By the time this patient visited the Asan Medical Center, he had developed mild hypoesthesia on the right side of the face and a palpable mass which resulted in right lower lid elevation, but his vision, intraocular pressure, and range of eyeball movement were within normal limits.
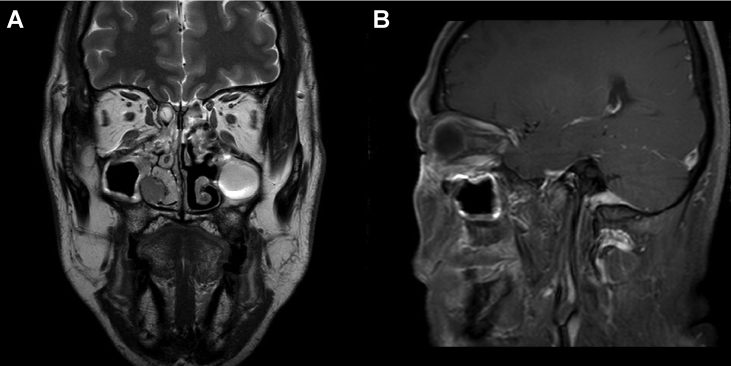
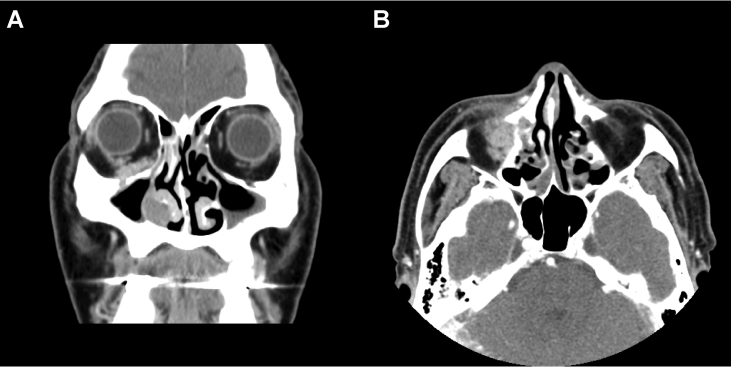
MRI with contrast enhancement showed a T2 intermediate enhancing mass extending from the right inferior extraconal space of the orbit to the right nasal cavity along the right nasolacrimal duct (Fig. 1). In-house computed tomography showed no significant interval change, but accentuated expansile bony change with destruction in the nasolacrimal duct when compared with MRI (Fig. 2). Positron emission tomography/computed tomography revealed no evidence of distant metastasis.
Fig. 1.
Magnetic resonance imaging with contrast enhancement, showing a T2 intermediate enhancing mass in the study patient. (A) The mass extended from the right inferior extraconal space of the orbit to the right nasal cavity along the right nasolacrimal duct (T2WI). (B) Invasion of the mass into the right inferior oblique muscle could be noted on T1WI.
Fig. 2.
Computed tomography scan of the paranasal sinuses in the study patient. Infiltrating enhancing lesion involving right orbit, nasolacrimal duct and nasal cavity was shown in coronal section (A) and axial section (B). Destructive bony change is noted.
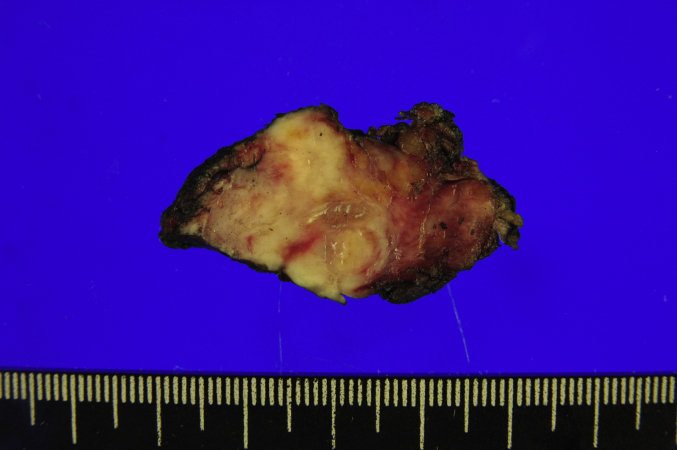
After multiple interdepartmental consultations, surgical resection was considered the appropriate first-line treatment. Right medial maxillectomy including removal of the inferior and middle turbinates and a partial inferior to medial orbital exenteration were performed, saving the patient's inferior rectus muscle and eyeball. The defect of the inferomedial orbital wall was repaired with a skin graft obtained from the right thigh of the patient. The surgical specimen was not en bloc. The largest specimen from the inferior orbit was a lump of soft tissue, measuring 4.2 × 2.0 × 1.0cm, containing an ill-demarcated, irregular solid mass, measuring 3.5 × 2.1 × 1.8cm. Its cut surface was whitish yellow to tan, firm and fibrotic (Fig. 3). Specimens from the medial wall of the maxillary sinus were also confirmed to be involved by the tumor on microscopic inspection.
Fig. 3.
The largest surgical specimen obtained from the inferior orbit. The specimen comprised soft tissue containing an ill-demarcated, irregular solid mass, measuring 3.5 × 2.1 × 1.8cm. Its cut surface was whitish yellow to tan, firm and fibrotic. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
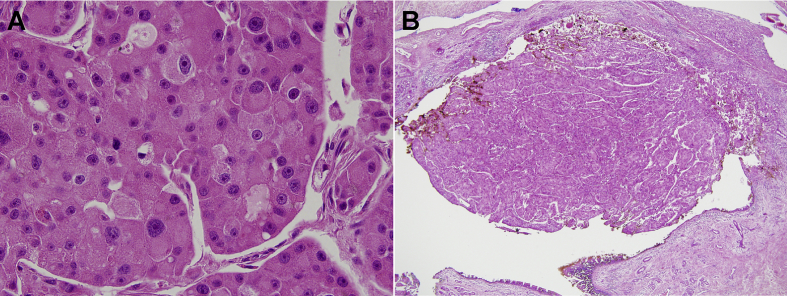
Microscopic examination of the neoplasm showed mainly solid sheet-like or nested growth of large epithelial cells. The tumor cells had distinct cell borders, abundant granular and eosinophilic cytoplasm, and eccentrically located large round nuclei. Each nucleus had an irregular nuclear membrane and a prominent single nucleolus, as well as considerable pleomorphism (Fig. 4A). No other types of cells were coexistent, and no glandular formation or mucin production was observed. Some areas showed moderate amount of lymphocytic infiltration and occasional formation of lymphoid aggregation. The histologic features were diagnostic of carcinoma with oncocytic features.
Fig. 4.
Histologic features of the neoplasm in the study patient. The most represented pattern consisted of solid sheet-like or nested growth of large epithelial cells. (A) The tumor cells showed distinct cell borders, abundant granular and eosinophilic cytoplasm, and eccentrically located large round nuclei with considerable pleomorphism ( × 400). (B) A part of the tumor protruding from the surface of the inferior meatal mucosa suggested the involvement of the inferior orifice of the nasolacrimal duct ( × 100).
The normal structures of the lacrimal drainage system were not recognizable in the upper part of the tumor around the inferior orbit, so that the relationship of the tumor with the lacrimal sac or the nasolacrimal duct could not be identified directly. However, a part of the tumor was protruding from the surface of the inferior meatal mucosa, so that the involvement of the inferior orifice of the nasolacrimal duct could be presumed (Fig. 4B). The tumor showed infiltrative periphery with lymphovascular invasion, but without perineural invasion.
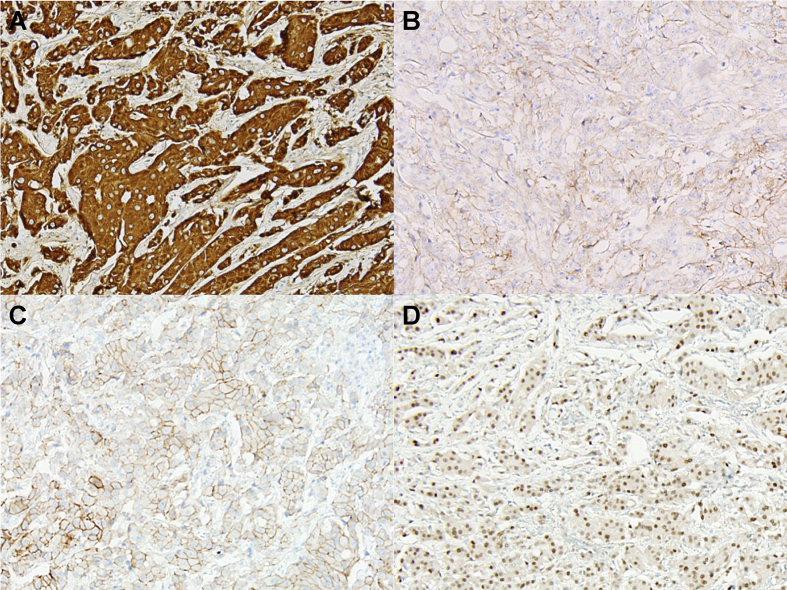
On immunohistochemistry (IHC), the tumor showed immunopositivity for cytokeratin7 (CK7), p53, epidermal growth factor receptor (EGFR), human epidermal growth factor receptor 2 (HER-2), and androgen receptor (AR), but was negative for CK20, caudal-type homeobox 2 (CDX2), p63, S100 protein, and CD117 (Fig. 5). These IHC results were consistent with either primary ductal adenocarcinoma with oncocytic change, or oncocytic carcinoma with AR positivity. Electron microscopic (EM) examination was performed using the formalin-fixed, paraffin-embedded tumor tissue for differential diagnosis. The ultrastructure did not show cytoplasmic accumulation of mitochondria (Fig. 6), which excluded the diagnosis of oncocytic carcinoma with AR positivity. Instead, tumor cells containing electron-dense cytoplasmic granules were observed, suggesting apocrine differentiation. The patient received 21 cycles of postoperative radiation therapy in an outside hospital. No local recurrence or distant metastases were identified at 25 months after surgery.
Fig. 5.
On immunohistochemical (IHC) staining, the tumor showed (A) diffuse and strong cytoplasmic immunopositivity for cytokeratin7(CK7). This lesion also showed circumferential membranous positivity for (B) epidermal growth factor receptor (EGFR), and (C) human epidermal growth factor receptor 2 (HER-2), and (D) intermediate nuclear positivity for androgen receptor (AR). IHC staining wasnegativeforCK20, caudal type homeobox 2 (CDX2), p63, S100 protein, and CD117(data not shown).
Fig. 6.
Electron microscopic examination of formalin-fixed, paraffin-embedded tumor tissue. The ultrastructure did not show cytoplasmic accumulation of mitochondria, which precluded a diagnosis of oncocytic carcinoma. Instead, the tumor cells showed electron-dense cytoplasmic granules suggesting apocrine differentiation. (A, × 6000; B, × 20,000).
3. Discussion
Primary ductal adenocarcinoma arising in the structures of the lacrimal drainage system is extremely rare. Since Katz et al. reported the first case of ductal adenocarcinoma of the lacrimal gland in 19964, only 16 further cases involvingthe lacrimal gland4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 and two involving the lacrimal sac16, 17 have been described (Table 1). IHC for AR was performed in seven lacrimal gland and two lacrimal sac tumors, all of which showed positive results (100%).8, 14, 15, 16 IHC for HER-2/neu was performed in eight cases, of which four were positive (50%).8, 10, 11, 15 The first case of primary ductal adenocarcinoma of the lacrimal sac was reported in 2013 and showed diffuse positivity for AR but was negative for HER-2/neu on IHC.17
Table 1.
Clinical data of the patients with primary ductal adenocarcinoma arising in the lacrimal drainage system.
| Literature | Age (year) | Sex | Epicenter | Immunohistochemistry |
|||
|---|---|---|---|---|---|---|---|
| AR | ER | PR | Her-2 | ||||
| Katz et al.4 (1996) | 68 | Male | Lacrimal gland | N/A | N/A | N/A | N/A |
| Nasu et al.13 (1998) | 67 | Male | Lacrimal gland | N/A | – | N/A | N/A |
| Krishnakumar et al.7 (2003) | 46 | Male | Lacrimal gland | N/A | N/A | N/A | N/A |
| Kurisu et al.9 (2003) | 67 | Male | Lacrimal gland | N/A | N/A | N/A | N/A |
| Milman et al.11 (2005) | 59 | Male | Lacrimal gland | N/A | – | N/A | – |
| Kim et al.6 (2008) | 47 | Male | Lacrimal gland | N/A | N/A | N/A | N/A |
| Lee et al.10 (2009) | 50 | Male | Lacrimal gland | N/A | – | – | – |
| Ishida et al.25 (2009) | 70 | Female | Lacrimal gland | N/A | N/A | N/A | N/A |
| Damasceno et al.5 (2012) | 78 | Male | Lacrimal gland | N/A | N/A | N/A | N/A |
| Kubota et al.8 (2013) | 75 | Male | Lacrimal gland | + | – | – | + |
| 67 | Male | Lacrimal gland | + | – | – | – | |
| 53 | Male | Lacrimal gland | + | – | – | – | |
| 39 | Male | Lacrimal gland | + | – | – | + | |
| 46 | Female | Lacrimal gland | + | – | – | + | |
| Ishida et al.17 (2013) | 79 | Female | Lacrimal sac | + | N/A | N/A | N/A |
| Min et al.12 (2014) | 46 | Male | Lacrimal gland | N/A | N/A | N/A | N/A |
| Vagia16 et al. (2015) | 65 | Male | Lacrimal sac | + | N/A | N/A | + |
| Zhu et al.15 (2015) | 49 | Female | Lacrimal gland | + | – | – | + |
| Lau et al.28 (2015) | 34 | Female | Lacrimal gland | N/A | N/A | N/A | N/A |
| Present study | 6th decade | Not consented | Nasolacrimal duct | + | N/A | N/A | + |
Abbreviations: AR, androgen receptor; ER, estrogen receptor; PR, progesterone receptor; Her-2, human epidermal growth factor receptor-2, N/A, not applicable.
In our present case, the tumor involved the entire route of the nasolacrimal duct, from the inferomedial aspect of the orbit to the inferior meatus of the nasal cavity. Although a relationship with the duct itself could not be observed due to extraductal extension, the origin of the tumor was assumed to be the nasolacrimal duct. The differential diagnosis of nasolacrimal ductal adenocarcinoma includes metastatic ductal carcinoma of breast origin, squamous carcinoma, mucoepidermoid carcinoma, and a rare form of oncocytic carcinoma with AR expression. The past medical history and a thorough physical examination can exclude the possibility of metastatic ductal carcinoma from breast, while immunonegativity for p63 excludes the diagnosis of squamous carcinoma and mucoepidermoid carcinoma. In our present case, our first diagnostic hypothesis was oncocytic carcinoma, on the basis of cytomorphologic features and the lack of comedo necrosis and perineural invasion, which are common in salivary duct carcinoma. In addition, oncocytic carcinoma of the lacrimal sac/nasolacrimal duct is reported to be more frequent than ductal adenocarcinoma.18, 19, 20, 21, 22, 23, 24 However, the tumor cells in our current patient proved by electron microscopy examination not to be oncocytic, but to be oncocytoid cells. The presence of intracytoplasmic secretory granules also suggested apocrine differentiation. In addition to our present case, previous AR-positive ductal adenocarcinomas have been reported to have an abundant granular eosinophilic cytoplasm.15, 17 It is thus possible that previously reported oncocytic carcinomas of the lacrimal apparatus may include ductal adenocarcinomas with apocrine differentiation. One case among reported ductal adenocarcinoma of the lacrimal gland was carcinoma ex pleomorphic adenoma.25 Our case has been meticulously examined, and shown no evidence of preexisting pleomorphic adenoma. To the best of our knowledge, our present patient is the first documented case of AR-positive primary ductal adenocarcinoma resembling oncocytic carcinoma arising in the nasolacrimal duct instead of the lacrimal sac or the lacrimal gland.
From a prognostic perspective, sufficient evidence to determine the treatment of choice is lacking when we encounter a carcinoma arising from the lacrimal drainage system, because of its rarity. El-Sawy et al. studied the therapeutic effects in 14 carcinomas of the lacrimal sac/nasolacrimal duct, and concluded that multidisciplinary therapy can preserve the eye and the visual function in most patients.26 One previously described patient with AR-positive lacrimal sac adenocarcinoma showed an impressive response to antiandrogen treatment (abiraterone).16 Our present patient was treated with a wide surgical resection followed by radiation therapy, and has survived for 16 months after surgery with no evidence of local recurrence or distant metastasis.
4. Conclusions
In conclusion, we here present the first case of AR-positive primary ductal adenocarcinoma arising from the nasolacrimal duct. As our patient histologically resembled a case of oncocytic carcinoma rather than ductal adenocarcinoma, the diffuse and strong immunopositivity for AR was the most diagnostic feature of his tumor. Since AR expression has been reported in many types of salivary gland and breast carcinomas, AR positivity alone cannot lead to the diagnosis of nasolacrimal ductal adenocarcinoma. It is thus necessary to document the nature of the oncocytoid cells by electron microscopy or the use of anti-mitochondrial antibodies. Nevertheless, IHC for AR is an important diagnostic step when a lacrimal apparatus carcinoma is encountered for both differential diagnosis and therapeutic planning.
Patient consent
Personal identifying information has been removed from this report because consent to publish such information was not obtained.
Funding
No funding or grant support.
Conflict of interest
The following authors have no financial disclosures: I.A.P., H-S.S., Y-S. C,.K-J. C.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgments
None.
References
- 1.Heindl L.M., Junemann A.G., Kruse F.E., Holbach L.M. Tumors of the lacrimal drainage system. Orbit (Amsterdam, Neth. 2010;29:298–306. doi: 10.3109/01676830.2010.492887. [DOI] [PubMed] [Google Scholar]
- 4.Katz S.E., Rootman J., Dolman P.J., White V.A., Berean K.W. Primary ductal adenocarcinoma of the lacrimal gland. Ophthalmology. 1996;103:157–162. doi: 10.1016/s0161-6420(96)30746-x. [DOI] [PubMed] [Google Scholar]
- 5.Damasceno R.W., Holbach L.M. Primary ductal adenocarcinoma of the lacrimal gland: case report. Arq Bras Oftalmol. 2012;75:64–66. doi: 10.1590/s0004-27492012000100014. [DOI] [PubMed] [Google Scholar]
- 6.Kim M.J., Hanmantgad S., Holodny A.I. Novel management and unique metastatic pattern of primary ductal adenocarcinoma of the lacrimal gland. Clin Exp Ophthalmol. 2008;36:194–196. doi: 10.1111/j.1442-9071.2008.01704.x. [DOI] [PubMed] [Google Scholar]
- 7.Krishnakumar S., Subramanian N., Mahesh L., Mohan E.R., Biswas J. Primary ductal adenocarcinoma of the lacrimal gland in a patient with neurofibromatosis. Eye Lond Engl. 2003;17:843–845. doi: 10.1038/sj.eye.6700476. [DOI] [PubMed] [Google Scholar]
- 8.Kubota T., Moritani S., Ichihara S. Clinicopathologic and immunohistochemical features of primary ductal adenocarcinoma of lacrimal gland: five new cases and review of literature. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle. Ophthalmologie. 2013;251:2071–2076. doi: 10.1007/s00417-013-2350-3. [DOI] [PubMed] [Google Scholar]
- 9.Kurisu Y., Shibayama Y., Tsuji M. A case of primary ductal adenocarcinoma of the lacrimal gland: histopathological and immunohistochemical study. Pathology, Res Pract. 2005;201:49–53. doi: 10.1016/j.prp.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Lee Y.J., Oh Y.H. Primary ductal adenocarcinoma of the lacrimal gland. Jpn J Ophthalmol. 2009;53:268–270. doi: 10.1007/s10384-008-0659-y. [DOI] [PubMed] [Google Scholar]
- 11.Milman T., Shields J.A., Husson M., Marr B.P., Shields C.L., Eagle R.C., Jr. Primary ductal adenocarcinoma of the lacrimal gland. Ophthalmology. 2005;112:2048–2051. doi: 10.1016/j.ophtha.2005.04.029. [DOI] [PubMed] [Google Scholar]
- 12.Min K.W., Park H.K., Kim W.Y. Primary ductal adenocarcinoma of the lacrimal gland, associated with abundant intracytoplasmic lumens containing some eosinophilic hyaline globules: cytological, histological and ultrastructural findings. Ultrastruct Pathol. 2014;38:363–366. doi: 10.3109/01913123.2014.921656. [DOI] [PubMed] [Google Scholar]
- 13.Nasu M., Haisa T., Kondo T., Matsubara O. Primary ductal adenocarcinoma of the lacrimal gland. Pathol Int. 1998;48:981–984. doi: 10.1111/j.1440-1827.1998.tb03870.x. [DOI] [PubMed] [Google Scholar]
- 14.Ricci M., Amadori E., Chiesa F. Single bone metastasis from adenocarcinoma of the lacrimal gland: a case report. Future Oncol Lond Engl. 2014;10:1735–1739. doi: 10.2217/fon.14.36. [DOI] [PubMed] [Google Scholar]
- 15.Zhu M.M., Cui H.G., Teng X.D. GCDFP-15, AR, and Her-2 as biomarkers for primary ductal adenocarcinoma of the lacrimal gland: a Chinese case and literature review. OncoTargets Ther. 2015;8:1017–1024. doi: 10.2147/OTT.S82168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vagia E., Economopoulou P., Oikonomopoulos N., Athanasiadis I., Dimitriadis G., Psyrri A. Androgen-receptor positive lacrimal sac adenocarcinoma demonstrating long-lasting response to LHRH analog plus abiraterone treatment. Front Oncol. 2015;5:10. doi: 10.3389/fonc.2015.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ishida M., Iwai M., Yoshida K. Primary ductal adenocarcinoma of the lacrimal sac: the first reported case. Int J Clin Exp pathology. 2013;6:1929–1934. [PMC free article] [PubMed] [Google Scholar]
- 18.Marglani O., Alherabi A., Corsten M. Malignant oncocytoma of the lacrimal sac with cervical metastasis: case report and literature review. J otolaryngology - head neck Surg = Le J d'oto-rhino-laryngologie de Chir cervico-faciale. 2008;37:E8–E10. [PubMed] [Google Scholar]
- 19.Peretz WL, Ettinghausen SE, Gray GF. Oncocytic adenocarcinoma of the lacrimal sac. Archives Ophthalmol (Chicago, Ill : 1960) 1978;96:303–304. [DOI] [PubMed]
- 20.Perlman J.I., Specht C.S., McLean I.W., Wolfe S.A. Oncocytic adenocarcinoma of the lacrimal sac: report of a case with paranasal sinus and orbital extension. Ophthalmic Surg. 1995;26:377–379. [PubMed] [Google Scholar]
- 21.Tomic S., Warner T.F., Brandenburg J.H. Malignant oncocytoma of the lacrimal sac: ultrastructure and immunocytochemistry. Ear, nose, throat J. 1995;74:717–720. [PubMed] [Google Scholar]
- 22.Villaret A.B., Lombardi D., Schreiber A., Farina D., Nicolai P. Oncocytic carcinoma of the nasolacrimal duct treated by transnasal endoscopic resection. Head neck. 2013;35:E24–E27. doi: 10.1002/hed.21803. [DOI] [PubMed] [Google Scholar]
- 23.Yuen H.K., Cheuk W., Cheng A.C., Anh C., Chan N. Malignant oncocytoma of the lacrimal sac as an unusual cause of epiphora. Ophthalmic plastic Reconstr Surg. 2007;23:70–72. doi: 10.1097/IOP.0b013e31802dd7f4. [DOI] [PubMed] [Google Scholar]
- 24.Zhang J., Jianmin Wang N., Shi J., Ge X. A case of primary oncocytic adenocarcinoma of the lacrimal sac. BMJ case Rep. 2009:2009. doi: 10.1136/bcr.04.2009.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ishida M., Hotta M., Kushima R., Tsuruoka S., Ohji M., Okabe H. Case of ductal adenocarcinoma ex pleomorphic adenoma of the lacrimal gland. Rinsho byori Jpn J Clin pathology. 2009;57:746–751. [PubMed] [Google Scholar]
- 26.El-Sawy T., Frank S.J., Hanna E. Multidisciplinary management of lacrimal sac/nasolacrimal duct carcinomas. Ophthalmic plastic Reconstr Surg. 2013;29:454–457. doi: 10.1097/IOP.0b013e31829f3a73. [DOI] [PMC free article] [PubMed] [Google Scholar]