Abstract
Purpose
To report a case of a giant retinal pigment epithelial (RPE) tear associated with fluid overload in a patient with diabetic macular edema (DME) and kidney disease.
Observations
A 60-year-old man with type 2 diabetes mellitus and end-stage diabetic kidney disease who had gained weight because of fluid overload complained of a visual disturbance in the left eye that had started a few days earlier. The left fundus showed a RPE defect in two temporal quadrants under an extensive serous retinal detachment (SRD) with exacerbation of the original DME. Seven days later, he was admitted for severe edema and pleural effusion. No overt signs of congestive heart failure were noted. On admission, the RPE defect had markedly widened to involve the macula. Spectral-domain optical coherence tomography images showed substantial intraretinal fluid and an extensive SRD with rolled edges of the retinal pigment epithelium, which led to the diagnosis of a RPE tear. The fluid under the SRD was absorbed on the fourth hospital day and the substantial intraretinal fluid resolved on the eleventh day after systemic management of fluid overload only without ophthalmic treatment. The change in the appearance of the RPE area was minimal and the visual field defect remained even after 6 months.
Conclusions and importance
A RPE tear may develop in association with fluid overload in patients with diabetes.
Keywords: Retinal pigment epithelial tear, Fluid overload, Diabetic macular edema, Serous retinal detachment
1. Introduction
Since Hoskin and associates1 first reported retinal pigment epithelial (RPE) tears, accumulated evidence has suggested that RPE tears could occur in the following situations: spontaneously,2 after treatment of choroidal neovascularization (CNV) and age-related macular degeneration (AMD)-associated pigment epithelial detachments by laser photocoagulation,2, 3 or after intravitreal drug injections of vascular endothelial growth factor (VEGF) inhibitors.4 In rare instances, a RPE tear can develop in patients with central serous chorioretinopathy,5 trauma,6 and Vogt–Koyanagi–Harada disease,7 and in those who undergo glaucoma drainage surgery8, 9 and laser photocoagulation for diabetic retinopathy.9, 10 However, no study to date has reported the development of a RPE tear in association with fluid overload, although fluid overload contributes to the worsening of diabetic macular edema (DME) in several clinical settings.11, 12, 13, 14 To our knowledge, this is the first reported case of a RPE tear that might have developed as the result of fluid overload due to end-stage diabetic kidney disease.
2. Case report
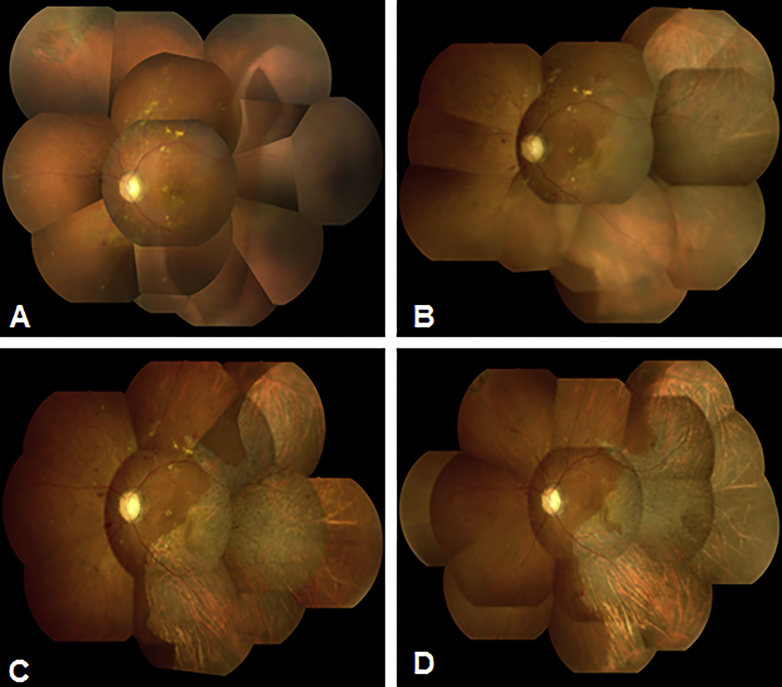
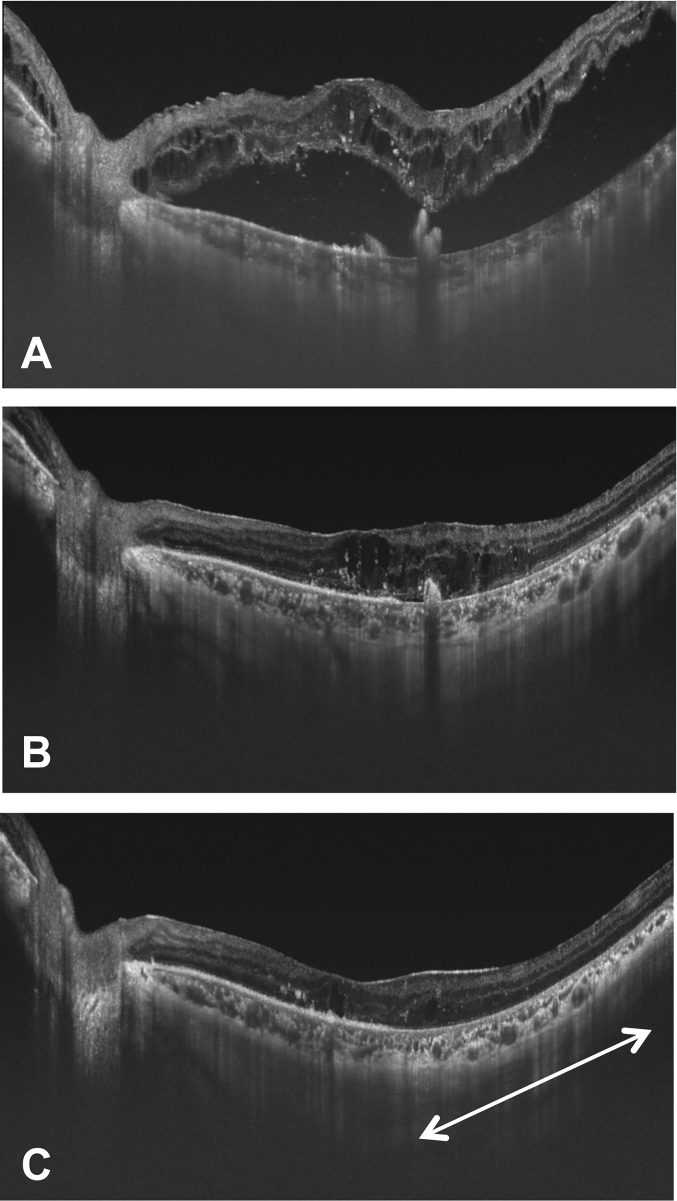
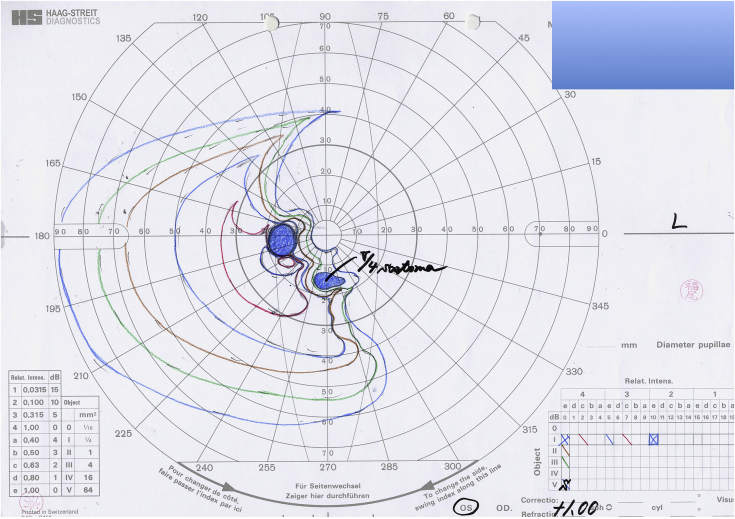
A 60-year-old man with a 9-year history of type 2 diabetes mellitus who was being followed up at our center complained of a visual disturbance in the left eye that had started a few days earlier. Seven months before presentation, he had received a posterior sub-Tenon injection of triamcinolone acetonide in the left eye for DME, but DME persisted. Six months before presentation, lattice degeneration with a retinal tear in the superonasal quadrant of the peripheral retina had been treated with laser photocoagulation. The left eye had not undergone any other ophthalmic treatment for several months prior to presentation. The patient had also gained approximately 9 kg because of fluid retention from end-stage diabetic kidney disease over the past 3 months despite taking 40 mg of furosemide daily. On examination, the best-corrected visual acuity (BCVA) was 0.2 in the right eye and 0.03 in the left eye. The fundus examination showed bilateral non-proliferative diabetic retinopathy and evidence of panretinal photocoagulation for rubeotic glaucoma that had been applied only in the right eye. The left fundus had a RPE defect in two temporal quadrants that lay under an extensive serous retinal detachment (SRD) (Fig. 1A). Optical coherence tomography (OCT) showed an increase in the substantial intraretinal fluid bilaterally and an extensive SRD in the left eye. No ophthalmic angiography was performed because of his poor general condition. Seven days later, he was admitted to the Department of Medicine in our center for severe fluid overload as evidenced by the increase in weight, pleural effusion, and lower limb edema. No overt signs of congestive heart failure were noted. On admission, widening of the RPE defect with involvement of the macula was observed (Fig. 1B). On spectral-domain (SD) OCT, the rolled edges of the retinal pigment epithelium in the left eye were noted (Fig. 2A). From the OCT findings, we diagnosed a RPE tear. At the same time, strict medical treatment, including an increased dose of furosemide, addition of a new diuretic, and a salt-restricted diet, was started to treat the fluid overload. He began to lose weight the next day. On the fourth hospital day, 10 days after detection of the RPE tear, the BCVA increased to 0.1 with absorption of fluid under the SRD in the left eye seen on SD-OCT images (Fig. 2B). The area of the RPE defect did not enlarge further (Fig. 1C). On the eleventh hospital day, 17 days after detection of the RPE tear, SD-OCT images of the left eye showed almost complete resolution of the substantial intraretinal fluid and direct attachment of the outer retina to Bruch's membrane (Fig. 2C). The BCVA remained 0.2 in the left eye, and Goldmann perimetry showed the presence of a visual field defect corresponding to the area where the RPE was lost. Eventually, the patient lost about 12 kg and returned to his usual weight after 7 weeks of hospital stay. However, the area where the RPE was absent showed little further change in appearance (Fig. 1D), and the visual field defect remained (Fig. 3) even after 6 months had passed after development of the RPE tear.
Fig. 1.
Composite fundus photography of the left fundus. Immediately (A), 7 days (B), 10 days (C), and 6 months (D) after the retinal pigment epithelial (RPE) tear developed.
Fig. 2.
Images of horizontal spectral-domain optical coherence tomography (SD-OCT) scan in the left fovea. (A) Seven days after the RPE tear developed, SD-OCT showed the presence of substantial intraretinal fluid and marked serous retinal detachment (SRD) with rolled edges of the retinal pigment epithelium. (B) Ten days after the RPE tear developed, SD-OCT showed rapid absorption of fluid under the SRD. (C) Seventeen days after the RPE tear developed, SD-OCT showed almost complete resolution of the substantial intraretinal fluid, and the direct attachment of the outer retina to Bruch's membrane without the RPE line (white arrow).
Fig. 3.
Goldmann perimetry of the left eye. Six months after the RPE tear developed, Goldmann perimetry revealed a visual field defect corresponding to the area where the retinal pigment epithelium was lost.
3. Discussion
Although the exact mechanism of RPE tear formation is still under debate, tangential shearing and tractional forces have been proposed as the underlying pathogeneses in some cases after treatment of CNV and AMD-associated pigment epithelial detachments with photocoagulation and anti-VEGF therapies.1, 2, 3, 4 However, in the current case, we observed little contractile forces that could have led to a RPE tear on ophthalmoscopy and OCT findings. Moreover, in the current case, a SRD and RPE tear developed with worsening of the DME during fluid overload. Under the conditions of fluid overload, an imbalance between the hydrostatic and colloid osmotic pressures favors fluid movement from the vascular to extravascular compartment, as stated in Starling's principle.15 Unfortunately, because of his poor general condition, we could not perform ophthalmic angiographies to further evaluate the retino-choroidal vascular permeability. Nevertheless, numerous studies have reported that fluid overload might be associated with exacerbation of DME, possibly resulting from the increased retinal vascular permeability.11, 12, 13, 14 Also, excessive tissue pressure due to choroidal vascular hyperpermeability may cause SRD, because such hydrostatic force might lead to mechanical disruption of the RPE barrier and abnormal fluid egress under the retina.16, 17, 18 Furthermore, Goldstein and Pavan reported that an abrupt increase in the amount of sub-RPE fluid could stretch the retinal pigment epithelium, leading to a blowout tear.19 For these reasons, we speculate that leakage in the choroid during fluid overload had led to an elevated fluid pressure beneath the retinal pigment epithelium, which then induced the extensive SRD and the widespread RPE tear that occurred simultaneously. Strict medical treatment for fluid overload during the hospitalization ameliorated the conditions associated with fluid overload, resulting in rapid resolution of the SRD. OCT performed after the recovery of systemic symptoms showed that the outer retina where the RPE tear developed appeared to have attached directly to Bruch's membrane, and fluid under the SRD had been rapidly absorbed. Consistent with our findings, Mukai et al. observed two different patterns of repair of RPE tears in AMD cases.20 First, the area where the retinal pigment epithelium was lost seems to be covered by thickened proliferative tissue when the subretinal fluid persisted after the development of a RPE tear. Second, the outer retina appears to attach directly to Bruch's membrane without ingrowth of proliferative tissue in a case of early and complete subretinal fluid resolution after the tear event.20 Though we did not find any apparent evidence of AMD in OCT images, we believe that the current case also followed the latter repair process. Despite the structural outcomes on imaging, the visual prognosis was poor in the present case, and Goldmann perimetry performed 6 month after the event showed a visual field defect corresponding to the area where the RPE tear had developed. In contrast, Iijima et al. reported a case of a RPE tear in which visual field of the affected eye was preserved.21 Intact retinal pigment epithelia surrounding a relatively small tear might enable maintenance of retinal sensitivity in the area of the RPE defect.21 Therefore, we speculate that the smaller area of the RPE defect might have favored the maintenance of visual function in their case (1.5 disc areas) compared with the current case (two-quadrant area).
4. Conclusion
We experienced a case of a RPE tear that developed in association with fluid overload due to diabetic end-stage kidney disease. Further studies are warranted to elucidate the exact mechanism by which the RPE tear progresses and is repaired.
Patient consent
The patient provided oral consent for publication of information that might identify the person, including medical record details and photographs. The oral consent was then documented in writing by the physician in charge.
Funding
No funding or grant support.
Conflict of interest
Y.K., A.H, T.I., Y.U. and S.K. have no financial disclosures.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgements
None.
References
- 1.Hoskin A., Bird A.C., Sehmi K. Tears of detached retinal pigment epithelium. Br J Ophthalmol. 1981;65(6):417–422. doi: 10.1136/bjo.65.6.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeo J.H., Marcus S., Murphy R.P. Retinal pigment epithelial tears: patterns and prognosis. Ophthalmology. 1988;95(1):8–13. [PubMed] [Google Scholar]
- 3.Gass J.D. Retinal pigment epithelial rip during krypton red laser photocoagulation. Am J Ophthalmol. 1984;98(6):700–706. doi: 10.1016/0002-9394(84)90684-6. [DOI] [PubMed] [Google Scholar]
- 4.Dhalla M.S., Blinder K.J., Tewari A., Hariprasad S.M., Apte R.S. Retinal pigment epithelial tear following intravitreal pegaptanib sodium. Am J Ophthalmol. 2006;141(4):752–754. doi: 10.1016/j.ajo.2005.10.053. [DOI] [PubMed] [Google Scholar]
- 5.Ishida Y., Kato T., Minamoto A., Yokoyama T., Jian K., Mishima H.K. Retinal pigment epithelial tear in a patient with central serous chorioretinopathy treated with corticosteroids. Retina. 2004;24(4):633–636. doi: 10.1097/00006982-200408000-00028. [DOI] [PubMed] [Google Scholar]
- 6.Levin L.A., Seddon J.M., Topping T. Retinal pigment epithelial tears associated with trauma. Am J Ophthalmol. 1991;112(4):396–400. doi: 10.1016/s0002-9394(14)76246-4. [DOI] [PubMed] [Google Scholar]
- 7.Milli-Boussen I., Letaief I., Dridi H., Ouertani A. Bilateral retinal pigment epithelium tears in acute Vogt-Koyanagi-Harada disease. Retin Cases Brief Rep. 2013;7(4):350–354. doi: 10.1097/ICB.0b013e3182964f68. [DOI] [PubMed] [Google Scholar]
- 8.Lois N., Sehmi K.S., Hykin P.G. Giant retinal pigment epithelial tear after trabculectomy. Arch Ophthalmol. 1999;117(4):546–547. doi: 10.1001/archopht.117.4.546. [DOI] [PubMed] [Google Scholar]
- 9.Lim J.I., Blair N.P., Liu S.J. Retinal pigment epithelial tear in a diabetic patient with exudative retinal detachment following panretinal photocoagulation and filtration surgery. Arch Ophthalmol. 1990;108:173–174. doi: 10.1001/archopht.1990.01070040025012. [DOI] [PubMed] [Google Scholar]
- 10.Kameda Y., Mori F., Masahara H., Suzuki M., Yamauchi Y., Eguchi E. Giant retinal pigment epithelial tear after laser photocoagulation for diabetic retinopathy. Br J Ophthalmol. 2009;93(1):11–12. doi: 10.1136/bjo.2007.130849. 123. [DOI] [PubMed] [Google Scholar]
- 11.Ciardella A.P. Partial resolution of diabetic macular oedema after systemic treatment with furosemide. Br J Ophthalmol. 2004;88(9):1224–1225. doi: 10.1136/bjo.2004.042580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perkovich B.T., Meyers S.M. Systemic factors affecting diabetic macular edema. Am J Ophthalmol. 1988;105(2):211–212. doi: 10.1016/0002-9394(88)90190-0. [DOI] [PubMed] [Google Scholar]
- 13.Ryan E.H., Jr., Han D.P., Ramsay R.C. Diabetic macular edema associated with glitazone use. Retina. 2006;26(5):562–570. doi: 10.1097/00006982-200605000-00011. [DOI] [PubMed] [Google Scholar]
- 14.Tokuyama T., Ikeda T., Sato K. Effects of haemodialysis on diabetic macular leakage. Br J Ophthalmol. 2000;84(12):1397–1400. doi: 10.1136/bjo.84.12.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Starling E.H. On the absorption of fluids from the connective tissue spaces. J Physiol (Lond) 1896;19(4):312–326. doi: 10.1113/jphysiol.1896.sp000596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marmor M.F. New hypotheses on the pathogenesis and treatment of serous retinal detachment. Graefes Arch Clin Exp Ophthalmol. 1988;226(6):548–559. doi: 10.1007/BF02169203. [DOI] [PubMed] [Google Scholar]
- 17.Iida T., Hagimura N., Takahashi K., Muraoka K. Study of choroidal vascular lesions in bullous retinal detachment by indocyanine green angiography. J Jpn Ophthalmol Soc. 1995;99:945–954. [PubMed] [Google Scholar]
- 18.Spaide R.F., Goldbaum M., Wong D.W., Tang K.C., Iida T. Serous detachment of the retina. Retina. 2003;23(6):820–846. doi: 10.1097/00006982-200312000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Goldstein B.G., Pavan P.R. Blow-outs in the retinal pigment epithelium. Br J Ophthalmol. 1987;71(9):676–681. doi: 10.1136/bjo.71.9.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mukai R., Sato T., Kishi S. Repair mechanism of retinal pigment epithelial tears in age-related macular degeneration. Retina. 2015;35(3):473–480. doi: 10.1097/IAE.0000000000000337. [DOI] [PubMed] [Google Scholar]
- 21.Iijima H., Gohdo T. Visual field change in eyes with retinal pigment epithelial tear. Retina. 1999;19(3):198–203. doi: 10.1097/00006982-199905000-00004. [DOI] [PubMed] [Google Scholar]