Abstract
Purpose
While there are many known etiologies of choroidal neovascularization (CNV), tuberculosis is not a well-known causative agent. In this case series, we highlight CNV occurring secondary to tuberculous chorioretinitis, its presentation, and its management.
Observations
We retrospectively reviewed the charts and imaging of four patients who presented with presumed tuberculous chorioretinitis and CNV. Three of these patients had signs of intraocular inflammation and were also found to have active macular CNV. The one remaining patient had chorioretinal scars from prior posterior uveitis and previously treated macular CNV membranes. The three patients with active disease were started on anti-tuberculosis medications and oral corticosteroids, and they also received intravitreal anti-vascular endothelial growth factor (VEGF) injections as needed for the CNV. There was a significant improvement in the clinical course of all three patients with active disease—the intraocular inflammation subsided, and CNV recurrences were mitigated. Upon completion of systemic treatment, all patients have remained quiescent.
Conclusions and importance
Our findings demonstrate that CNV may occur in the course of tuberculous chorioretinitis with marked loss of vision, and management with anti-tuberculosis medications, oral corticosteroids, and intravitreal anti-VEGF injections results in notable improvement in their clinical course.
Keywords: Choroidal neovascularization, Tuberculosis, Tuberculous chorioretinitis, Aflibercept
1. Introduction
Choroidal neovascularization (CNV) may lead to significant visual morbidity when left untreated. There are multiple known causes of CNV. Broadly, the categories include degenerative conditions (age-related macular degeneration, pathologic myopia, angioid streaks), hereditary degenerative conditions (vitelliform macular dystrophy), inflammatory conditions (ocular histoplamosis syndrome, multifocal choroiditis), infectious etiologies (toxoplasmosis), tumors, traumatic, and idiopathic. Elucidating the underlying etiology of a CNV membrane (CNVM) is important for determining the treatment and prognosis of the eye.
Tuberculosis (TB) is overall an uncommon etiology for CNV, which is driven by vascular endothelial growth factor (VEGF). However, experimental animal models of intraocular TB reveal VEGF expression in the RPE, which has been shown to harbor Mycobacterium tuberculosis that thrive by inhibiting phagolysosome fusion.1, 2, 3 In this case series, we highlight four patients who developed CNV with tuberculous chorioretinitis and describe their subsequent management.
2. Findings
2.1. Case 1
A 57-year-old Korean female was diagnosed with “tuberculous uveitis” in both eyes (OU) by an outside ophthalmologist based on a positive tuberculin skin test and interferon-gamma release assay. The patient was started on quadruple anti-tuberculosis therapy and oral prednisone 10mg daily, but pyrimethamine and ethambutol were discontinued after two weeks due to intolerable side effects. The patient received an intravitreal triamcinolone injection in the left eye (OS) for distorted central vision.
Three months later, she presented with a best-corrected visual acuity (BCVA) of 20/30 in the right eye (OD) and 20/40 OS. Exam was notable for vitritis OU, macular CNV, subretinal tissue, cystoid edema, and peripheral chorioretinal scars OS (Fig. 1). Fluorescein angiography (FA), optical coherence tomography (OCT), and indocyanine green revealed a Type 1 choroidal neovascular membrane (CNVM) parafoveally OS (Fig. 1). Autofluorescence (AF) was notable for peripapillary hypoautofluorescence with hyperfluorescence at the edges of the chorioretinal lesions OS. The patient was continued on anti-tuberculosis therapy and corticosteroids. After three intravitreal bevacizumab injections with persistent sub- and intraretinal fluid OS, the patient was switched to aflibercept with notable improvement. Six weeks later, the fluid recurred, and the patient's oral prednisone was increased to 20mg daily for one week, followed by 15mg daily thereafter. There was a slight improvement in the macular edema, and after nine months of anti-tuberculosis treatment with an ongoing oral prednisone taper, the patient has remained quiescent.
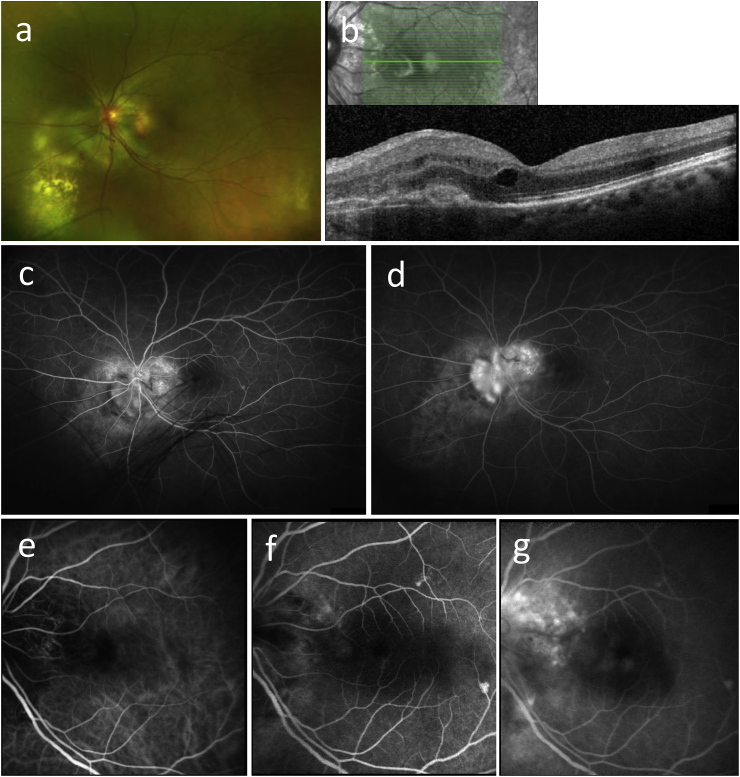
Fig. 1.
Case 1. a) Wide-field optos fundus photo of the left eye is notable for peripapillary subretinal tissue hypopigmented chorioretinal lesions. b) Optical coherence tomography of the left eye demonstrating macular, subretinal tissue, and cystoid macular edema. c, d) Fluorescein angiogram of the left eye with mid (C) and late (d) phase images, respectively, demonstrating peripapillary staining and leakage. e–g) Indocyanine green angiogram of the left eye with early (e), mid (f), and late (g) phase images from left to right. There is an area of juxtapapillary leakage in the nasal macula. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2.2. Case 2
A 76-year-old Indian male with rheumatoid arthritis on methotrexate was referred for “tuberculous serpiginoid chorioretinitis” diagnosed two years ago based on a positive interferon-gamma release assay. The patient underwent triple anti-tuberculosis therapy for six months, after which he developed CNV OS then OD. He received multiple intravitreal bevacizumab injections OU with limited response.
Upon referral, the patient's BCVA was 20/100 OD and 20/200 OS. Examination revealed vitritis and scattered hypopigmented chorioretinal scars throughout the macula and periphery OU (Fig. 2). AF and FA showed active inflammation OU and a large macular CNVM OS. OCT revealed Type 2 choroidal neovascularization and extensive subretinal fibrosis. Because of concern for resistant mycobacteria, the patient was placed on quadruple therapy and oral prednisone 20mg daily and received an intravitreal bevacizumab injection OS. After six months of anti-tuberculosis treatment and continuing on a slow prednisone taper, there persisted an area of subretinal fluid OS. Given the poor visual potential and lack of active inflammation, the patient elected to observe.
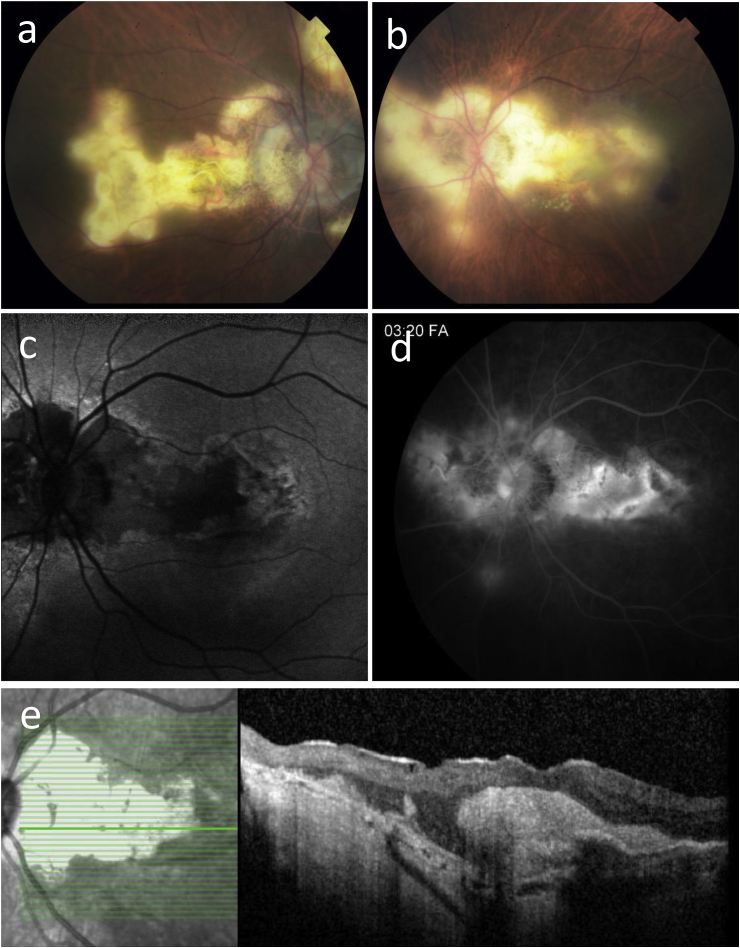
Fig. 2.
Case 2. a, b) Fundus photos of the right (a) and left (b) eyes demonstrate serpiginoid hypopigmented chorioretinal lesions in both eyes. c) Autofluorescence of the left eye reveals hypoautofluorescence of the chorioretinal lesions with hyperautofluorescence at the lesion edges. (d) Late-phase fluorescein angiography of the left eye shows peripapillary staining and a large area of macular leakage corresponding to choroidal neovascularization. e) Optical coherence tomography of the left eye is notable for dense subretinal fibrosis and hemorrhage consistent with Type 2 choroidal neovascularization.
2.3. Case 3
A 39-year-old Vietnamese female without past medical history who immigrated to the United States at an early age presented complaining of a “blind spot” and photopsias OD for three weeks. She reported a similar blind spot OS which never resolved. On initial presentation, BCVA was 20/30 OU. Examination revealed trace vitritis OU, hypopigmented peripapillary lesions OD, and scattered old chorioretinal scars OS (Fig. 3). FA did not demonstrate any leakage. Based on a positive tuberculin skin test, she was diagnosed with tuberculous multifocal choroiditis and started on a nine-month course of triple therapy and oral prednisone 40mg daily. Ethambutol was discontinued two weeks later due to dizziness and nausea. The intraocular inflammation subsequently subsided, but six months into the course of treatment, macular CNV developed OD (Fig. 3). This responded well to a single intravitreal bevacizumab injection, after which she received two more as a loading dose.
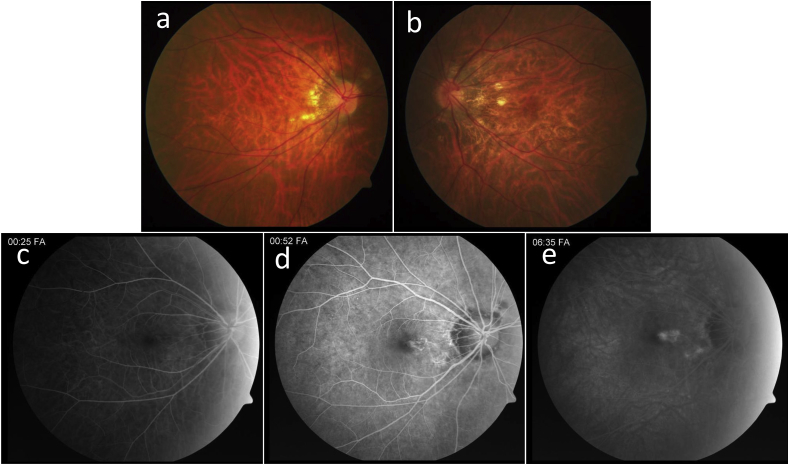
Fig. 3.
Case 3. a, b) Fundus photos of the right (a) and left (b) eyes demonstrate well-demarcated peripapillary hypopigmented chorioretinal lesions. c–e) Fluorescein angiogram of the right eye with early (a), mid (b), and late (c) phase images reveals an area of leakage nasal to the fovea with staining at the peripapillary chorioretinal scars.
Four years later, she developed intermittent CNVMs OU, which were treated with bevacizumab eight times OD and two times OS. New active chorioretinal lesions were seen on autofluorescence OU, and the patient was re-started on rifampin, isoniazid, and oral prednisone 40mg daily. Anti-VEGF injections were switched to aflibercept, of which she received four injections OD and two OS. After completing one year of anti-tuberculosis therapy, the patient has since remained quiescent with BCVA 20/30 OU.
2.4. Case 4
A 65 year-old Indian male with history of a positive tuberculin skin test in 1986 for which he underwent one year of quadruple therapy presented with a history of tuberculous chorioretinitis OU. Based on review of outside records, the patient had developed a recurrent macular CNVM OS for which he received five ranibizumab injections, most recently two years ago. On presentation, BCVA was hand motions OD and 20/100 OS. Exam was notable for extensive subretinal scars, chorioretinal atrophy, and pigmentary changes OD greater than OS (Fig. 4). There was currently no evidence of active uveitis on evaluation, and OCT images did not demonstrate the presence of subretinal or intraretinal fluid. He has remained quiescent for the past year.
Fig. 4.
Case 4. a) Wide-field Optos fundus photo of the right eye demonstrating extensive chorioretinal atrophy and retinal pigment epithelial migration. b) Fundus photo of the left eye has a similar but less extensive appearance of peripapillary chorioretinal atrophy and pigmentary changes. c) Autofluorescence demonstrates hypoautofluorescence at the area of chorioretinal atrophy. d) Optical coherence tomography of the left eye reveals subretinal fibrosis without subretinal or intraretinal fluid.
3. Discussion
TB chorioretinitis likely develops as an exuberant immune response to reactivation of dormant Mycobacterium tuberculosis harbored in the RPE.2, 3 Similarly, choroidal seeding secondary to reactivation elsewhere in the body may also lead to intraocular TB inflammation.1 Experimental animal models of ocular TB reveal localized tissue hypoxia and enhanced VEGF levels at foci of TB infections,4 which may account for the occurrence of CNV in cases of tuberculous uveitis.
The earliest mention of TB-associated CNV in the literature was in a 1987 case report, where “subretinal neovascularization” occurred 34 years after the initial TB diagnosis and was treated successfully with isoniazid and laser photocoagulation.5
Several therapeutic strategies have been attempted for uveitis-related CNV: focal laser photocoagulation,6 photodynamic therapy with verteporfin,7 local and systemic corticosteroids,8 systemic immunosuppression,9 surgery,10 and most recently, intravitreal bevacizumab injections.11, 12 Given the elevated VEGF levels associated with intraocular TB infections, anti-VEGF therapy may effectively suppress TB-related CNV.4
In a study by Julián et al., 15 patients with CNV secondary to uveitis, including one tuberculous uveitis, received intravitreal bevacizumab injections for CNV treatment.11 After 17 months follow-up, nearly 80% of eyes showed significant improvement in visual acuity and OCT macular thickness after a mean 4.25 injections. However, most patients had been started on systemic immunosuppressive agents prior to initiation of bevacizumab, which included intravenous methylprednisolone pulse therapy, oral prednisone (∼1mg/kg/day), azathioprine, and interferon alpha. The authors suggested that systemic immunosuppression is critical for targeting the underlying inflammatory process.
One cannot overlook the multiple deleterious side effects associated with immunosuppressive agents, which further compound the potential hepatotoxicity of anti-tuberculosis medications. In light of this, we recommend treating TB-related CNV with intravitreal anti-VEGF injections coupled with systemic anti-tuberculosis medications and oral corticosteroids. Failed efficacy of intravitreal bevacizumab leads one to consider other anti-VEGF agents such as aflibercept, as we describe the earliest use of this newer agent with CNV due to TB uveitis. It is important to recognize CNV as a potential sequelae of TB chorioretinitis to ensure proper management with both local and systemic agents.
4. Conclusions
This retrospective case series highlights four patients presenting with active choroidal neovascularization or prior choroidal neovascular membranes as a result of tuberculous chorioretinitis. The three patients with active choroidal neovascular membranes were all treated with anti-tuberculosis medications and oral corticosteroids, in addition to anti-VEGF injections. Intravitreal aflibercept was found to be particularly efficacious, and we describe the earliest use of this newer agent in these cases. All three patients had a notable improvement in their clinical course with retention of their baseline visual acuity. This study emphasizes the importance of identifying tuberculosis as a cause of choroidal neovascularization to ensure proper management of these patients with a combination of local and systemic therapy.
5. Patient consent
The Institutional Review Board at USC approved the study protocol, and all participants provided written consent for inclusion in this study.
Funding
None.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Conflict of interest
D.R. is a paid consultant for Allergan, Inc. The following authors have no financial disclosures: E.K. and N.R.
Acknowledgements
None.
Footnotes
The authors have not published or submitted any related papers from this study.
Contributor Information
Esther Lee Kim, Email: kim.esther.lee@gmail.com.
Damien C. Rodger, Email: damien.rodger@med.usc.edu.
Narsing A. Rao, Email: narsing.rao@med.usc.edu.
References
- 1.Rao N.A., Albini T.A., Kumaradas M., Pinn M.L., Fraig M.M., Karakousis P.C. Experimental ocular tuberculosis in Guinea pigs. Arch Ophthalmol. 2009;127:1162–1166. doi: 10.1001/archophthalmol.2009.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nazari H., Karakousis P.C., Rao N.A. Replication of Mycobacterium tuberculosis in retinal pigment epithelium. JAMA Ophthalmol. 2014;132:724–729. doi: 10.1001/jamaophthalmol.2014.270. [DOI] [PubMed] [Google Scholar]
- 3.Rao N.A., Saraswathy S., Smith R.E. Tuberculous uveitis: distribution of Mycobacterium tuberculosis in the retinal pigment epithelium. Arch Ophthalmol. 2006;124:1777–1779. doi: 10.1001/archopht.124.12.1777. [DOI] [PubMed] [Google Scholar]
- 4.Thayil S.M., Albini T.A., Nazari H. Local ischemia and increased expression of vascular endothelial growth factor following ocular dissemination of Mycobacterium tuberculosis. PLoS One. 2011;6:e28383. doi: 10.1371/journal.pone.0028383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gur S., Silverstone B.Z., Zylberman R., Berson D. Chorioretinitis and extrapulmonary tuberculosis. Ann Ophthalmol. 1987;19:112–115. [PubMed] [Google Scholar]
- 6.Fine S.L., Wood W.J., Isernhagen R.D. Laser treatment for subfoveal neovascular membranes in ocular histoplasmosis syndrome: results of a pilot randomized clinical trial. Arch Ophthalmol. 1993;111:19–20. doi: 10.1001/archopht.1993.01090010021006. [DOI] [PubMed] [Google Scholar]
- 7.Parodi M.B., Di Crecchio L., Lanzetta P., Polito A., Bandello F., Ravalico G. Photodynamic therapy with verteporfin for subfoveal choroidal neovascularization associated with multifocal choroiditis. Am J Ophthalmol. 2004;138:263–269. doi: 10.1016/j.ajo.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 8.Flaxel C.J., Owens S.L., Mulholland B., Schwartz S.D., Gregor Z.J. The use of corticosteroids for choroidal neovascularisation in young patients. Eye (Lond) 1998;12(Pt 2):266–272. doi: 10.1038/eye.1998.62. [DOI] [PubMed] [Google Scholar]
- 9.Dees C., Arnold J.J., Forrester J.V., Dick A.D. Immunosuppressive treatment of choroidal neovascularization associated with endogenous posterior uveitis. Arch Ophthalmol. 1998;116:1456–1461. doi: 10.1001/archopht.116.11.1456. [DOI] [PubMed] [Google Scholar]
- 10.Hawkins B.S., Bressler N.M., Bressler S.B. Surgical removal vs observation for subfoveal choroidal neovascularization, either associated with the ocular histoplasmosis syndrome or idiopathic: I. Ophthalmic findings from a randomized clinical trial: submacular Surgery Trials (SST) Group H Trial: SST Report No. 9. Arch Ophthalmol. 2004;122:1597–1611. doi: 10.1001/archopht.122.11.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Julian K., Terrada C., Fardeau C. Intravitreal bevacizumab as first local treatment for uveitis-related choroidal neovascularization: long-term results. Acta Ophthalmol. 2011;89:179–184. doi: 10.1111/j.1755-3768.2010.02046.x. [DOI] [PubMed] [Google Scholar]
- 12.Tran T., Fardeau C., Terrada C., Ducos De Lahitte G., Bodaghi B., Lehoang P. Intravitreal bevacizumab for refractory choroidal neovascularization (CNV) secondary to uveitis. Graef Arch Clin Exp. 2008;246:1685–1692. doi: 10.1007/s00417-008-0906-4. [DOI] [PubMed] [Google Scholar]