Summary
Action potentials clustered into high-frequency bursts play distinct roles in neural computations. However, little is known about ionic currents that control the duration and probability of these bursts. We found that, in cartwheel inhibitory interneurons of the dorsal cochlear nucleus, the likelihood of bursts and the interval between their spikelets were controlled by Ca2+ acting across two nanodomains, one between plasma membrane P/Q Ca2+ channels and endoplasmic reticulum (ER) ryanodine receptors and another between ryanodine receptors and large-conductance, voltage- and Ca2+-activated K+ (BK) channels. Each spike triggered Ca2+-induced Ca2+-release (CICR) from ER immediately beneath somatic, but not axonal or dendritic, plasma membrane. Moreover, immunolabeling demonstrated close apposition of ryanodine receptors and BK channels. Double nanodomain coupling between somatic plasma membrane and hypolemmal ER cisterns provides a unique mechanism for rapid control of action potentials on the millisecond time scale.
Introduction
Spike bursts are clusters of 2–5 high-frequency (>100 Hz) action potentials (spikelets), and the capacity to generate bursts is fundamental to signaling in the brain. Spike bursts play roles in synaptic plasticity, sleep and arousal, reward-related behavior, extraction of sensory features, and motor learning (Cooper, 2002; Huerta and Lisman, 1995; Ito, 2001; Krahe and Gabbiani, 2004; Thomas et al., 1998). Bursts increase release of transmitter in presynaptic terminals (Roberts et al., 2008; Williams and Stuart, 1999), and enhance spine Ca2+ in dendrites (Kampa et al., 2006). To maintain the depolarization that drives emission of multiple spikelets, sustained inward current is necessary. While in some cases inward current is provided by EPSPs (Grienberger et al., 2014; Schmolesky et al., 2002), the burst may also be initiated by intrinsic conductances, including somato/dendritic Ca2+ channels, Ca2+ channels of the axon initial segment (AIS), or persistent Na+ current of AIS or first node (Bender and Trussell, 2009; Kim and Trussell, 2007; Kole, 2011; McCormick and Huguenard, 1992; Williams and Stuart, 1999). However, to temper the burst, the burst-specific excitatory current must be countered by outward current, and this outward current is thus likely to determine burst duration, frequency and spikelet interval.
Given the prominence of Ca2+ in triggering and sustaining spike bursts, it is plausible that Ca2+-activated K+ currents play a significant role in shaping the burst (Benhassine and Berger, 2009; Bock and Stuart, 2016; Kim and Trussell, 2007; Womack and Khodakhah, 2004). For example, spontaneous and synaptically driven bursts in cartwheel interneurons of the dorsal cochlear nucleus are strongly dependent on large-conductance, voltage- and Ca2+-activated K+ channels (BK channels); blockade of these channels, or reduction in extracellular Ca2+, profoundly increases burst duration and frequency (Kim and Trussell, 2007). For bursts like these, with spikelet frequencies of 200 Hz or more, recruitment of BK channels requires that the intracellular Ca2+ rise be sufficiently rapid and close to the BK channels to control spikelet probability and interspikelet interval in the millisecond timescale.
Rapid, precise spatiotemporal control of intracellular Ca2+ concentration is found in local Ca2+ signaling domains called nanodomains, in which Ca2+ channels and Ca2+ binding sites are within 100 nm (Augustine et al., 2003; Eggermann et al., 2012). Example of such nanodomains, or the somewhat wider microdomains, are found in presynaptic nerve terminals, where transmitter release is efficiently triggered by Ca2+ sensors located short distances from voltage-gated Ca2+ (Cav) channels (Eggermann et al., 2012). A candidate postsynaptic Ca2+ domain that could regulate spike bursts is the region between the plasma membrane and Ca2+ stores in endoplasmic reticulum (ER). ER varies in morphology and functional properties (Verkhratsky, 2005), and portions of the ER are molded into specialized structures known as subsurface or hypolemmal cisterns, which come into extremely close contact with the plasma membrane (Rosenbluth, 1962) and function as Ca2+ stores (Blaustein and Golovina, 2001). In principle, Ca2+-induced Ca2+-release (CICR), the release of ER Ca2+ through ryanodine receptors (RyRs) (Endo et al., 1970), could act on plasma membrane Ca2+-activated K+ channels to control bursts. Indeed, at the cell bodies and proximal dendrites of cerebellar Purkinje cells, subsurface cisterns are overlaid by plasma membrane BK channels colocalized with Cav2.1 (α1A subunit of P/Q-type Ca2+ channels) within about 40 nm, suggesting the clustered Cav2.1 and BK channels form Ca2+ nanodomains (Henkart et al., 1976; Indriati et al., 2013; Kaufmann et al., 2009; Takahashi and Wood, 1970). From electrophysiological studies, postsynaptic CICR has been associated with alterations in baseline firing rates in neurons and the generation of a slow afterhyperpolarization, often by activation of SK channels (Cui et al., 2004; Kakizawa et al., 2007; Sah and McLachlan, 1991). However, these effects on SK channels manifest over hundreds of milliseconds, and it remains unknown if Ca2+ cisterns can participate in rapid spike-by-spike control of voltage necessary to regulate the spike burst.
Cartwheel cells in dorsal cochlear nucleus are characterized by prominent subsurface cisterns immediately beneath somatic membrane (Ryugo et al., 1995; Wouterlood and Mugnaini, 1984). Given the significance of spike bursts to signaling in cartwheel cells, we examined the role of CICR in determining the threshold and inter-spikelet interval of bursts in cartwheel cells. RyRs responded to Ca2+ entering through P/Q-type Ca2+ channels. Remarkably, Ca2+ released from stores activated BK channels within the time course of a single spike, and could maintain CICR on a spike-by-spike basis. Two-photon Ca2+ imaging revealed that rapid CICR was triggered by individual spikes immediately beneath somatic but not dendritic or axonal membrane. CICR-induced BK currents were blocked by intracellular injection of BAPTA but not by EGTA, demonstrating nanodomain coupling of RyRs with both Cav and BK channels. Accordingly, immunohistochemical labeling revealed colocalization of RyRs and BK channels, suggesting close apposition of ER and somatic membrane. Thus, nanodomain coupling between Cav, BK and RyR controls the probability and fine structure of spike bursts.
Results
RyR-mediated CICR control of action potentials
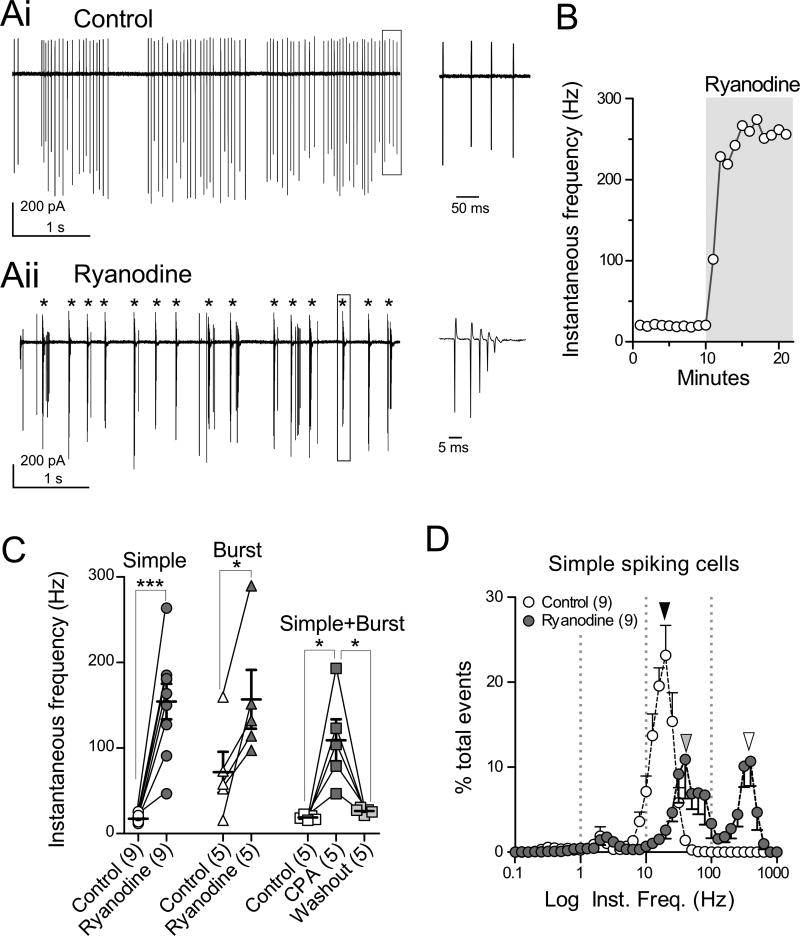
Blockade of CICR profoundly altered spontaneous action potential firing in cartwheel cells. Loose cell-attached recordings were made from cell bodies while blocking synaptic transmission with NBQX, MK-801, picrotoxin, and strychnine. Of 19 spontaneous-firing cartwheel cells, 12 showed ongoing simple spike firing (simple-firing cells, Fig. 1Ai) while the others exhibited a mix of spontaneous simple spikes and spike bursts (burst-firing cells, Fig. S1Ai), as previously reported (Bender et al., 2012; Kim and Trussell, 2007). RyR-mediated CICR was blocked by bath application of 20 µM ryanodine (Saito and Yanagawa, 2013; Verkhratsky, 2005). For simple-firing cells, ryanodine transformed the firing pattern into burst firing and increased spike frequency within 5 min (Fig. 1Aii, burst indicated by *), and the instantaneous frequency calculated from the intervals between action potentials increased from 20 to about 260 Hz (Fig. 1B). When a spontaneously burst-firing cell was exposed to ryanodine, the cell exhibited a sharp increase in burst frequency (Fig. S1Ai and Aii, asterisks). Figure 1C summarizes the increases of instantaneous spike frequency by ryanodine or by 10 µM cyclopiazonic acid (CPA), an ER Ca2+-ATPase inhibitor which depletes Ca2+ stores (Verkhratsky, 2005). Both compounds significantly increased the instantaneous firing frequency in simple-firing and burst-firing cells [ryanodine: Fig. 1C; simple-firing, 17.5±1.4 Hz to 154.3±20.7 Hz, n=9, p<0.001; burst-firing, 71.8±23.9 Hz to 157.0±34.4 Hz, n=5, p<0.05; CPA: Fig. 1C, data obtained from 3 simple-firing and 2 burst-firing cells, 18.8±1.3 Hz (control), 109.1±24.6 Hz (CPA), and 26.4±1.5 Hz (washout), n=5, p<0.05 between control and CPA, or between CPA and washout].
Figure 1. Blockade of CICR induces spontaneous spike bursts.
(A) Loose cell-attached recordings of a spontaneously firing cell in the presence of synaptic blockers. (Ai, left) Control, all action potentials are simple spikes. (Ai, right) The boxed region in (Ai, left) with expanded time base. (Aii, left) Bursting (*) observed in the presence of 20 µM ryanodine. (Aii, right) The boxed region (Aii, left), showing one burst of 5 spikelets.
(B) Instantaneous firing frequency over time. The data were obtained from the same cell in (A). Ryanodine was bath-applied during time marked by gray box.
(C) Summarized data of instantaneous frequencies in ryanodine or CPA (10 µM). Simple: data from spontaneous simple spike-firing cells; Burst: data from spontaneous burst-firing cells; Simple+Burst: pooled data from both firing types of cells. Here and elsewhere, error bars indicate SEM, and statistical significance was tested using paired t-test unless otherwise stated (significance, p<0.05). *p<0.05 and ***p<0.001.
(D) Normalized histograms of instantaneous spike frequencies from cells showing only simple spikes in the absence of ryanodine. Black arrowhead, the main peak of the event in control at 20 Hz (23.2 %); gray and white arrowheads, peaks at 40 Hz (10.9%) and 398 Hz (10.7%) in ryanodine, respectively. Here and elsewhere, numbers in parentheses indicate the number of cells.
To quantify the firing pattern, instantaneous frequencies were plotted as normalized histograms. Spikelets within a burst were counted as discrete action potentials, and the distributions of spike/spikelet frequencies recorded from 9 simple-firing cartwheel cells were plotted (Fig. 1D). Group data from these cells exhibited a major peak at 20 Hz (23.2% of total events, black arrowhead in Fig. 1D). After application of ryanodine, the histogram showed 2 major peaks, one at ~ 40 Hz and the other at ~ 398 Hz (Fig. 1D, gray and white arrowheads, 10.9±4.6% and 10.7±2.9% of total events, respectively). The former peak corresponds to a somewhat higher rate of simple spike firing and the latter peak reflects the short-interval spikelets in spike bursts induced by ryanodine. Similar analyses were done using data obtained from burst-firing cells (Fig. S1A–D). In these, ryanodine significantly enhanced the peak corresponding to high-frequency spikelets within the bursts (Fig. S1A, asterisks; Fig. S1B, white arrowhead, 398 Hz, 3.4±1.6% in control and 13.4±4.6% in ryanodine, n=5, p<0.05). Across all cells, control activity was dominated by simple spiking, and after ryanodine bursts account for half the spike activity in the cell (Fig. S1E). To examine the effects of ryanodine on the fine structure of the burst, averaged number of spikelets in a burst (e.g., left panel in Fig. S1Ai) and inter-spikelet intervals were measured. Ryanodine did not affect the average number of spikelets in a burst (Fig. S1C, n=5, p=0.685), but markedly shortened intervals between 1st and 2nd spikelets in those bursts consisting of 4 or 5 spikelets (4 spikelet bursts, Fig. S1Di, 9.52±1.57 ms in control, 7.22±0.33 ms in ryanodine, n=5, p<0.05; 5 spikelet bursts, Fig. S1Dii, 11.74±1.53 ms in control, 4.64±0.47 ms in ryanodine, n=5, p<0.01). Thus, ryanodine has specific effects on the burst, increasing its onset probability and modulating the interval between spikelets.
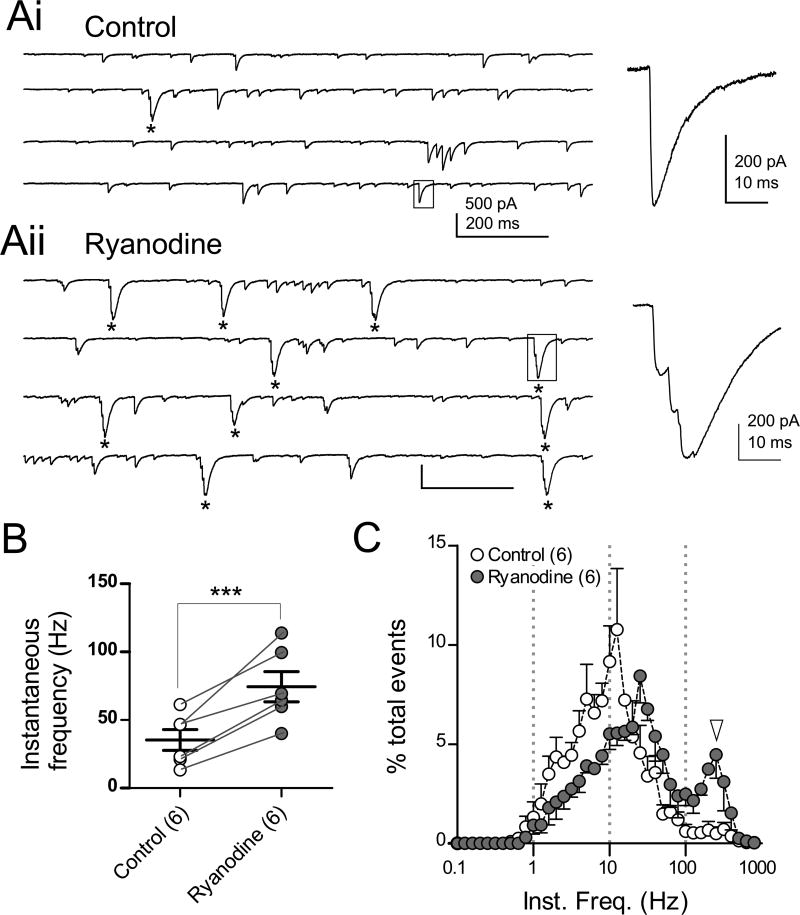
Spike bursts in cartwheel cells propagate into synaptic terminals and transmit a cluster of postsynaptic inhibitory currents (Roberts et al., 2008), and it was clear that ryanodine caused a sharp increase in these clusters. Glycinergic spontaneous inhibitory postsynaptic currents (sIPSCs) were recorded by using a CsCl-based internal solution in the presence of NBQX, MK-801, and SR95531. Because cartwheel cells are predominantly glycinergic and make synaptic contact with all neighboring neurons, presynaptic cartwheel cell firing can be assayed by recording cartwheel cell sIPSCs (Mancilla and Manis, 2009; Roberts et al., 2008). In control, few clusters of sIPSCs were observed (Fig. 2Ai, one cluster indicated by *), corresponding to rare presynaptic spike bursts. Ryanodine dramatically increased clusters of sIPSCs (Fig. 2Aii, 10 clusters indicated by *). Accordingly, the instantaneous frequency of action potential-induced sIPSCs (see STAR methods) was significantly increased by ryanodine (Fig 2B; 35.4±7.6 Hz in control, 74.4±11.1 Hz in ryanodine, n=6, p<0.001). Instantaneous IPSC frequency for control and ryanodine was plotted as histograms (Fig. 2C); the plot for ryanodine had two peaks, and the right-most peak at 251 Hz (4.4% of total events, Fig. 2C, white arrowhead) almost matched that observed for spike bursts in ryanodine (398 Hz, white arrowheads Fig. 1D and S1B). Thus, CICR tempers the occurrence of bursts that would otherwise propagate to axon terminals and generate enhanced postsynaptic inhibition.
Figure 2. Ryanodine induces an increase of IPSC bursts.
(A) Spontaneous glycinergic IPSCs recorded in the presence of NBQX, MK-801, and 10 µM SR95531. The cell was voltage-clamped at −65 mV and filled with a CsCl-based internal solution so that IPSCs were inward. Spike bursts led to IPSCs with multiple inflections on their rising phase. In control traces, one high-frequency IPSC burst can be seen (* in left traces in Ai), whereas 10 IPSC bursts are seen in ryanodine (Aii, *). Right panels in (Ai) and (Aii), expanded sIPSCs from corresponding boxed regions of left traces.
(B) Summary of instantaneous frequencies of spontaneous spike-driven IPSCs. Miniature IPSCs were excluded (see STAR methods).
(C) Normalized histograms of instantaneous frequencies of action potential-evoked spontaneous IPSCs. White arrowhead indicates a peak at 251 Hz in ryanodine (4.4%) of total events, similar to the spikelet frequency within spike bursts (Fig. 1 and S1).
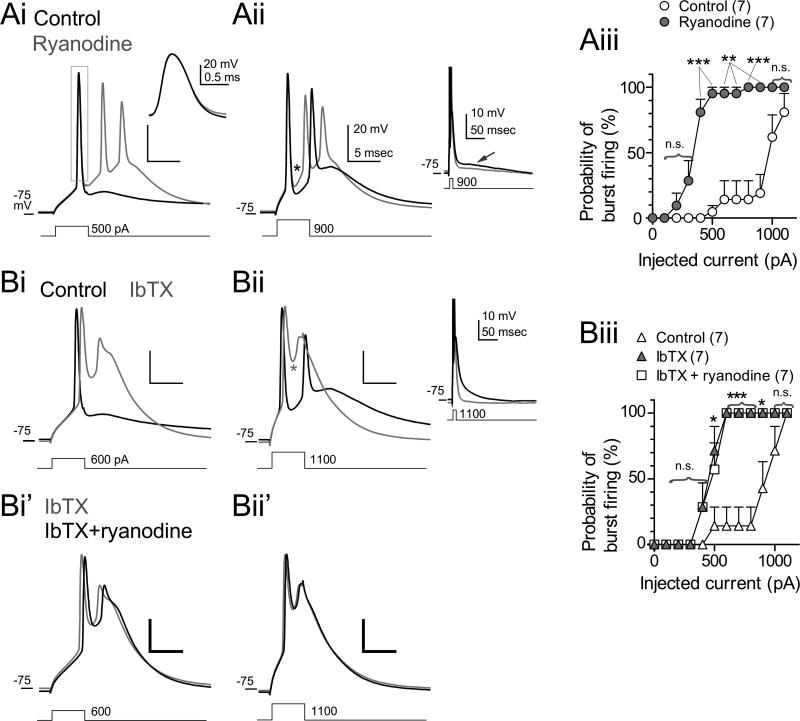
To understand how CICR controls spike bursts, perforated patch recordings were made, and resting potentials were adjusted to between −75 and −85 mV by injecting negative bias currents to suppress spontaneous firing. As reported previously, cartwheel cells emit single action potentials in response to small depolarizing current injections (Fig. 3Ai, Bi) and bursts in response to large current injections (Fig. 3Aii, Bii) (Kim and Trussell, 2007). Two clear effects of ryanodine were observed. First, ryanodine affected spike time course, reducing the fast afterhyperpolarization (fAHP) apparent after the 1st spikelet (asterisk in Fig. 3Aii) by 4.2 mV (n=10, p<0.01), increasing the half-width of the 1st spikelet by 0.011 ms (n=10, p<0.05), and reducing the maximum rate of spike decay by 10.9 V/s (n=10, p<0.001). Second, ryanodine markedly increased the likelihood of spike bursts elicited in response to small currents (Fig. 3Ai, iii; n=7, p<0.05, Wilcoxon signed rank test). The effects of ryanodine on spike waveforms and intrinsic membrane properties are summarized in Table S1. By contrast, a late afterpotential seen on a time scale much slower than the burst (inset in Fig. 3Aii, indicated by an arrow) was hyperpolarized slightly in the presence of ryanodine, possibly due to increased Ca2+ influx via Cav during the spikelets and subsequent activation of SK channels. Together, these data suggest that CICR enhances fast spike repolarization and thereby opposes inward currents that promote the generation of the burst.
Figure 3. The effects of ryanodine and IbTX on action potential properties.
(A and B) Evoked action potentials recorded in perforated patch mode. Resting potential was slightly hyperpolarized by injecting negative current to suppress spontaneous firing (A). Traces recorded in control (black) and ryanodine (gray) are superimposed. Injected currents in (Ai) and (Aii) were 500 and 900 pA, respectively. Duration: 5 ms. Asterisk indicates fAHP between 1st and 2nd spikelets. The region surrounded with box in (Ai) was expanded in the inset. The inset in (Aii) includes a longer segment of the recording to illustrate the slow afterpotential.
(Aiii) Summary of the change of burst firing probability by ryanodine. Three to four successive trials were used to obtain averaged probability in each experiment.
(B) The effect of ryanodine was occluded by IbTX. Bath application of IbTX (100 nM) alone broadened 1st action potentials (Bi and Bii, gray traces) and made the fAHP less negative (Bii,, asterisk). Subsequent application of ryanodine in the presence of IbTX did not affect the waveform (Bi’ and Bii’, black traces). Inset in (Bii) is the same sweep but displayed with longer time base, with spikes truncated. (Biii) Summary of the change of burst firing probabilities by IbTX. Statistical significance was tested between control and IbTX.
P/Q-type Ca2+ channels trigger CICR leading to activation of BK channels
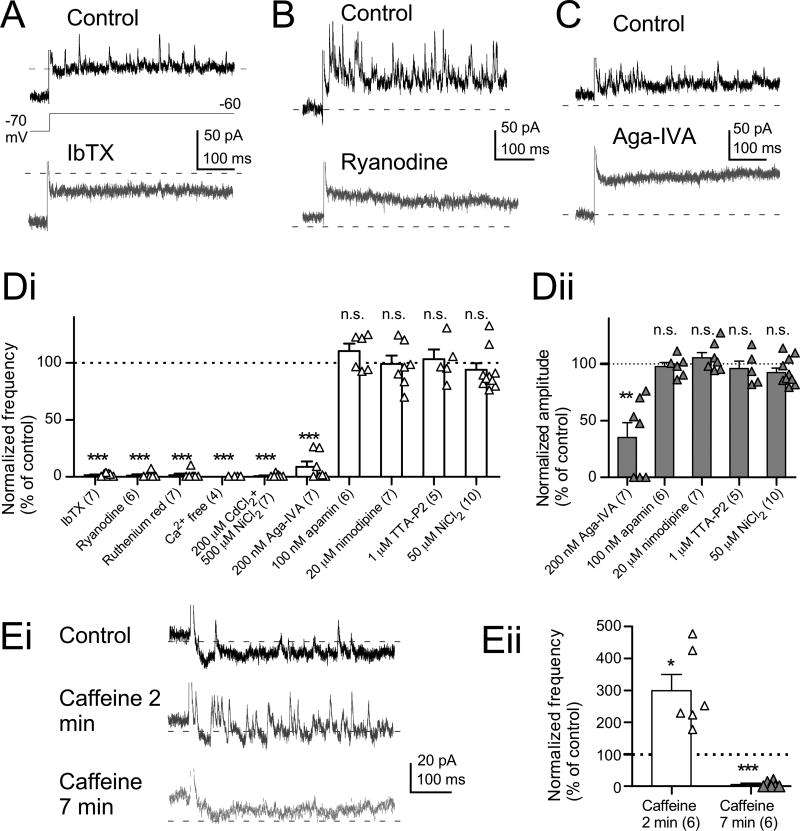
As ryanodine reduced the fAHP but did not affect resting membrane potential (Table S1), we asked whether BK channels, which are both voltage- and Ca2+- dependent, were activated by CICR, leading to deepening of the fAHP. Action potentials were induced by 5-ms depolarizing current injections, and the effect of ryanodine was then tested in the presence of iberiotoxin, a BK channel blocker (IbTX, 100 nM). By itself, IbTX broadened action potential waveforms, as reported previously (Fig. 3Bi and Bii, Table S2) (Bender and Trussell, 2009; Kim and Trussell, 2007), and made the fAHP less negative (asterisk in Fig. 3Bii, Table S2). Like ryanodine, IbTX also reduced the current threshold for bursts (Fig. 3 Bi and Biii). No additional effects on spike parameters were seen when ryanodine was applied in the presence of IbTX (n=7, Fig. 3Bi’, Bii’, Biii, Table S2), suggesting that spike triggered CICR leads to BK channel activation.
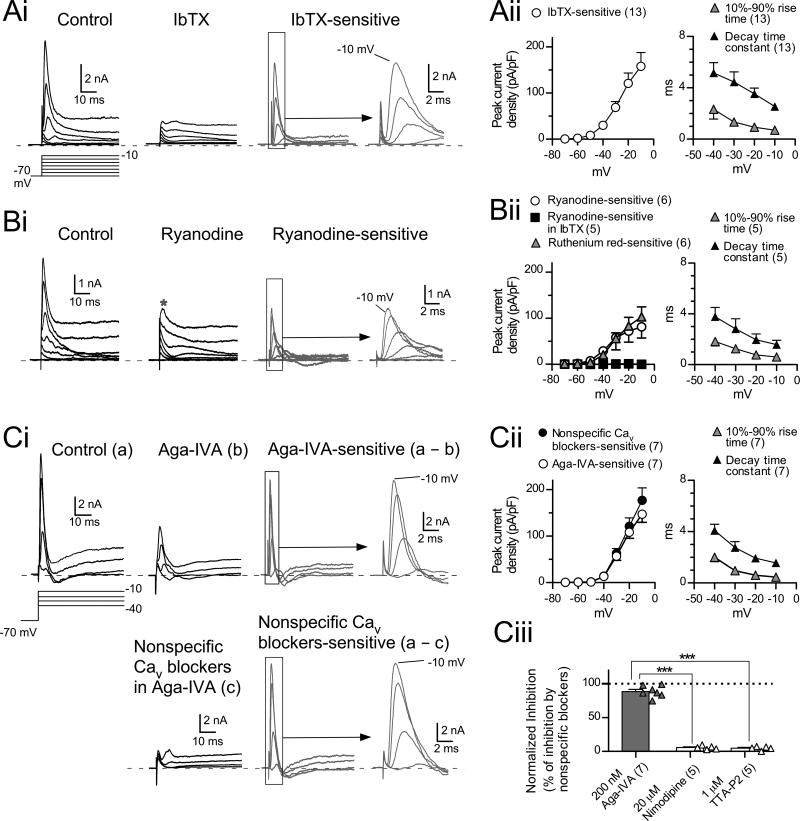
More direct evidence for a link between BK channels and CICR was obtained by recording under voltage clamp in the presence of TTX and synaptic blockers. Large depolarizing voltage steps (from −70 mV to between −30 mV and −10 mV) evoked a rapid transient outward current followed by a sustained current (Fig. 4Ai, control). The transient currents were inhibited by IbTX (Fig. 4Ai), and IbTX-sensitive currents, obtained by subtracting IbTX from control traces, showed a clear voltage dependence (Fig. 4Ai, subtraction; gray circles in in Fig. 4Aii, n=13), and had fast activation and inactivation (10%-90% rise time, 0.70±0.05 ms at −10 mV, n=13, gray triangles in Fig. 4Aii; single exponential decay constant was 2.54±0.24 ms at −10 mV, n=13, black triangles in Fig. 4Aii). Transient currents were completely blocked by bath-perfusion of Ca + free ACSF (2.2±2.2% of control at −10 mV, n=4, not shown) or application of a mixture of nonspecific Cav channel blockers (200 mM CdCl2 and 500 mM NiCl2, 2.2±1.5% of control at −10 mV, n=7; Fig. 4Ci, lower panel), consistent with activation of BK channels through Ca + influx via Cav channels. Importantly, transient currents were almost completely suppressed by ryanodine (Fig. 4Bi and Bii). The mean ryanodine-sensitive current density was similar in amplitude to that of IbTX-sensitive current at −10 mV (ryanodine-sensitive, 81.0±24.0 pA/pF, n=6; IbTX-sensitive, 157.8±24.0 pA/pF, n=13; p=0.063, unpaired t-test with Welch’s correction). Accordingly, ryanodine did not suppress currents evoked by depolarizing steps in the presence of IbTX (black squares in Fig. 4Bii). These results demonstrate that BK channels are the primary currents activated by CICR. To confirm the effect of blockade of RyRs on the transient current, another RyR inhibitor, ruthenium red (200 µM) was used with the combination of dual-electrode recordings. After the recording of a control response using a pipette containing normal K-gluconate intracellular solution, the giga-ohm seal formed by second pipette containing pipette solution with ruthenium red was ruptured to dialyze the cell. The transient outward currents were almost completely suppressed by 20-min dialysis with this solution (n = 6, gray triangles in Fig. 4Bii, Fig. S2A).
Figure 4. CICR triggers BK channel-mediated transient outward currents.
(A) IbTX-sensitive transient outward currents. (Ai) In control, transient currents followed by sustained currents were evoked by depolarizing voltage steps (−30 to −10 mV from −70 mV holding potential, 10-mV increment). All transient current was inhibited by 100 nM IbTX (traces in IbTX and subtraction). IbTX-sensitive currents were obtained by subtracting traces in IbTX from control traces. (Aii) Summary of the peak current densities (left panel), and rise time and decay time constant of IbTX-sensitive currents (right panel). In (A) and (B), capacitive artifacts were blanked for clarity. Here and in following figures, dashed lines in current traces indicate zero current levels.
(B) RyRs are involved in the transient outward currents. Same voltage protocol as in (A). (Bi) Note that some transient outward currents remain in ryanodine (Bi, ryanodine). (Bii) Summary of peak current densities (left panel) and rise time and decay time constant of ryanodine-sensitive currents (right panel).
(Ci) Most of the transient current is suppressed by ω-Agatoxin-IVA (Aga-IVA, a P/Q-type blocker, 200 nM; trace in Aga-IVA and Aga-IVA-sensitive). Subsequent application of nonspecific Cav channel blockers (200 µM CdCl2 and 500 µM NiCl2) blocked transient currents almost completely (traces in lower panel in Ci). Data in (C) were recorded in the presence of TTX, synaptic blockers, and 1 mM 4-AP. (Cii) Summary of peak current densities (left panel), and the rise time and decay time constant of Aga-VIA-sensitive currents (right panel). (Ciii) Summary of effects of subtype-specific Cav blockers on transient currents. Aga-VIA inhibited transient currents more potently than nimodipine or TTA-P2. Effects are expressed as 100 × (selective blocker-sensitive current)/(nonspecific Cav blockers-sensitive current). ***p<0.001, unpaired t-test.
Cartwheel cells express P/Q-, L-, and T- type Ca2+ channels (Kim and Trussell, 2007). To determine which subtypes are involved in triggering CICR, we tested the effects of selective Cav antagonists on transient outward currents. Application of ω-agatoxin-IVA (Aga-IVA, a P/Q blocker, 200 nM) markedly suppressed transient currents evoked by depolarization (Fig. 4Ci, Aga-IVA and Aga-IVA-sensitive). Subsequent application of the nonspecific blockers CdCl2 and NiCl2 then eliminated the remaining transient current (lower panel in Fig. 4Ci). The peak current densities, 10%-90% rise time, and the decay time constant of Aga-IVA-sensitive currents (Fig. 4Cii) had features similar to those of IbTX-sensitive currents (Fig. 4Aii). The reduction of the transient currents by specific blockers was expressed as a percentage of the block by the nonspecific Cav blockers (Fig. 4Ciii). Aga-VIA blocked almost all of the CaV-dependent outward current (88.4±3.2%, n=7, p<0.001, one-sample t-test). By contrast, nimodipine and TTA-P2 reduced the outward current only slightly (20 µM nimodipine, 5.65±2.7%, n=5; 1 µM TTA-P2, 4.65±2.8%, n=5), and thus were significantly less effective than Aga-IVA (Aga-VIA vs nimodipine effect, p<0.001; Aga-VIA vs TTA-P2, p<0.001, unpaired t-test, Fig. 4Ciii). Apamin-sensitive SK currents did not contribute to the transient outward current (Fig. S2B). Furthermore, SK currents could be observed even in the presence of ryanodine (Fig. S2Bii), suggesting that the source of intracellular Ca2+ for the activation of SK channels is not derived from CICR. In conclusion, these results indicate that P/Q Ca2+ channels are the principal subtype of Cav involved in CICR and BK channel activation.
Rapid CICR at somatic plasma membrane
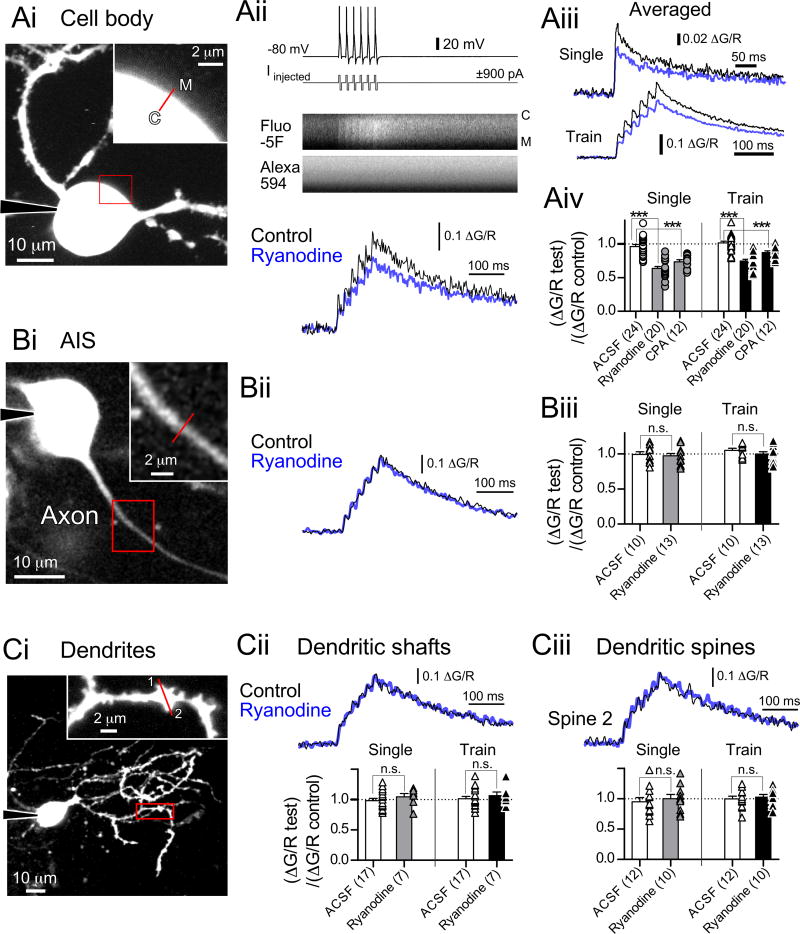
Since CICR-triggered BK current occurs during single spikes, ryanodine-sensitive Ca2+ transients must be very rapid and therefore arise immediately adjacent to plasma membrane. We therefore tested the effects of ryanodine and CPA on spike-triggered Ca2+ transients using two-photon microscopy. Current-clamped cells were dialyzed with an intracellular solution containing Fluo-5F and Alexa 594. Ca2+ transients were evoked by single spikes or spike trains (6 spikes at 50 Hz) induced by biphasic current injections adjusted to prevent induction of burst firing (Bender and Trussell, 2009).
Short, rapid line scans were made across the somatic membrane (Fig. 5Ai) synchronized with evoked action potentials (Fig. 5Aii). A train of action potentials induced summating Ca2+ transients (Fig. 5Aii, bottom panel) (Bender and Trussell, 2009). Notably, the peak of each transient was suppressed by ryanodine (Fig. 5Aii, bottom panel; Fig. 5Aiii, averaged responses from 10 regions of interest of 5 cells, bottom panel). The change in Ca2+ transient amplitude induced by ryanodine or CPA were compared with control cells [(ΔG/R test)/(ΔG/R control)] to exclude that CICR was attenuated by rundown of Ca2+ channels. Ryanodine reduced the peak amplitude of single spike-evoked Ca2+ transients by 33.7% (Fig. 5Aiv, p<0.001, unpaired t-test), and Ca2+ transients evoked by trains of action potentials (reduction by 26.6%, Fig. 5Aiv, train, p<0.001, unpaired t-test). Moreover, CPA also inhibited Ca2+ transients (Fig. 5Aiv, single action potentials, reduced by 23.6% of the vehicle, p<0.001, unpaired t-test; a train of action potentials, reduced by 14.1%, p<0.001). The changes of decay time constants of Ca2+ transients by ryanodine or CPA were further examined by fitting double-exponential functions to the decay of the transients (Table S3). Ryanodine did not affect the time constants but reduced the contribution of the fast decay component following single action potentials, whereas CPA slowed the decay of Ca2+ transients evoked by the train of action potentials and decreased the contribution of the fast decay component. Since CPA blocks ER Ca2+-ATPase, an increased time constant may reflect slowed clearance of Ca2+ from the cytosol.
Figure 5. Action potential-induced CICR at somatic plasma membrane but not AIS or dendrites.
(A) Two-photon Ca2+ imaging at somatic plasma membrane. (Ai) Maximum intensity projection of Alexa-594-filled cartwheel cell. The red boxed region is enlarged in Ai, inset. Regions of interest for segmented line scans are indicated by red lines. C: cytosolic side. M: membrane side. (Aii, top and middle panels) Spike trains evoked by current injection (top) elicited an increase of Fluo-5F fluorescence with no change in Alexa-594. (Aii, bottom) Ca2+ transients induced by spike trains (6 simple spikes at 50 Hz). The transients are expressed as ΔG/R (change in Fluo-5F intensity divided by Alexa-594 intensity). Black, control; blue, in ryanodine. (Aiii) Averaged Ca2+ transients from 10 regions of interest of 5 cells. Single spike or trains of simple spikes (6 spikes at 50 Hz) evoked by current injection. (Aiv) Summary of the changes in Ca2+ transients. ***p<0.001, unpaired t-test.
(B) Ca2+ imaging at AIS. (Bi) Single image plane of cell body and AIS. (Bii) Ca2+ transients at AIS in (Bi) induced by spike trains (6 spikes at 50Hz). (Biii) Summary of the changes of Ca2+ transients.
(C) Ca2+ imaging at dendrites. (Ci) Maximum intensity projection of a cartwheel cell. Spines in the region of interest are numbered. (Cii, top) Ca2+ transients at the dendritic shaft in (Ci). (Cii, bottom) Summary of the changes of Ca2+ transients at shafts. (Ciii, top) Ca2+ transients at the spine 2 (Ci). (Ciii, bottom) Summary of Ca2+ transients at spines.
In sharp contrast to the effects of blockade of CICR at somatic membrane, action potential-induced Ca2+ transients at AISs, dendritic shafts, and dendritic spines were not affected by ryanodine (AISs, Fig. 5Biii, single, p=0.690, unpaired t-test; train, p=0.264; dendritic shafts, bottom panel in Fig. 5Cii, single, p=0.370; train, p=0.494; dendritic spines, bottom panel in Fig. 5Ciii, single, p=0.603; train, p=0.634). Thus, spike evoked CICR is restricted to somatic membrane, despite the presence of burst induced Ca2+ flux in the AIS (Bender and Trussell, 2009) and backpropagation of bursts into dendrites (Roberts et al., 2008).
Spontaneous currents mediated by CICR
In Fig. 4B, large depolarizing voltage steps evoked CICR-mediated transient BK currents. When smaller voltage steps were applied (to −60 or −50 mV from a −70 mV holding potential) in the presence of TTX and synaptic blockers, spontaneous miniature outward currents (SMOCs) were apparent (Fig. 6 and Fig. S3) (Satin and Adams, 1987). SMOCs were blocked by IbTX, ryanodine, or intracellularly applied ruthenium red, demonstrating that the SMOCs reflect BK channels activation by CICR (Fig. 6 A, B, and Di; IbTX, 1.51 ± 0.56 % of control, n=7, p<0.001, one-sample t-test; ryanodine, 1.18 ± 1.18% of control, n=6, p<0.001; ruthenium red, 1.42± 1.42% of control, n=7, p<0.001, Fig. S3D). SMOCs observed at −60 mV appeared as random, discrete events, suggesting each SMOC was triggered by a Ca2+ spark from a RyR, as previously described in cardiac myocytes (Perez et al., 1999; ZhuGe et al., 1999). SMOCs were transiently enhanced by caffeine (10 mM), which induces release from Ca2+ stores by acting on RyRs, eventually depleting the store (Kuba, 1994). The maximum increase in SMOC frequency was observed around 2 min after wash-in of caffeine (Fig. 6Ei, middle trace; Fig. 6Eii, caffeine 2 min, 299.3±50.3% of control, n=6, p<0.05, one-sample t-test) followed by a sharp decrease (Fig. 6Ei, lower trace; Fig. 6Eii, caffeine 7 min, 5.65±3.67% of control, n=6).
Figure 6. SMOCs are induced by CICR triggered by P/Q-type Ca2+ channels.
(A–C) Representative current traces containing SMOCs evoked by 10-mV depolarization from −70 mV in the presence of TTX and synaptic blockers. SMOCs were blocked completely by IbTX (A), ryanodine (B), and Aga-IVA (P/Q-type Ca2+ blocker, 200 nM).
(D) Summary of change of SMOC frequency (Di) and amplitude (Dii). Frequencies and amplitudes were normalized by using control data obtained before drug application. **p<0.01 and ***p<0.001, one-sample t-test.
(E) Caffeine-induced (10 mM) changes of SMOC frequencies. In (E), SMOCs were recorded in the presence of TTX, synaptic blockers and 1 mM 4-AP. (Eii) Summary of caffeine-induced changes of frequencies. *p<0.05 and ***p<0.001, one-sample t-test.
The frequency and amplitude of SMOCs were voltage dependent (Fig. S3A and B; −60 mV, 24.6±3.4 Hz, n=7; 28.9±1.7 pA amplitude, n=7; −50 mV, 48.5±2.1 Hz, n=7; 43.7±4.8 pA amplitude, n=7), and SMOCs were rarely observed at −70 mV (Fig. S3A and B; −70 mV, 1.4±0.6 Hz, n=7; 16.9±1.7 pA amplitude, n=4), consistent with the observation that ryanodine had no effect on resting potential (Table S1). Bath perfusion of Ca + free ACSF or a mixture of nonspecific Cav channel blockers (200 µM CdCl2 and 500 µM NiCl2) also inhibited SMOCs (Fig. 6Di; Ca + free, 0.0±0.0% of control, n=5, p<0.001, one-sample t-test; 200 µM CdCl2 and 500 µM NiCl2, 0.7% of control, n=7, p<0.001). The decay time of SMOCs was 1.86±0.15 ms (n=5; Fig. S3A, right lower panel), and was not significantly different from the decay of the ryanodine-sensitive transient current evoked by large voltage steps (Fig. 4Aii, p=0.10 at −30 mV, p=0.088 at −20 mV, p=0.45 at −10 mV, p=0.63 at 0 mV, unpaired t-test).
Both the increase in SMOC frequency with voltage and the Ca2+-dependence of SMOCs suggest that Cav channel openings provide Ca2+ that triggers RyR opening and generation of SMOCs. Therefore, we explored what Cav subtypes were involved in SMOCs using selective antagonists. Aga-IVA (200 nM) inhibited the frequency and amplitude of the SMOCs (Fig. 6C; frequency, Fig. 6Di, Aga-IVA, 8.9±4.6% of control, n=7, p<0.001, one-sample t-test; amplitude, Fig. 6Dii, 35.2±13.0% of control, n=7, p<0.01), whereas nimodipine (L-type blocker), TTA-P2 (T-type blocker), or a low concentration (50 µM) of NiCl2 (R- and T-type blocker) did not affect SMOCs frequency or amplitudes (frequency, Fig. 6Di, 200 µM nimodipine, 98.9±7.5% of control, n=7, p=0.89, one-sample t-test; 1 µM TTA-P2, 103.1±8.5% of control, n=5, p=0.73; 50 µM NiCl2, 93.8±5.7% of control, n=10, p=0.309; amplitude, Fig. 6Dii, nimodipine, 105.2±4.7% of control, n=7, p=0.31; TTA-P2, 95.8±6.5% of control, n=5, p=0.55; 50 µM NiCl2, 93.4±3.8% of control, n=10, p=0.114). Apamin (100 nM) did not affect the frequency or amplitude of SMOCs (frequency, Fig. 6Di, 110.3±6.6% of control, n=6, p=0.18, one-sample t-test; amplitude, Fig. 6Dii, 97.5±3.6% of control, n=6, p=0.53). These results confirm no involvement of SK channels in the SMOCs, although SMOCs recorded from several other neuronal types are mediated by SK channels (Cui et al., 2004; Saito and Yanagawa, 2013). Thus, RyRs, P/Q-type Ca2+, and BK channels work together in generation of the SMOC.
Nanodomain coupling of Cav, RyR and BK channels
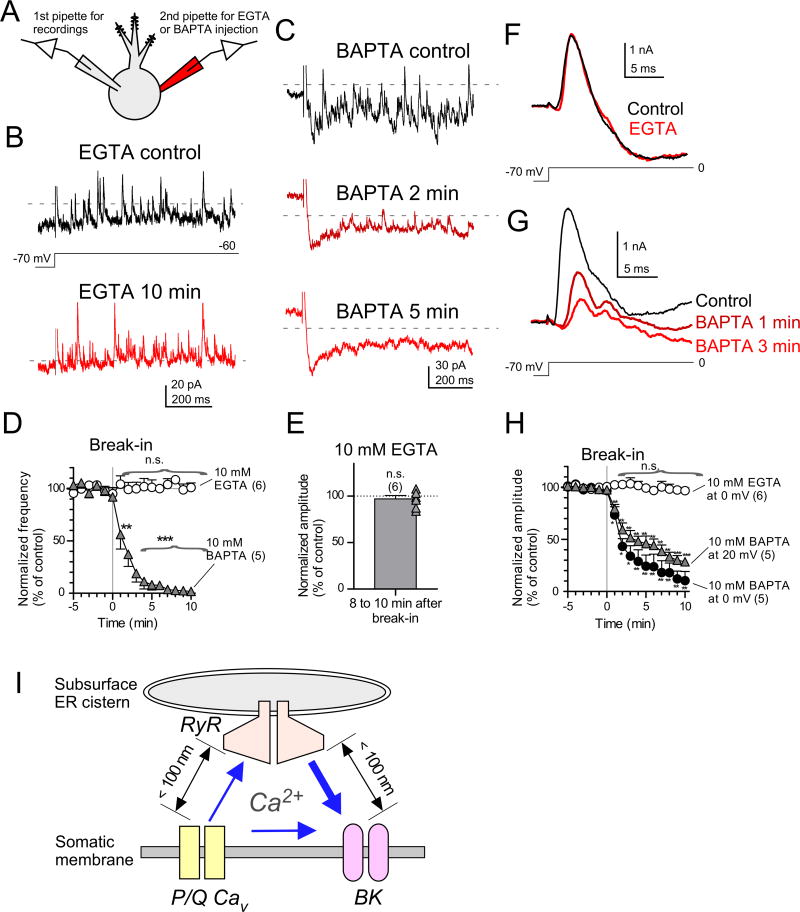
The SMOC may represent the unitary current that shapes the spike burst. Thus, a key question is the functional distance between the three channels involved in generation of the SMOC. Ca2+ stores in rough and smooth ER, subsurface cisterns, and the outer leaflet of the nucleus provide Ca2+ to the membrane over very different distances and latencies. Experimentally, Ca2+ signaling distances can be probed using Ca2+ chelators. When the distance between Ca2+ channels and the Ca2+ sensors of effectors is in the nanodomain (< 100 nm), only the fast Ca2+ chelator BAPTA at mM levels can capture Ca2+ and block signaling; longer distance signals can be also be intercepted by the slower chelator EGTA (Adler et al., 1991; Eggermann et al., 2012; Naraghi and Neher, 1997). In designing experiments using high concentrations of these chelators, we were concerned that prolonged recordings could lead to depletion of store Ca2+, thereby confounding interpretations about the size of Ca2+ domains. Therefore, we tested the effects of the chelators using dual-electrode recordings (Fig. 7A). SMOCs were recorded under voltage-clamp with a pipette containing 0.1 mM EGTA. A second pipette containing 10 mM EGTA or 10 mM BAPTA was sealed onto the cell and a 5-min control period of SMOCs were recorded; after this period the membrane under the second electrode was ruptured and whole-cell current clamp mode (I=0) was established for the second electrode (Fig. 7A; break-in in Fig. 7D and H). In this way, the first pipette could maintain voltage clamp while the second could rapidly dialyze in the chelator beginning at a defined time point. The results were very clear: SMOCs evoked by 10-mV depolarization were virtually unaffected by intracellular dialysis of high EGTA (Fig. 7B; frequency, Fig, 7D, 101.2±4.3% of control at 10 min, n=6, p=0.80, one-sample t-test; amplitude, Fig. 7E, 96.9±3.8% of control, n=6, p=0.44). By contrast, SMOCs were quickly eliminated by dialysis of BAPTA (Fig. 7C; Fig, 7D, 10 mM BAPTA, 56.2±13.9% at 1 min, n=5, p<0.05, one-sample t-test; 1.85±1.95% at 10 min, n=5, p<0.001). Assuming the two pipettes have equal efficiency in dialyzing the cell, the final chelator concentration in the cell should be about half that in the second pipette, or 5 mM. The dramatic difference between the effects of the chelators on frequency and amplitude of SMOCs suggests that all three channels, Cav, RyRs and BK channels, are positioned within nanodomains, despite the Ca2+ sources and targets being in different membrane compartments.
Figure 7. Activation of BK channels mediated by nanodomain coupling.
(A) Experimental configuration. Recordings made in the presence of TTX, synaptic blockers, 100 nM apamin, and 1 mM 4-AP.
(B) No effects of intracellularly applied 10 mM EGTA on SMOCs. Representative traces before rupturing the membrane patch with the 2nd pipette (B, top) and 10 min after rupturing (B, bottom). In (B) and (C), capacitive artifacts were blanked for clarity.
(C) The effects of 10 mM BAPTA on SMOCs. Traces before break-in (C, top) and 2 min (C, middle) and 5 min (C, bottom) after break-in.
(D) Time course of SMOC frequencies recorded from cells dialyzed with EGTA or BAPTA. The dialysis was done at 0 min (break-in). The frequencies were normalized to the average frequency between −5 and 0 min. **p<0.01 and ***p<0.001, one-sample t-test.
(E) No effect of EGTA on SMOC amplitude. Average amplitude of SMOCs between 8 and 10 min after break-in was normalized to the average between −5 and 0 min.
(F) No effects of EGTA on BK transient currents. The currents were evoked by depolarization from −70 mV to −60 mV, and leak and capacitive currents were subtracted by using the p/4 protocol with opposite polarity. Control, trace before break-in (black). EGTA, trace recorded 10 min after break-in (gray).
(G) Time-dependent effects of BAPTA on the transient currents. Traces before break-in (control) and 1 min (BAPTA 1 min) and 3 min (BAPTA 3 min) after break-in.
(H) Time course of peak amplitudes of the transient currents. Amplitudes were normalized to the average amplitude between −5 and 0 min. *p<0.05, **p<0.01, and ***p<0.001, one-sample t-test.
(I) Subsurface ER cisterns expressing RyRs are located just beneath somatic plasma membrane. The distances between RyRs and P/Q-type Ca2+ channels or BK channels are less than 100 nm. Ca2+_influx likely reaches both RyR and BK channels, but Ca2+ are boosted significantly by RyR channel activation.
While SMOCs appear to operate using double nanodomain Ca2+ signals, we predicted that the much larger Ca2+ currents and CICR associated with action potentials or large voltage steps might act over microdomain distances. We therefore examined the effect of EGTA and BAPTA transient BK outward currents evoked by voltage steps to 0 mV (Figs. 7F–H). Unexpectedly, large transient outward currents were resistant to a 10-min dialysis of EGTA (Fig. 7F and H, 97.0±1.5% of control at 10 min, n=6, p=0.104, one-sample t-test). By contrast, transient outward currents were strongly attenuated by dialysis of BAPTA (Fig. 7G; Fig. 7H, at 20 mV, 79.6±4.0% at 1 min, n=5, p<0.01, one-sample t-test; 28.5±4.0% at 10 min, n=5, p<0.001). These results demonstrate that both SMOCs and CICR-mediated transient outward currents are driven by nanodomain coupling of the three channel types and suggest that the intracellular Ca2+ stores for the CICR are the subsurface cisterns positioned only tens of nm from plasma membrane (Fig. 7I; Ryugo et al., 1995; Wouterlood and Mugnaini, 1984). Moreover, because SMOCs and the transient outward current share similar pharmacology, Ca2+ domain size, and time course, it is likely that the SMOC represents the quantal unit of the large BK currents that control the likelihood of the spike burst and the properties of its spikelets.
Colocalization of BK channels and RyRs at somatic membranes
We used immunohistochemistry to localize components of the RyR-BK coupling, taking advantage of reliable antibodies for BK and RyR (Mandikian et al., 2014; Misonou et al., 2006) and a transgenic mouse line expressing ChR2(H134R)-EYFP under the control of GlyT2 promoter (Lu and Trussell, 2016) to pinpoint the location plasma membrane in relation to ion channels.
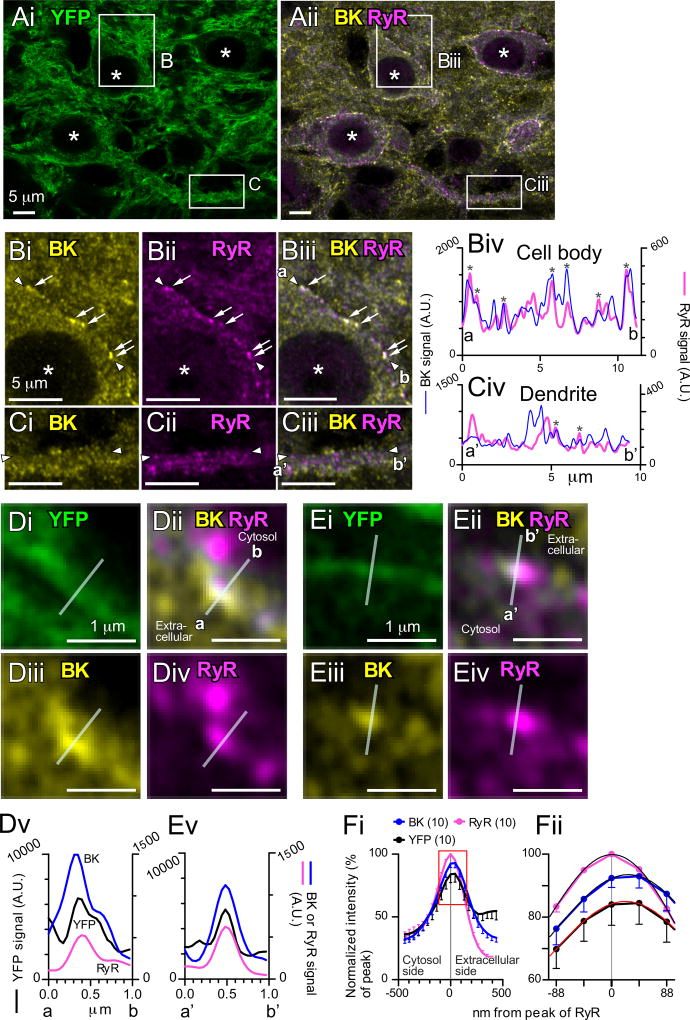
Figure 8A shows a representative fluorescent image containing three cartwheel cells. The membrane and processes of the glycinergic neurons are strongly labeled with YFP, and intracellular components, presumably Golgi and ER surrounding the nuclei (white asterisks) are more faintly labeled. Regions marked B (cell body) and C (secondary dendrite) are expanded in Fig. 8B and C. Puncta of BK and RyR labeling are clearly apparent (Fig. 8Bi, ii), and many of these show clear overlap (arrows in Fig. 8Bi–Biii). The profiles of signal intensity along membranes of the cell body or dendrite were measured by drawing linear regions of interest along the cells’ membrane guided by the YFP signal. The BK and RyR intensity along these linear regions, were plotted in Fig. 8Biv (cell body) or Civ (dendrite). The profile at the cell body had at least 7 peaks that overlapped between the BK and RyR signals (asterisks in Fig. 8Biv), while the profile of the dendrite had less overlap and smaller peaks (asterisks in Fig. 8Civ). To quantify colocalization, normalized signal intensities were measured from membrane regions and plotted in Fig. S4A. Figure S4Aiii shows that 41.6% of colocalized points in somatic membrane had normalized intensities higher than 0.2 in both axes (shaded area, 1057 out of 2538 blue points), whereas only 1.9% of points in dendritic membrane had intensities higher than 0.2 (shaded area, 48 out of 2538 magenta points). Thus, somatic membranes contain more colocalizing points of high intensity (shaded area in Fig. S4Aiii) than dendritic membranes, and strongly suggesting that nanodomain coupling of RyRs and BK via CICR is restricted to areas of somatic membrane, consistent with the absence of action potential-induced CICR at dendritic processes (Fig. 5C).
Figure 8. Colocalization of BK channels and RyRs at somatic membranes.
Glycine transporter 2 (GlyT2)-Cre;Ai32 mouse brainstem sections containing DCN were triple-immunofluorescence labeled for EYFP (green), BK channels (yellow), and RyRs (magenta) and imaged with a Zeiss Airyscan super-resolution confocal microscope. (A) Single optical section images containing three cartwheel cells. Asterisks indicate nuclei of cartwheel cells. BK and RyR signals are merged in (Aii). The regions marked (B) and (C) are expanded in Fig. 8B and C.
(B and C) Expanded images of cell body (B) and dendrite (C). Arrows in (B) indicate obvious colocalization of BK and RyR signals. Signal intensities between (a) and (b) in (Biii) along the cell membrane, and between (a’) and (b’) in (Ciii) was plotted in (Biv) and (Civ), respectively. The cell membrane marked by EYFP, which was expressed as a fusion protein with a membrane protein ChR2. (Bi and Ci) Labeling for BK. (Bii and Cii) Labeling for RyRs. (Biii and Ciii) Merged image of BK and RyR labeling. (Biv and Civ) Histograms of signal intensities along somatic membrane (Biv) and dendritic membrane (Civ). The overlapping of the two signals is highlighted by *. A.U.: arbitrary unit.
(D and E) Colocalization of BK and RyR signals with membrane EYFP. Images in (D) and (E) were expanded from the marked regions in Fig. S4B. Regions of interests (transparent white lines) were drawn perpendicular to cell membranes labeled by EYFP. (Di and Ei) Labeling for EYFP. (Dii and Eii) Merged image of BK and RyR labeling. (Diii and Eiii) Labeling for BK. (Div and Eiv) Labeling for RyRs. (Dv and Ev) Histograms of signal intensities along the regions of interests.
(F) Average signal intensities vs location. (Fii) The intensity around the peaks were fitted by sixth-order polynomial regression curves.
The lines used above to define regions of interest along the membrane had a width of 0.22 µm, just wider than the point-spread function for the Zeiss Airyscan confocal fluorescence signals (0.186 µm for BK and 0.211 µm for RyRs), and thus overlap of signals indicate colocalization at least within that width. An alternative measure of colocalization was made by measuring fluorescence along short lines drawn perpendicular to the cell membrane (defined by YFP) and across overlapping BK and RyR puncta (Fig. 8D and E, transparent white lines). Figure 8D and E are expanded images containing somatic membranes (see Fig. S4B). The signal profiles are shown in Fig. 8Dv and Ev. The positions of the peaks for these signals were nearly identical, within the resolution of the confocal. To detect potential differences between peaks, averaged profiles were made from 10 lines (Fig. 8F). Profiles from these lines were aligned by using the x-axis value of the peak location of RyR signals, and the signal intensity was averaged. 7–8 data points around the peaks (rectangle in Fig. 8Fi) were fitted by a sixth-order polynomial regression curves to estimate the position of maximal fluorescence for each channel (Fig. 8Fii). While positions of the RyR signal peak differed from that of the other peaks (RyR to YFP, 45.7 ± 13.5 nm, n = 10, p < 0.01, one-sample t-test; RyR to BK, 28.5 ± 11.2 nm, n = 10, p < 0.001), all curves were within the confocal point spread function, indicating close apposition of ER and plasma membrane channels, and suggesting that the CICR originates from the subsurface cisterns previously found using electron microscopy (Ryugo et al., 1995; Wouterlood and Mugnaini, 1984).
Discussion
We have shown that three channels, P/Q, BK and RyR, interact within nanodomains defined by the distances between plasma membrane channels and subsurface ER cisterns (Fig. 7I). Ca2+ from P/Q channels targets ER RyR over a nanodomain, and Ca2+ released from RyR then targets BK channels across a similar distance. Together, these channels and membranes constitutes an organelle that rapidly controls the likelihood of action potential bursts as well as the fine structure of spikelets within the burst (Fig. 7I). Although the coupling between P/Q-type Ca2+ and BK channels has been proposed based on anatomical grounds (Indriati et al., 2013), and ryanodine effects on spike activity have been described in other cells, this study demonstrates that this double nanodomain mechanism operates remarkably quickly, and is thereby suitable for control of action potentials in the millisecond time scale of the spike burst. Each of the molecular elements, the channel subtypes, cisterns, and spike bursts, as well as the presence of SMOCs, have been described in neurons throughout the central nervous system, and thus it is likely that the mechanism we describe may be utilized in diverse brain regions where spike bursts occur. Importantly, because this mechanism requires convergence of multiple cellular components, pathological changes in any one component could bias neurons to a ‘ryanodine-like’ condition of elevated firing. Indeed, recent studies indicate roles of altered function of neuronal ryanodine receptor type 1 and 2 (RyR1, RyR2) in some models of seizure susceptibility (Aiba et al., 2016; Mikami et al., 2016).
A channel triad
The striking differences in the effects of EGTA and BAPTA on the frequency of SMOCs or on the size of evoked transient outward current strongly suggests nanodomain communication by Ca2+ between the three channel subtypes. It has been suggested previously that Cav and BK channels interact through a nano- or micro-domain (Fakler and Adelman, 2008), but the potential dependence of this interaction on a third channel type in a different membrane compartment, i.e., RyR in ER, is less well appreciated. SMOCs appear during small membrane depolarizations. These are voltage levels which might be expected to slightly raise the open probability of P/Q-type Ca2+ channels and thus only gradually raise cytosolic Ca2+ (Awatramani et al., 2005). Surprisingly, given that EGTA does not affect SMOC frequency or amplitude, SMOC events may be triggered immediately upon gating of nearby P/Q-type Ca2+ channels, and rather than by the elevation of background Ca2+ by repeated channel openings. Random opening of a small number of P/Q-type Ca2+ channels, possibly even a single channel, during weak voltage steps could be sufficient to activate a RyR.
The close apposition of the subsurface cistern to the plasma membrane apparently serves multiple functions in the regulation of action potential activity. Besides acting as a source of Ca2+, the close distance of the cistern to the plasma membrane defines a narrow “cleft” region in which Ca2+ ions can be rapidly concentrated, thereby ensuring rapid and reliable activation of both the RyR and BK channels. Moreover, since subsurface cisterns typically have lateral widths of only ~1 µm, enhanced diffusion at the edges of the cistern-plasma membrane cleft into the cytosol may ensure rapid clearance of Ca2+ following channel closure, a process likely to be far faster than reuptake by Ca2+-ATPase. Although EGTA at ~5 mM (our estimate of the final concentration of chelator in the double perfusion experiments) had little effect on BK channel gating in cartwheel cells, the expression and distribution of endogenous Ca2+ buffers in different cell types might significantly impact the time course of CICR-induced BK currents and determine their role in controlling action potential activity. More generally, the fact that SMOCs induced by small voltage steps and large BK transient currents induced by large voltage steps have similar time course and buffer sensitivity suggests that SMOCs compose the transient outward currents and by extension that the number and dimensions of the cisterns may be a critical, and potentially regulated, determinant of spike activity in neurons.
This mechanism requiring nanodomain coupling appears to differ from that described in some previous studies in which Ca2+ flux and RyR activity leads to activation of SK channels and membrane potential hyperpolarization (Cui et al., 2004; Kakizawa et al, 2007; Sah and McLachlan, 1991). In those cases, SK currents are comparatively slow to rise, and may last hundreds of milliseconds, eliminating a nanodomain requirement. Indeed, RyRs associated with this mechanism in cerebellar Purkinje cells are more centrally located in cytoplasm of dendrites and spines (Kakizawa et al., 2007), rather than at the membrane of cell soma as described here. Thus, coupling between RyR and plasma membrane channels may arise across different spatial domains and use different channel effectors dependent upon the required kinetics of the electrical response.
CICR and spike bursts
It was striking that rapid CICR is restricted to the cell soma. CICR is well established to play roles in synaptic plasticity in the brain, and indeed IP3 receptors are highly concentrated in cartwheel cells (Ryugo et al., 1995), and blockade of CICR prevents induction of long-term plasticity in these neurons (Fujino and Oertel, 2003). Moreover, CICR has been shown to give rise to Ca2+ “waves” in dendrites, which may also function to trigger experience dependent change in synaptic function (Ross, 2012). The ER membrane compartment is thought to be continuous throughout the full extent of a neuron (Okubo et al., 2015; Verkhratsky, 2005). The use of somatic CICR-mediated control of spiking, and dendritic CICR-mediated control of plasticity or slower voltage changes, indicates that the functional specializations of CICR may vary depending on its location in the cell.
However, this regional restriction of function raises an additional question: if CICR mediated spike regulation requires rapid gating of BK channels, why is it absent from the AIS, where spikes are triggered? It is well established that spikes initiate in the AIS due to a higher concentration of Na+ channels there relative to the soma, and it is also known that axonal K+ channels are critically distributed to determine axonal spike shape and frequency (Bender and Trussell, 2012; Kole and Stuart, 2012). Interestingly, studies in multiple cell types highlight the metabolic need for critical displacement in time of Na+ and K+ channel activation, so that Na+ influx, which must be removed by ATPase activity, is not “wasted” by simultaneous K+ efflux (Alle et al., 2009; Carter and Bean, 2009). Recent studies also indicate that not only single spikes, but spike bursts too are initiated in the AIS, due to the localized expression of T-type Ca2+ channels (Bender and Trussell, 2009), which provide the sustained inward current necessary to drive multiple Na+ spikelets in each burst. In order for the balance of inward and outward currents to determine spike burst probability, CICR-mediated BK current must necessarily occur nearly simultaneously with the Ca2+ current that drives the burst. Since these two currents cannot be separated in time, the cartwheel cell apparently separates these processes in space, and depends on rapid charge transfer between soma and AIS over the time course of the burst to control axonal membrane voltage near threshold. Moreover, restricting CICR to the soma minimizes the amount of ER packed into axonal cytosol, which might otherwise elevate axial resistance and slow conduction.
STAR METHODS
CONTACT FOR REAGENT AND RESOURCE SHARING
Further information and requests for resources should be directed to and will be fulfilled by the Lead Contact Laurence Trussell (trussell@ohsu.edu).
EXPERIMENTAL MODEL AND SUBJECT DETAILS
Animals
All animal care and handling procedures used in this study were approved by the Oregon Health & Science University Institutional Animal Care and Use Committee. Animals were housed in the OHSU Department of Comparative Medicine animal quarters and cared for by OHSU staff. C57BL/6J mice (16- to 26-day-old, either sex) were used for electrophysiological recordings. Glycine transporter 2 (GlyT2)-Cre;Ai32 mice (C57BL/6J background strain; 24-days-old), which express ChR2(H134R)-EYFP under the control of GlyT2 promoter, were used for fluorescent immunohistochemistry to label somatic and dendritic membranes of cartwheel cells (Lu and Trussell, 2016).
METHOD DETAILS
Electrophysiological recordings and data analysis
Brain-stem slices containing DCN were prepared as follows: mice were anesthetized with isoflurane and decapitated. Parasagittal slices of brain stems (200-mm thickness) were prepared using a vibrating blade microtome (VT1200S, Leica Microsystems, Wetzlar, Germany) in ice-cold, cutting solution containing the following (in mM): 93 N-methyl-D-glucamine (NMDG), 2.5 KCl, 1.2 NaH2PO4, 30 NaHCO3, 20 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), 0.5 CaCl2, 10 MgSO4, 5 sodium L-ascorbate, 2 thiourea, 3 sodium pyruvate, 3 myo-inositol, 25 glucose, pH adjusted to 7.4 with HCl (~305 mOsm), and bubbled with 5% CO2/95% O2. Slices were subsequently recovered at 34°C in the cutting solution for 10 min and transferred to room temperature artificial cerebrospinal fluid (ACSF) solution containing (in mM): 125 NaCl, 2.1 KCl, 1.7 CaCl2, 1 MgCl2, 1.2 KH2PO4, 20 NaHCO3, 3 HEPES-Na, 10 glucose, 0.4 ascorbic acid, 3 myo-inositol, 2 sodium pyruvate, and bubbled with 5% CO2/95% O2 (~310 mOsm). The slices were further incubated for 40 min before use. In electrophysiological recordings, ascorbic acid was omitted from the ACSF. The ACSF was supplemented with fast synaptic blockers {10 µM 2,3-Dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide disodium salt (NBQX, Alomone labs, Jerusalem, Israel), 3 µM (5S,10R)-(+)-5-Methyl-10,11-dihydro-5H–dibenzo[a,d]cyclohepten-5,10-imine maleate (MK-801), 100 mM picrotoxin, and 1 µM strychnine} unless otherwise stated. When CdCl2 and/or NiCl2 was added to ACSF to block Ca2+ channels, KH2PO4 was exclude and replaced with equimolar KCl to avoid precipitation. Parasagittal slices of the cerebellum (200-µm thickness) were prepared and recovered by using similar methods.
The brain slices were transferred to a recording chamber, and continuously perfused at 2 ml/min with ACSF at 33–34°C. Neurons were visualized with Zeiss Axioskop 2 microscope equipped with a 60×/0.9 numerical aperture water-immersion objective lens (LUMPlanFI, Olympus, Tokyo, Japan), infrared CCD video camera (IR-1000, DAGE-MTI, Michigan City, IN), and custom-made Dodt gradient contrast optics. Cartwheel cells were identified based on location within the DCN, somatic size, morphology, characteristic responses to current injections (Golding and Oertel, 1996; Manis et al., 1994; Tzounopoulos et al., 2004). In loose cell-attached recordings, spontaneously active cartwheel cells could be easily identified by their irregular action potential firing patterns (Bender et al., 2012; Kim and Trussell, 2007). Data were collected with Molecular Devices (Sunnyvale, CA) hardware and software (Multiclamp 700B, Digidata 1322A, and Clampex 9.2 and 10.3). Signals were low-pass filtered at 6 kHz and digitized at 10 kHz for loose cell-attached recordings, or filtered at 6–10 kHz and digitized at 20–50 kHz for other experiments.
Loose cell-attached recordings were made using patch pipettes (tip diameter: 2–3 µm) made from borosilicate glass capillaries (1B150F-4, World precision instrument, Sarasota, Florida). The pipettes were filled with a modified ACSF containing (in mM): 142 NaCl, 2.1 KCl, 1.7 CaCl2, 1.0 MgSO4, 1.2 KH2PO4, 10 HEPES, 11 glucose, and pH adjusted to 7.4 with NaOH (~300 mOsm). Loose cell-attach configuration was achieved by applying slight negative pressure on the pipette. Seal resistances were in the range of 20 to 300 MΩ. Recordings were done in voltage-clamp configuration with the pipette potential held at 0 mV.
For recording of spontaneous glycinergic IPSCs (sIPSCs), a CsCl-based internal solution containing following was used (in mM): 105 CsCl, 5 CsF, 10 tetraethylammonium-Cl, 5 ethylene glycol tetraacetic acid (EGTA), 2 Mg-ATP, 3 Na2-ATP, 0.3 Na2-GTP, 13 Tris2-phosphocreatine, 3 N-(2,6-Dimethylphenylcarbamoylmethyl)triethylammonium chloride (QX-314, Alomone labs), 10 HEPES, and pH adjusted to 7.3 with CsOH (~290 mOsm). Glycinergic sIPSCs were recorded in the presence of NBQX, MK-801, and 10 µM 6-Imino-3-(4-methoxyphenyl)-1(6H)-pyridazinebutanoic acid hydrobromide (SR95531, a GABAA receptor blocker, Abcam, Cambridge, MA) to block excitatory inputs from granule cells and reduce inhibitory inputs from superficial stellate cells (Apostolides and Trussell, 2014). Membrane potentials were held at −65 mV under whole-cell voltage clamp configuration and IPSCs were recorded as inward currents. Action potential-induced sIPSCs was defined as follows: at the end of the recording of sIPSCs, 0.5 µM tetrodotoxin (TTX, Abcam) was bath-applied to record 200–800 miniature IPSCs (mIPSCs), which were used to obtain a value for mean mIPSC plus 3 standard deviations of the mean (mean + 3SD). When a sIPSC had a single peak (e.g., inset in Fig. 2Ai) and its amplitude was larger than mean + 3SD, the IPSC was regarded as action potential-induced sIPSC. When a cluster of sIPSC were composed of summation of sIPSCs (e.g., inset in Fig. 2Aii), and at least one of the sIPSC had the amplitude larger than mean + 3SD, the cluster was defined as action potential-induced. These action potential-induced sIPSCs were used for the calculation of instantaneous frequency (Fig. 2B and C).
Most of the somatic whole-cell patch clamp recordings from cartwheel cells were performed using a K-gluconate-based internal solution containing (in mM): 125 K-gluconate, 10 KCl, 0.1 EGTA, 2 Mg-ATP, 3 Na2-ATP, 0.3 Na2-GTP, 13 Tris2-phosphocreatine, 10 HEPES, and pH adjusted to 7.3 with KOH (~295 mOsm). The pipette resistances were 3–4 MΩ when filled with the internal solution. In current clamp recordings, series resistance was compensated using bridge balance, pipette capacitance was neutralized, and resting membrane potentials were adjusted to suppress spontaneous firings by injecting negative bias currents. Action potentials were evoked by depolarizing current injections. In some experiments (Fig. 3), perforated-patch clamp recordings were made as reported previously (Kim and Trussell, 2007). The K-gluconate intracellular solution containing 240 µg/mL of amphotericin-B was used. When voltage clamp experiments were made, series resistance was compensated by 60–90% (bandwidth: 3–4 kHz). Outward currents were evoked by voltage steps from −70 mV holding potential up to 0 ~ 20 mV in 10-mV increments. In some experiments, ruthenium red (200 µM; Wako pure chemical industries, Osaka, Japan) was added to the K-gluconate-based internal solution to inhibit the opening of RyRs (Smith et al, 1988; Xu et al., 1999). Here, dual-patch clamp experiments were made using one pipette containing normal K-gluconate solution and the other pipette had a K-gluconate solution containing ruthenium red.
When the effects of several blockers on SMOCs were tested, SMOCs were evoked every 1 min by applying 10-mV depolarizing voltage steps (2.5 sec duration, from −70 mV to −60 mV), and the frequencies and amplitudes of SMOCs were averaged from the data recorded for 3 min. Thereafter, the frequencies and amplitudes were normalized by using control data obtained before application of the blockers (Fig. 6A–D). The sensitivity of SMOCs to caffeine was monitored every 5 sec by applying 10-mV voltage steps (1-sec duration, from −70 mV holding potential to −60 mV, Fig. 6E); the frequencies and amplitudes of events were then averaged every 1 min, and normalized by using control data obtained before caffeine application (Fig. 6Eii). The outward currents including SMOCs were recorded in the presence of fast synaptic blockers and TTX. In some recording, 1 mM 4-aminopyridine (4-AP) was also added in the ACSF to block voltage-gated K+ channels (Fig. 4Bii, black squares; Fig. 4C; Fig. 6C and D excluding IbTX, ryanodine, Ca2+ free, and apamin, Fig. 6E) (Benton et al., 2013).
When the effects of calcium chelators on the SMOCs and transient outward currents were examined, dual-patch clamp experiments were used. One pipette was filled with the normal K-gluconate solution and used for voltage clamp recordings, and the other was filled with the following EGTA or 1,2-Bis(2-aminophenoxy)ethane-N,N,N’,N’-tetraacetic acid (BAPTA)-containing K-gluconate solution to inject the chelators and to monitor the change of the membrane potential after rupturing the gigaohm seal (break-in) under current clamp configuration (in mM): 110 K-gluconate, 10 KCl, 10 EGTA, 2 Mg-ATP, 3 Na2-ATP, 0.3 Na2-GTP, 13 Tris2-phosphocreatine, 10 HEPES, and pH adjusted to 7.3 with KOH (~295 mOsm), or 115 K-gluconate, 11 KCl, 10 BAPTA, 2 Mg-ATP, 3 Na2-ATP, 0.3 Na2-GTP, 13 Tris2-phosphocreatine, 11 HEPES, and pH adjusted to 7.3 with KOH (~295 mOsm). SMOCs were induced every 10 s by 10-mV depolarization step from −70 mV holding potential. Transient outward currents were evoked every 20 s by depolarization step to 0 or 20 mV from −70 mV holding potential in the presence of fast synaptic blockers, 4-AP, and 100 nM apamin [a small-conductance Ca2+-activated K+ (SK) channel blocker]. The leak and capacitive currents were subtracted on-line by using a p/4 protocol with opposite polarity.
Data were analyzed using Clampfit 10.3 software (Molecular Devices) and Igor Pro 6 software (Wavemetrics, Lake Oswego, OR) with the added import functionality provided by ReadPclamp XOP of the NeuroMatic software package (http://www.neuromatic.thinkrandom.com/) (Rothman et al., 2009). Event detections of spontaneous firings were done by using the spike detection function of NeuroMatic. For the detection of events, 1000–3000 spikes in control and 2000–4000 in ryanodine or cyclopiazonic acid (CPA) were used from each experiment. Locations of sIPSC peaks were detected by using the event detection function of Neuromatic, and 500–1500 events in control and 800–2000 in ryanodine were counted. mIPSCs and SMOCs were analyzed by using the template search function in the Clampfit. Liquid junction potentials (K-gluconate-based, −10 mV; CsCl-based, −4 mV) were corrected off-line. All data are provided as the means ± standard error of the mean unless otherwise stated. Numbers in parentheses in figures and n in the text and table indicate the number of experiments.
Peptide blocker application
When peptide blockers [apamin, Iberiotoxin (IbTX), and ω-Agatoxin-IVA (Aga-IVA) (all from Peptide Institute, Osaka, Japan)] were used, 0.5 mg/ml cytochrome C was added to all ACSF to reduce nonspecific binding. These peptide blockers were perfused at least for 15 min by using recirculation before data recording.
Two-photon Ca2+ imaging
The imaging experiments were performed in conjunction with whole-cell patch clamp recordings using the same current clamp method stated as above except that the intracellular solution replaced EGTA with 250 µM Fluo-5F pentapotassium salt (Fluo-5F, Thermo Fisher Scientific, Waltham, MA) and 20 µM Alexa Fluor 594 hydrazide (AF594, Thermo Fisher Scientific). Cells were dialyzed with the pipette solution at least for 20 min. Calcium transients were evoked by single or a train of action potentials (6 action potentials at 50 Hz) induced by somatic current injections (biphasic square wave pulse, 4-ms duration, 700 to 1000 pA amplitude). The amplitudes of current pulses were carefully adjusted so as not to induce burst firings even in the presence of ryanodine (Alomone labs) or CPA, and therefore each pulse evoked single action potential. Intracellular Ca2+ imaging was performed as described previously with some modifications (Bender et al., 2010; Bender and Trussell, 2009). The fluorescence signals from of Fluo-5F and AF594 were captured with H8224 and R9110 photomultiplier tubes (Hamamatsu Photonics, Hamamatsu, Japan), respectively. Data were corrected in segmented linescan mode (2–2.2 ms/line including mirror flyback) using Prairie View 5.3 software (Bruker, Billerica, MA). The scan areas were drawn at 1 to 2 regions of interests (ROIs). The ROIs on cell bodies were placed across the cell membranes and on axon initial segments (AISs) were done between 15 to 20 mm from axon hillocks (Bender and Trussell, 2009). The imaging at dendritic shafts and spines was made by drawing ROIs across the processes, which were more than 20 mm away from the center of the cell bodies. The effects of ryanodine or CPA on Ca2+ transient amplitudes were compared with vehicle control experiments to exclude the possibility that the changes were caused by the rundown of Ca2+ currents. Data were obtained by averaging 20–40 successive trials (interval of the trials: 5 s) and showed as ΔG/R in traces or (ΔG/R test)/(ΔG/R control) in graphs. Data were imported by using Bio-Formats plugin installed in Fiji software (https://fiji.sc/) (Schindelin et al., 2012), and then analyzed using Fiji and Igor Pro 6. The decays of Ca2+ transients were fit by double-exponential function: D(t) = Afast × e−t/τfast + Aslow × e−t/τslow, where D(t) is the decay of the Ca2+ transients as a function of time (t); Afast and Aslow are amplitude constants, and τfast and τslow are fast and slow constants, respectively. Percentage of fast component was calculated as %Fast = [Afast/(Afast + Aslow)] × 100. When the fast component could not be fitted, Afast was defined as 0, and the τfast was not measured and was not counted in the number of experiments.
Fluorescent immunohistochemistry and super-resolution microscopy imaging
Transgenic mice were anesthetized with isoflurane and fixed by transcardial perfusion of warmed (~34°C) phosphate buffered saline (PBS, pH 7.4 adjusted by NaOH) followed by 4% (wt/vol) ice-cold formaldehyde in PBS. The brains were removed from skulls, post-fixed in the formaldehyde at room temperature for 30 min, and cryoprotected in 30% (wt/wt) sucrose-containing PBS overnight at 4°C. Using a cryostat (Microm HM550, Thermo Fisher Scientific), the brainstems or cerebellum were sliced into 20 µm-thick coronal or sagittal sections, respectively. The sections were washed with PBS containing 0.3% (vol/vol) Triton X-100 (PBS-X), and then incubated overnight at 4°C in incubation buffer [1% (vol/vol) goat and donkey serum, 0.25% (wt/vol) λ-carrageenan, and 0.02% (wt/vol) sodium azide in PBS-X] containing the following primary antibodies: chicken polyclonal anti-GFP antibody (1 µg/mL, GFP-1020, Aves Labs, Tigard, OR), mouse IgG2a monoclonal anti-BK channel α subunit antibody [1 µg/mL, clone L6/60, UC Davis/NIH NeuroMab Facility, Davis, CA, (Misonou et al., 2006)], and mouse IgG1 monoclonal anti-ryanodine receptor antibody (1 µg/mL, clone 34C, MA3–925, Thermo Fisher Scientific; This detects all RyR isoforms in mouse tissue). The slices were washed with PBS-X, and then incubated for 6 hr at room temperature in the incubation buffer containing the following secondary antibodies at the dilution of 1/500: AF 488 donkey anti-chicken IgY (703-545-155, Jackson Immuno Research, West Grove, PA, USA), AF 568 goat anti-mouse IgG2a (A21134, Thermo Fisher Scientific), and AF 647 goat anti-mouse IgG1 (A21240, Thermo Fisher Scientific). The slices were washed with PBS-X, fixed again in the formaldehyde, then washed with PBS-X, and finally coverslipped with Fluoromount-G (Southern Biotech, Birmingham, AL).
Immunofluorescence images were acquired using an Airyscan super-resolution microscope (LSM710 with Airyscan, Carl Zeiss, Jena, Germany) with the following appropriate settings: AF 488 (excitation, 488 nm laser; emission, the combination of 545 nm short-pass filter and 495–550 nm band-pass), AF 568 (excitation, 561 nm; emission, 610 nm short-pass and 495–620 nm band-pass), AF 647 (excitation, 633 nm; emission, 605 nm long-pass). Images were obtained with a 63×/1.4 numerical aperture oil-immersion objective lens, and the pixel size was 44 nm (x axis) × 44 nm (y) × 187 nm (z). The confocal pinhole size was 2.0 Airy unit. Channel alignments among different channels were done by imaging microspheres coated with multiple fluorophores (TetraSpeck Microspheres, T7279, Thermo Fisher Scientific).
The profiles of the signal intensities along cell membranes were measured by drawing 0.22-mm-width lines (region of interests, ROIs) by the aid of the YFP signal, which highlighted cell membranes of cartwheel cells (Fig. 8Biv and Civ, and Fig. S3A). The graphs in Fig. S4A were made by measuring signal intensities from somatic or dendritic membrane regions having 110 µm-length in total (12 cell bodies and 12 dendrites, total 2538 points in both). Each point had 0.22 µm × 0.044 µm area. When the distances among cell membranes, BK, and RyRs were measured, linear ROIs which were perpendicular to the cell membrane and crossed co-localizations of BK and RyRs, were drawn (Fig. 8D and E). Averaged profiles for the measurement of the signal distances were obtained from 10 ROIs as follows (Fig. 8F): signal intensities in each profile were normalized by the most intense signal value in the same focal plane. The individual, normalized profiles were aligned by using the x-axis value of the peak location of RyR signal, and then averaged. The averaged or individual profiles around the peaks were fitted by sixth order polynomial regression curves (Fig. 8Fii, black and red lines). Data were analyzed using Fiji and GraphPad Prism 5 software.
QUANTIFICATION AND STATISTICAL ANALYSIS
Mean values are reported as ±SEM, along with number (n) of replications (cells) or events (spikes, SMOCs, puncti, as indicated). Blinding to identity of applied drug was not employed. No data were excluded. Unless otherwise stated, statistical significance was tested using paired t-tests (significance, p < 0.05) using GraphPad Prism 5 (GraphPad Software, San Diego, CA), based on an assumption of normality of sample population distributions.
Supplementary Material
KEY RESOURCES TABLE
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| Alexa Fluor 647 goat anti-mouse IgG1 | Thermo Fisher Scientific | A21240; RRID:AB_2535809 |
| Alexa Fluor 488 donkey anti-chicken IgY | Jackson Immuno Research | 703-545-155; RRID:AB_2340375 |
| Alexa Fluor 568 goat anti-mouse IgG2a | Thermo Fisher Scientific | A21134; RRID:AB_2535773 |
| Chicken polyclonal anti-GFP antibody | Aves Labs | GFP-1020; RRID:AB_10000240 |
| Mouse IgG2a monoclonal anti-BK channel a subunit antibody | UC Davis/NIH NeuroMab Facility | 73-022; RRID:AB_10698180 |
| Mouse IgG1 monoclonal anti-ryanodine receptor | Thermo Fisher | MA3-925; |
| antibody | Scientific | RRID:AB_2254138 |
| Bacterial and Virus Strains | ||
| Biological Samples | ||
| Chemicals, Peptides, and Recombinant Proteins | ||
| w-Agatoxin-IVA | Peptide Institute | 4256-s |
| Alexa Fluor 594 hydrazide | Thermo Fisher Scientific | A10438 |
| Apamin | Peptide Institute | 4257-v |
| Cyclopiazonic acid | Sigma-Aldrich | C1530 |
| Fluo-5F | Thermo Fisher Scientific | F14221 |
| Iberiotoxin | Peptide Institute | 4235-s |
| MK-801 | Sigma-Aldrich | M107 |
| NBQX disodium salt | Alamone | N-186 |
| Picrotoxin | Sigma-Aldrich | P1675 |
| QX-314 | Alomone labs | Q-150 |
| Ryanodine | Alomone | R-500 |
| SR95531 | Abcam | ab120042 |
| Strychnine | Sigma-Aldrich | S0532 |
| TetraSpeck Microspheres | Thermo Fisher Scientific | T7279 |
| TTA-P2 | Alomone | T-155 |
| TTX | Abcam | ab120055 |
| Ruthenium red | Wako | 189-03181 |
| Critical Commercial Assays | ||
| Deposited Data | ||
| Experimental Models: Cell Lines | ||
| Experimental Models: Organisms/Strains | ||
| Mouse (C57BL/6J) | Jackson Labs | RRID:IMSR_JAX:0006 64 |
| Mouse (GlyT2-Cre) | MMRRC | 030730-UCD; RRID:MMRRC_03073 0-UCD |
| Mouse (Ai32) | Jackson Labs | 012569; RRID:IMSR_JAX:0125 69 |
| Oligonucleotides | ||
| Recombinant DNA | ||
| Software and Algorithms | ||
| Igor Pro 6 | Wavemetrics | RRID:SCR_000325 |
| Neuromatic | http://www.neuromatic.thinkrandom.com | RRID:SCR_004186 |
| Fiji | https://fiji.sc/ | RRID:SCR_002285 |
| GraphPad Prism 5 | GraphPad Software | RRID:SCR_002798 |
| Other | ||
Highlights.
High-frequency spike bursts are controlled by calcium inducted calcium release (CICR)
CICR rapidly activates BK potassium channels in a nanodomain region
Subsurface ER cisterns may act as quantal units of CICR-BK signaling in neurons
Acknowledgments
This work was supported by National Institutes of Health Grants NS028901 and DC004450 (L.O.T.) and by Japan-U.S. Brain Research Cooperation Program (T.I.). The OHSU Imaging Core used for confocal imaging is supported by NIH grant P30NS061800. We thank Drs. Craig Jahr and Stefanie Kaech-Petrie for technical advice on imaging experiments, Drs. Brett Carter, Timothy Balmer, and Zhengquan Tang for comments on the manuscript, Trussell lab members for helpful discussions, and Michael Bateschell and Ruby Larisch for help with mouse colony management.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
T.I. performed research; T.I. and L.O.T. designed research, analyzed data, and wrote the paper.
The authors have no conflicts of interest.
References
- Adler EM, Augustine GJ, Duffy SN, Charlton MP. Alien intracellular calcium chelators attenuate neurotransmitter release at the squid giant synapse. J Neurosci. 1991;11:1496–1507. doi: 10.1523/JNEUROSCI.11-06-01496.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aiba I, Wehrens XH, Noebels JL. Leaky RyR2 channels unleash a brainstem spreading depolarization mechanism of sudden cardiac death. Proc Natl Acad Sci U S A. 2016;113:E4895–4903. doi: 10.1073/pnas.1605216113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alle H, Roth A, Geiger JR. Energy-efficient action potentials in hippocampal mossy fibers. Science. 2009;325:1405–1408. doi: 10.1126/science.1174331. [DOI] [PubMed] [Google Scholar]
- Apostolides PF, Trussell LO. Chemical synaptic transmission onto superficial stellate cells of the mouse dorsal cochlear nucleus. J Neurophysiol. 2014;111:1812–1822. doi: 10.1152/jn.00821.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine GJ, Santamaria F, Tanaka K. Local calcium signaling in neurons. Neuron. 2003;40:331–346. doi: 10.1016/s0896-6273(03)00639-1. [DOI] [PubMed] [Google Scholar]
- Awatramani GB, Price GD, Trussell LO. Modulation of transmitter release by presynaptic resting potential and background calcium levels. Neuron. 2005;48:109–121. doi: 10.1016/j.neuron.2005.08.038. [DOI] [PubMed] [Google Scholar]
- Bender KJ, Trussell LO. Axon initial segment Ca2+ channels influence action potential generation and timing. In Neuron. 2009:259–271. doi: 10.1016/j.neuron.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender KJ, Ford CP, Trussell LO. Dopaminergic modulation of axon initial segment calcium channels regulates action potential initiation. Neuron. 2010;68:500–511. doi: 10.1016/j.neuron.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender KJ, Trussell LO. The physiology of the axon initial segment. Annu Rev Neurosci. 2012;35:249–265. doi: 10.1146/annurev-neuro-062111-150339. [DOI] [PubMed] [Google Scholar]
- Bender KJ, Uebele VN, Renger JJ, Trussell LO. Control of firing patterns through modulation of axon initial segment T-type calcium channels. J Physiol. 2012;590:109–118. doi: 10.1113/jphysiol.2011.218768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benhassine N, Berger T. Large-conductance calcium-dependent potassium channels prevent dendritic excitability in neocortical pyramidal neurons. Pflugers Arch. 2009;457:1133–1145. doi: 10.1007/s00424-008-0569-3. [DOI] [PubMed] [Google Scholar]
- Benton MD, Lewis AH, Bant JS, Raman IM. Iberiotoxin-sensitive and -insensitive BK currents in Purkinje neuron somata. J Neurophysiol. 2013;109:2528–2541. doi: 10.1152/jn.00127.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein MP, Golovina VA. Structural complexity and functional diversity of endoplasmic reticulum Ca(2+) stores. Trends Neurosci. 2001;24:602–608. doi: 10.1016/s0166-2236(00)01891-9. [DOI] [PubMed] [Google Scholar]
- Bock T, Stuart GJ. The Impact of BK Channels on Cellular Excitability Depends on their Subcellular Location. Frontiers in cellular neuroscience. 2016;10:206. doi: 10.3389/fncel.2016.00206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter BC, Bean BP. Sodium entry during action potentials of mammalian neurons: incomplete inactivation and reduced metabolic efficiency in fast-spiking neurons. Neuron. 2009;64:898–909. doi: 10.1016/j.neuron.2009.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper DC. The significance of action potential bursting in the brain reward circuit. Neurochem Int. 2002;41:333–340. doi: 10.1016/s0197-0186(02)00068-2. [DOI] [PubMed] [Google Scholar]
- Cui G, Okamoto T, Morikawa H. Spontaneous opening of T-type Ca2+ channels contributes to the irregular firing of dopamine neurons in neonatal rats. J Neurosci. 2004;24:11079–11087. doi: 10.1523/JNEUROSCI.2713-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggermann E, Bucurenciu I, Goswami SP, Jonas P. Nanodomain coupling between Ca(2)(+) channels and sensors of exocytosis at fast mammalian synapses. Nat Rev Neurosci. 2012;13:7–21. doi: 10.1038/nrn3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M, Tanaka M, Ogawa Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature. 1970;228:34–36. doi: 10.1038/228034a0. [DOI] [PubMed] [Google Scholar]
- Fakler B, Adelman JP. Control of K(Ca) channels by calcium nano/microdomains. Neuron. 2008;59:873–881. doi: 10.1016/j.neuron.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Fujino K, Oertel D. Bidirectional synaptic plasticity in the cerebellum-like mammalian dorsal cochlear nucleus. Proc Natl Acad Sci U S A. 2003;100:265–270. doi: 10.1073/pnas.0135345100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golding NL, Oertel D. Context-dependent synaptic action of glycinergic and GABAergic inputs in the dorsal cochlear nucleus. J Neurosci. 1996;16:2208–2219. doi: 10.1523/JNEUROSCI.16-07-02208.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grienberger C, Chen X, Konnerth A. NMDA receptor-dependent multidendrite Ca(2+) spikes required for hippocampal burst firing in vivo. Neuron. 2014;81:1274–1281. doi: 10.1016/j.neuron.2014.01.014. [DOI] [PubMed] [Google Scholar]
- Henkart M, Landis DM, Reese TS. Similarity of junctions between plasma membranes and endoplasmic reticulum in muscle and neurons. J Cell Biol. 1976;70:338–347. doi: 10.1083/jcb.70.2.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huerta PT, Lisman JE. Bidirectional synaptic plasticity induced by a single burst during cholinergic theta oscillation in CA1 in vitro. Neuron. 1995;15:1053–1063. doi: 10.1016/0896-6273(95)90094-2. [DOI] [PubMed] [Google Scholar]
- Indriati DW, Kamasawa N, Matsui K, Meredith AL, Watanabe M, Shigemoto R. Quantitative localization of Cav2.1 (P/Q-type) voltage-dependent calcium channels in Purkinje cells: somatodendritic gradient and distinct somatic coclustering with calcium-activated potassium channels. J Neurosci. 2013;33:3668–3678. doi: 10.1523/JNEUROSCI.2921-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito M. Cerebellar long-term depression: characterization, signal transduction, and functional roles. Physiol Rev. 2001;81:1143–1195. doi: 10.1152/physrev.2001.81.3.1143. [DOI] [PubMed] [Google Scholar]
- Kampa BM, Letzkus JJ, Stuart GJ. Requirement of dendritic calcium spikes for induction of spike-timing-dependent synaptic plasticity. J Physiol. 2006;574:283–290. doi: 10.1113/jphysiol.2006.111062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakizawa S, Kishimoto Y, Hashimoto K, Miyazaki T, Furutani K, Shimizu H, Fukaya M, Nishi M, Sakagami H, Ikeda A, Kondo H, Kano M, Watanabe M, Iino M, Takeshima H. Junctophilin-mediated channel crosstalk essential for cerebellar synaptic plasticity. EMBO J. 2007;26:1924–1933. doi: 10.1038/sj.emboj.7601639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann WA, Ferraguti F, Fukazawa Y, Kasugai Y, Shigemoto R, Laake P, Sexton JA, Ruth P, Wietzorrek G, Knaus HG, et al. Large-conductance calcium-activated potassium channels in purkinje cell plasma membranes are clustered at sites of hypolemmal microdomains. J Comp Neurol. 2009;515:215–230. doi: 10.1002/cne.22066. [DOI] [PubMed] [Google Scholar]
- Kim Y, Trussell LO. Ion channels generating complex spikes in cartwheel cells of the dorsal cochlear nucleus. J Neurophysiol. 2007;97:1705–1725. doi: 10.1152/jn.00536.2006. [DOI] [PubMed] [Google Scholar]
- Kole MH. First node of Ranvier facilitates high-frequency burst encoding. Neuron. 2011;71:671–682. doi: 10.1016/j.neuron.2011.06.024. [DOI] [PubMed] [Google Scholar]
- Kole MH, Stuart GJ. Signal processing in the axon initial segment. Neuron. 2012;73:235–247. doi: 10.1016/j.neuron.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Krahe R, Gabbiani F. Burst firing in sensory systems. Nat Rev Neurosci. 2004;5:13–23. doi: 10.1038/nrn1296. [DOI] [PubMed] [Google Scholar]
- Kuba K. Ca(2+)-induced Ca2+ release in neurones. Jpn J Physiol. 1994;44:613–650. doi: 10.2170/jjphysiol.44.613. [DOI] [PubMed] [Google Scholar]
- Lu HW, Trussell LO. Spontaneous Activity Defines Effective Convergence Ratios in an Inhibitory Circuit. J Neurosci. 2016;36:3268–3280. doi: 10.1523/JNEUROSCI.3499-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancilla JG, Manis PB. Two distinct types of inhibition mediated by cartwheel cells in the dorsal cochlear nucleus. J Neurophysiol. 2009;102:1287–1295. doi: 10.1152/jn.91272.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandikian D, Bocksteins E, Parajuli LK, Bishop HI, Cerda O, Shigemoto R, Trimmer JS. Cell type-specific spatial and functional coupling between mammalian brain Kv2.1 K+ channels and ryanodine receptors. J Comp Neurol. 2014;522:3555–3574. doi: 10.1002/cne.23641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manis PB, Spirou GA, Wright DD, Paydar S, Ryugo DK. Physiology and morphology of complex spiking neurons in the guinea pig dorsal cochlear nucleus. J Comp Neurol. 1994;348:261–276. doi: 10.1002/cne.903480208. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Huguenard JR. A model of the electrophysiological properties of thalamocortical relay neurons. J Neurophysiol. 1992;68:1384–1400. doi: 10.1152/jn.1992.68.4.1384. [DOI] [PubMed] [Google Scholar]
- Mikami Y, Kanemaru K, Okubo Y, Nakaune T, Suzuki J, Shibata K, Sugiyama H, Koyama R, Murayama T, Ito A, et al. Nitric Oxide-induced Activation of the Type 1 Ryanodine Receptor Is Critical for Epileptic Seizure-induced Neuronal Cell Death. EBioMedicine. 2016;11:253–261. doi: 10.1016/j.ebiom.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misonou H, Menegola M, Buchwalder L, Park EW, Meredith A, Rhodes KJ, Aldrich RW, Trimmer JS. Immunolocalization of the Ca2+-activated K+ channel Slo1 in axons and nerve terminals of mammalian brain and cultured neurons. J Comp Neurol. 2006;496:289–302. doi: 10.1002/cne.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naraghi M, Neher E. Linearized buffered Ca2+ diffusion in microdomains and its implications for calculation of [Ca2+] at the mouth of a calcium channel. J Neurosci. 1997;17:6961–6973. doi: 10.1523/JNEUROSCI.17-18-06961.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo Y, Suzuki J, Kanemaru K, Nakamura N, Shibata T, Iino M. Visualization of Ca2+ Filling Mechanisms upon Synaptic Inputs in the Endoplasmic Reticulum of Cerebellar Purkinje Cells. J Neurosci. 2015;35:15837–15846. doi: 10.1523/JNEUROSCI.3487-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez GJ, Bonev AD, Patlak JB, Nelson MT. Functional coupling of ryanodine receptors to KCa channels in smooth muscle cells from rat cerebral arteries. J Gen Physiol. 1999;113:229–238. doi: 10.1085/jgp.113.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MT, Bender KJ, Trussell LO. Fidelity of complex spike-mediated synaptic transmission between inhibitory interneurons. J Neurosci. 2008;28:9440–9450. doi: 10.1523/JNEUROSCI.2226-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluth J. Subsurface cisterns and their relationship to the neuronal plasma membrane. J Cell Biol. 1962;13:405–421. doi: 10.1083/jcb.13.3.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross WN. Understanding calcium waves and sparks in central neurons. Nat Rev Neurosci. 2012;13:157–168. doi: 10.1038/nrn3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryugo DK, Pongstaporn T, Wright DD, Sharp AH. Inositol 1,4,5-trisphosphate receptors: immunocytochemical localization in the dorsal cochlear nucleus. J Comp Neurol. 1995;358:102–118. doi: 10.1002/cne.903580107. [DOI] [PubMed] [Google Scholar]
- Sah P, McLachlan EM. Ca(2+)-activated K+ currents underlying the afterhyperpolarization in guinea pig vagal neurons: a role for Ca(2+)-activated Ca2+ release. Neuron. 1991;7:257–264. doi: 10.1016/0896-6273(91)90264-z. [DOI] [PubMed] [Google Scholar]
- Saito Y, Yanagawa Y. Ca(2+)-activated ion currents triggered by ryanodine receptor-mediated Ca(2+) release control firing of inhibitory neurons in the prepositus hypoglossi nucleus. J Neurophysiol. 2013;109:389–404. doi: 10.1152/jn.00617.2012. [DOI] [PubMed] [Google Scholar]
- Satin LS, Adams PR. Spontaneous miniature outward currents in cultured bullfrog neurons. Brain Res. 1987;401:331–339. doi: 10.1016/0006-8993(87)91417-x. [DOI] [PubMed] [Google Scholar]
- Schmolesky MT, Weber JT, De Zeeuw CI, Hansel C. The making of a complex spike: ionic composition and plasticity. Ann N Y Acad Sci. 2002;978:359–390. doi: 10.1111/j.1749-6632.2002.tb07581.x. [DOI] [PubMed] [Google Scholar]
- Smith JS, Imagawa T, Ma J, Fill M, Campbell KP, Coronado R. Purified ryanodine receptor from rabbit skeletal muscle is the calcium-release channel of sarcoplasmic reticulum. J Gen Physiol. 1988;92:1–26. doi: 10.1085/jgp.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Wood RL. Subsurface cisterns in the Purkinje cells of cerebellum of Syrian hamster. Z Zellforsch Mikrosk Anat. 1970;110:311–320. doi: 10.1007/BF00321144. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Watabe AM, Moody TD, Makhinson M, O’Dell TJ. Postsynaptic complex spike bursting enables the induction of LTP by theta frequency synaptic stimulation. J Neurosci. 1998;18:7118–7126. doi: 10.1523/JNEUROSCI.18-18-07118.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzounopoulos T, Kim Y, Oertel D, Trussell LO. Cell-specific, spike timing-dependent plasticities in the dorsal cochlear nucleus. Nat Neurosci. 2004;7:719–725. doi: 10.1038/nn1272. [DOI] [PubMed] [Google Scholar]
- Verkhratsky A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol Rev. 2005;85:201–279. doi: 10.1152/physrev.00004.2004. [DOI] [PubMed] [Google Scholar]
- Williams SR, Stuart GJ. Mechanisms and consequences of action potential burst firing in rat neocortical pyramidal neurons. J Physiol. 1999;(521 Pt 2):467–482. doi: 10.1111/j.1469-7793.1999.00467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Womack MD, Khodakhah K. Dendritic control of spontaneous bursting in cerebellar Purkinje cells. J Neurosci. 2004;24:3511–3521. doi: 10.1523/JNEUROSCI.0290-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wouterlood FG, Mugnaini E. Cartwheel neurons of the dorsal cochlear nucleus: a Golgi-electron microscopic study in rat. J Comp Neurol. 1984;227:136–157. doi: 10.1002/cne.902270114. [DOI] [PubMed] [Google Scholar]
- Xu L, Tripathy A, Pasek DA, Meissner G. Ruthenium red modifies the cardiac and skeletal muscle Ca(2+) release channels (ryanodine receptors) by multiple mechanisms. J Biol Chem. 1999;274:32680–32691. doi: 10.1074/jbc.274.46.32680. [DOI] [PubMed] [Google Scholar]
- ZhuGe R, Tuft RA, Fogarty KE, Bellve K, Fay FS, Walsh JV., Jr The influence of sarcoplasmic reticulum Ca2+ concentration on Ca2+ sparks and spontaneous transient outward currents in single smooth muscle cells. J Gen Physiol. 1999;113:215–228. doi: 10.1085/jgp.113.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.