Abstract
Objective
Oral etoposide has been used as a later line therapy for metastatic breast cancer for more than twenty years. Its efficacy and clinical usefulness has been suggested in small phase II studies in the metastatic breast cancer population and the drug has also the added advantage of convenient oral administration. Despite these advantages, the place of oral etoposide in treatment of metastatic breast cancer has been challenged in the last decade due to introduction of several other chemotherapeutics, including options available orally, as well as novel targeted therapies. This report pools the data on response rates and survival from all available oral etoposide studies in order to reach a more precise estimate of the clinical benefit of the drug.
Materials and methods
A review of the literature was performed for studies of oral etoposide in metastatic breast cancer. Data were extracted from eligible studies and summary statistics derived. Calculations of pooled response rates and survival estimates were performed according to a random or fixed effect model as appropriate.
Results
The pooled estimate of Response Rate derived from twelve studies found in the English literature was 18.5% (95% CI 11.5–25.5%). The pooled estimate of Clinical Benefit Rate (CBR) was 45.8% (95% CI 38.6–53.0%) and median Overall Survival (OS) approached 1 year. Summarized adverse effects profile data show an overall manageable toxicity.
Conclusion
This pooled analysis provides evidence of a moderate clinical effectiveness of oral etoposide in metastatic breast cancer that could be useful in situations that options are limited but active treatment still appropriate.
Keywords: Breast cancer, metastatic, chemotherapy, oral etoposide, metronomic
Introduction
Several chemotherapy options exist for the treatment of patients with metastatic breast cancer. Newer drugs such as capecitabine (Xeloda, Roche, Basel, Switzerland), vinorelbine and eribulin (Halaven, Eisai, Tokyo, Japan) have been introduced in recent years to supplement anthracyclines and taxanes and help control disease and prolong the life of these patients (1). Before the introduction of these newer options, as well as several targeted treatments such as Cyclin-Dependent Kinase 4/6 inhibitors and mechanistic Target of Rapamycin (mTOR) inhibitors, oral etoposide had been one of the few available systemic treatments in the oncology armamentarium for advanced breast cancer. This drug can be given in daily low doses with a brief interruption of a few days at every three-weekly or monthly cycle. It has the advantage of oral administration that makes it a preferred option in the palliative setting and is still used, although less commonly, when other options are not available or had already failed but a treatment is appropriate. Nevertheless the efficacy of oral continuous dose etoposide in metastatic breast cancer has only been studied in small phase II trials with a few patients in each of them. These studies have been one arm, non-randomized and non-blinded with all possible biases associated with this design. The current paper reports a pooled analysis of all available trials of oral low dose daily etoposide in advanced breast cancer in order to obtain a more accurate efficacy evaluation of the drug. This estimation, although not negating the above limitations associated with the source studies, will inform better the clinician on the benefits that could be expected with the use of the drug in the palliative setting. In addition an overview of the toxicity profile of oral etoposide derived from the whole body of data in these studies will be discussed.
Materials and Methods
The two essential databases of medical literature, Medline/PubMed and Embase were searched for articles related to oral etoposide in advanced breast cancer. Search terms used were “oral etoposide” and “metastatic breast cancer” or “advanced breast cancer”. Studies were retained for further data extraction and data analysis if they were published in English, were describing the use of low dose daily etoposide as monotherapy in metastatic breast cancer patients and included more than twenty patients. Articles in other languages, case reports or case series of less than 20 patients and studies describing pre-clinical, pharmacokinetic or pharmacodynamics data were excluded. Also excluded were studies using etoposide in other cancers, in the adjuvant setting, in combination with other chemotherapy drugs, in high dose intermittent schedules or with an intravenous administration. In addition to the electronic search, a scanning of references of retained articles manually for additional publications fulfilling the inclusion criteria was performed.
Data obtained from the retrieved studies pertaining to patients’ population characteristics and treatment efficacy and adverse effects were tabularized and stored in a database. Patients’ data extracted for this pooled analysis included age of the patients, Eastern Cooperative Oncology Group (ECOG) performance status, number and type of previous lines of treatment for metastatic disease, number and site (visceral versus non-visceral) of organs involved, and biologic type of the breast cancer [Estrogen Receptor (ER) and Human Epithelial Growth Factor Receptor family member 2 (Her2) receptors expression], when available. Efficacy outcomes of interest included Response Rate (RR), Clinical Benefit Rate (CBR), median Overall Survival (OS), median Progression-Free Survival (PFS) or Time to Progression (TTP). Data on all grades and grade 3 and 4 toxicity rates were also obtained from the included studies for this pooled analysis.
There have been no conflicts of interest regarding this work. As this study involves only analysis of previously published data and no new data with human participants, no informed consent was required and no approval by the institution Ethics Committee.
Statistical analysis
Summary statistics were calculated for outcomes of interest measurements. Only part of the characteristics and outcomes of interest were available from each study included in the analysis. Thus, presented data in each occasion as well as efficacy and toxicity outcomes and their means and confidence intervals were calculated with the total number of patients in the studies with the given characteristic or outcome of interest as the denominator. The number of studies from which each outcome of interest was derived is also presented in the results tables. Pooled outcomes rates calculations were weighted according to the number of patients in each study. As several studies provided TTP instead of PFS as the survival measure of treatment efficacy, for the purpose of the current analysis, a pooled estimate of both PFS and TTP was calculated. In cases where TTP was provided instead of PFS, estimated 95% confidence intervals were calculated from the range according to the formula: variance=range/4 (2). Heterogeneity among the studies was evaluated with the Cochran’s Q and I2 tests. The fixed or random effect model was employed as appropriate according to the degree of heterogeneity (3). Calculations were performed in Excel (Microsoft Corp., Redmond, WA) with some modifications of a previously described method (4).
Results
Review of the literature led to the retrieval of sixty two articles (Figure 1). After exclusion of preclinical, pharmacokinetics, pharmacodynamics reports and reviews or case reports and publications not in English, thirty eight clinical reports remained. Twenty six of them were further excluded because they were not referring to breast cancer (nine publications), were studying etoposide in combination with other drugs or in the adjuvant setting (fifteen publications), were probably partially overlapping with one of the included studies (one publication) (5) or used higher dose intermittent schedule (one study) (6). Twelve publications were retained for the current analysis (Table 1). Included studies were published between 1993 and 2015 and reported on a total of 483 patients (Table 2). All studies except one that was retrospective (7), had a prospective phase II, non-randomized, one arm design (8–18). Besides two studies that were from China (17, 18), all other reports originated from Europe and the United States (Table 1). The median age of patients in most included studies was between 50 and 60 year-old with a wide range. Most patients for whom data were available had a performance status of 0 (33%) or 1 (44%) (Table 2). The median number of previous lines of chemotherapy ranged from 1 to 8 in the different studies with older studies published before 2001 including mostly first and second line patients while the few studies published more recently including later line patients, mostly third line and beyond. Information on previous treatments was reported in only six studies for anthracyclines and four studies for taxanes. In these studies most patients had previously received the two drugs (Table 2). In two studies from the era after the introduction of capecitabine, previous exposure to this drug was observed in 54% of the patients. ER status of patients was reported in five studies and was positive in 63.6%. Only three studies dated from the era after the introduction of routine use of HER2 evaluation and reported a HER2 positivity of about 27%. The dose and schedule of oral etoposide used most commonly was 50 mg/m2 for 21 days of a 28-day cycle in seven studies, while the two Chinese studies used a dose of 60 mg/m2 for 10 days of a 21-day cycle and two others used a fixed dose of 100 mg for 10 days of a 21-day cycle and of 50 mg for 20 days in a 28-day cycle, respectively.
Figure 1.
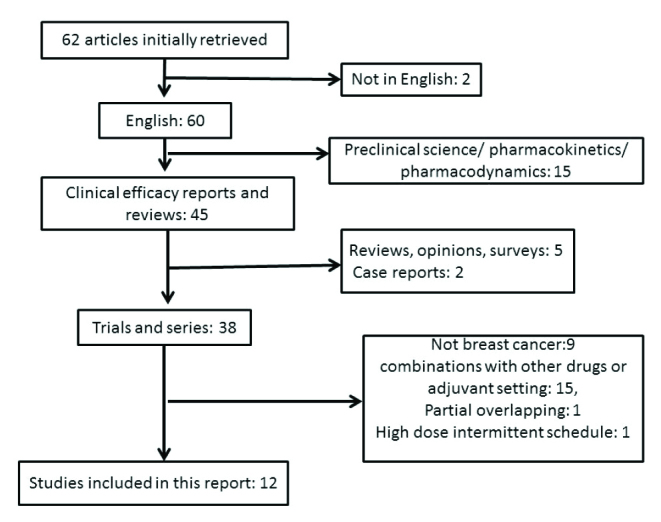
Schematic diagram of studies initially evaluated for the current pooled analysis and reasons for exclusion
Table 1.
The twelve studies included in this pooled analysis of oral daily etoposide in metastatic breast cancer patients. Question mark denotes that CBR is not reported in the study
| Study [Reference] | Year of publication | Country | Number of patients | RR (%) | CBR (%) |
|---|---|---|---|---|---|
| Saphner et al. [8] | 2000 | U.S.A., S. Africa | 30 | 30.0 | ? |
| Pusztai et al. [9] | 1998 | U.S.A. | 30 | 4.2 | 33.3 |
| Atienza et al. [10] | 1995 | U.S.A. | 30 (26 evaluable for response) | 19.2 | 42.3 |
| Palombo et al. [11] | 1994 | Spain | 18 | 22.2 | 55.6 |
| Martin et al. [12] | 1994 | Spain | 43 | 34.9 | ? |
| Bontenbal et al. [13] | 1995 | The Netherlands | 25 | 9.5 | 42.9 |
| Calvert et al. [14] | 1993 | U.K. | 38 | 21.1 | 36.8 |
| Neskovic et al. [15] | 1996 | Serbia | 21 (18 evaluable for response) | 33.3 | 88.9 |
| Jagodic et al. [16] | 2001 | Slovenia | 75 | 37.3 | 50.7 |
| Yuan et al. [17] | 2012 | China | 32 | 25.0 | 68.8 |
| Valabrega et al. [7] | 2015 | Italy | 66 | 4.5 | 37.9 |
| Yuan et al. [18] | 2015 | China | 75 | 9.3 | 48.0 |
CBR: Clinical Benefit Rate; RR: response rate
Table 2.
The twelve studies included in this pooled analysis of oral daily etoposide in metastatic breast cancer patients. Question mark denotes that CBR is not reported in the study
| Pooled studies (%) | Total patients with data | Number of series with data | |
|---|---|---|---|
| Age (median, range) | 50–62 (26–83) | 483 | 12 |
| ECOG PERFORMANCE STATUS | |||
| 0 | 77 (32.9%) | 234 | 6 |
| 1 | 71 (44.1%) | 161 | 5 |
| 2 | 20 (10.5%) | 191 | 6 |
| 3 | 9 (2.8%) | 321 | 9 |
| # PRIOR LINES OF CHEMO | |||
| 0 | 64 (16.5%) | 387 | 10 |
| 1 | 119 (33.3%) | 357 | 9 |
| 2 | 111 (31.1%) | 357 | 9 |
| ≥3 | 97 (25.1%) | 387 | 10 |
| Median # | 0–8 | 483 | 12 |
| Range # | 0–13 | 423 | 10 |
| TYPES OF PRIOR CHEMOTHERAPY | |||
| Anthracyclines | 215 (80.2%) | 268 | 6 |
| Taxanes | 116 (74.8%) | 155 | 4 |
| Capecitabine | 27 (54%) | 50 | 2 |
| Hormonal | 136 (59.1%) | 230 | 6 |
| # ORGANS INVOLVED | |||
| 1 | 54 (31.6%) | 171 | 4 |
| 2 | 49 (32.7%) | 150 | 3 |
| 3 | 33 (22.0%) | 150 | 3 |
| ≥4 | 24 (16.0%) | 150 | 3 |
| SITES INVOLVED | |||
| Visceral | 87 (56.5%) | 154 | 4 |
| Non-visceral only | 67 (43.5%) | 154 | 4 |
| ER STATUS | |||
| Positive | 138 (63.6%) | 217 | 5 |
| Negative | 71 (32.8%) | 217 | 5 |
| Unknown | 8 (3.7%) | 217 | 5 |
| HER2 STATUS | |||
| Positive | 47 (27.2%) | 173 | 3 |
| Negative | 118 (68.2%) | 173 | 3 |
| Unknown | 8 (13.7%) | 173 | 3 |
| Triple negative | 18 (12.8%) | 141 | 2 |
| EFFICACY | |||
| Median OS (months) (95% CI) | 11.7 (9.6–13.8) | 228 | 5 |
| Median PFS or TTP (months) (95% CI) | 3.6 (2.6–4.6) | 303 | 6 |
| RR% (95% CI) | 18.5 (11.5–25.5) | 483 | 12 |
| CBR% (95% CI) | 45.8 (38.6–53.0) | 410 | 10 |
PFS: Progression-Free Survival; ER: Estrogen Receptor; RR: Response Rate; CBR: Clinical Benefit Rate
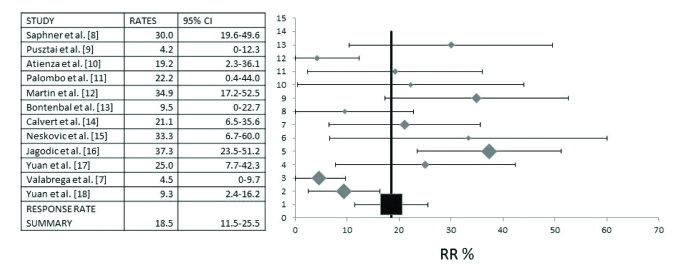
A Response Rate (RR) pooled analysis has included all twelve studies that provided RR data and referred to a total of 466 evaluable patients. Pooled RR was 18.5% (95% CI 11.5–25.5%) (Figure 2). Evaluation for heterogeneity between studies disclosed a high I2 value of 74 (Cochran’s Q=42.44, χ2 p=0.0001). Thus, calculations were made under a random effect model.
Figure 2.
Diagram of pooled analysis of Response Rates (RR) and 95% Confidence Intervals (CI) of studies of oral etoposide in metastatic breast cancer. Twelve studies that included a total of 466 patients that provided information on the RR were analyzed. Overall, RR was 18.5% (95% CI 11.5–25.5%)
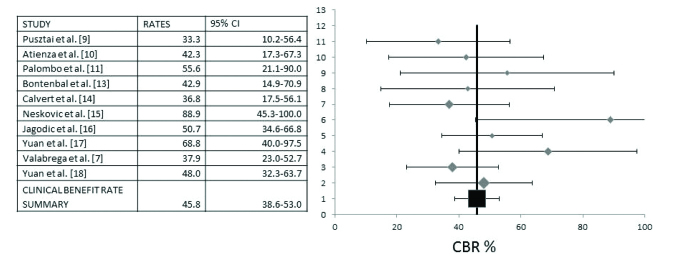
Information on Clinical Benefit Rate (CBR) was provided in ten studies with 393 patients, which formed the basis for a pooled analysis presented in Figure 3. This analysis disclosed a CBR of 45.8% (95% Confidence Interval (CI) 38.6–53.0%). Heterogeneity between studies was low (I2=10.8, Cochran’s Q=10.09, χ2 p=0.34) and both fixed and random models produced similar results. Results presented in Figure 3 depict the analysis with the random effect model.
Figure 3.
Pooled analysis of Clinical Benefit Rates (CBR) and 95% Confidence Intervals (CI). Ten studies with a total of 393 patients that provided information for CBR were included in this analysis. The overall CBR was 45.8% (95% CI 38.6–53.0%)
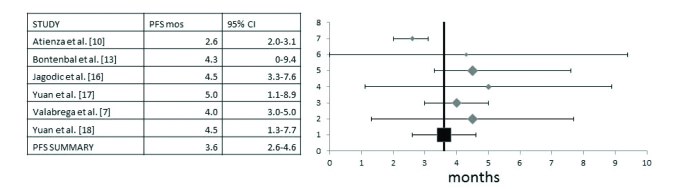
Progression-Free Survival (PFS) data were available in three studies (13, 17, 18) and three additional studies provided Time to Progression (TTP) data instead (7, 10, 16). The total number of patients in these six studies was 295. For the pooled analysis PFS and TTP were analyzed together as, although they are not identical, they are of similar clinical value. Heterogeneity between studies was intermediate (I2=49, Cochran’s Q=9.9, χ2 p=0.07) and a random effect model was used. The pooled PFS derived was 3.6 months (95% CI 2.6–4.6 months) (Figure 4).
Figure 4.
A pooled analysis of Progression-Free Survival (PFS) (three studies with TTP instead) and 95% Confidence Intervals (CI) includes six studies with a total of 295 patients. Overall PFS was 3.6 months (95% CI 2.6–4.6 months)
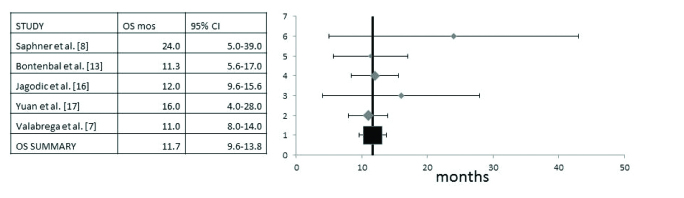
Overall Survival (OS) data were available in five of the studies that included a total number of 224 patients (7, 8, 13, 16, 17). Heterogeneity between studies was low and both random and fixed models gave similar results (I2=0, Cochran’s Q=2.35, χ2 p=0.67). Results presented in Figure 5 show the analysis with the random effect model. The pooled OS was 11.7 months (95% CI 9.6–13.8 months). One of the studies (8) had a clearly higher median OS of 24 months, although with a wide range than the other four. About half of the patients in this trial were treated in the first line metastatic setting, while the others mostly contained patients in second or later line setting.
Figure 5.
Pooled analysis of Overall Survival (OS) and 95% Confidence Intervals (CI). Five studies with a total of 224 patients that provided information for the OS were included. Pooled OS was 11.7 months (95% CI 9.6–13.8 months)
Three studies presented RR according to number of previous chemotherapies (8, 10, 15). In the first of these, RR was 57.1% (eight of 14 patients) in patient treated with first line oral etoposide and 6.25% (one of 16 patients) in patients receiving the drug as a second or later line treatment (8). In another study the respective RR in first and later lines were 20% and 18.75% (10). In the third study that included only patients receiving etoposide as a second or later line treatment, RR were 28.6% (four of 14 patients) in the second line, 35.8% (19 of 53 patients) in the third line, and 54.2% in the fourth or later line (15). Three studies reported on responses in different metastatic sites (11, 12, 15) and noticed that a variety of metastatic sites were among the responding sites. Another study performed a PFS analysis according to metastatic site and reported that patients with visceral metastases had a worse PFS than the rest of the populations in the study (18). No study provided any information on responses of brain metastases to oral etoposide.
Common adverse effects of all grades reported with oral etoposide included alopecia (59%), nausea and other GI toxicities (58.8%), anemia (42.4%), neutropenia (52%), mucositis (31.8%), asthenia/fatigue (28.2%), and anorexia (25%) (Table 3). Neutropenia was the most common grade 3 or 4 toxicity (19.7%) and it was febrile in 7.5% of patients and fatal in 1.7% of patients. No other type of grade 3 or 4 toxicity was observed in more than 10% of patients (Table 3).
Table 3.
Toxicity of oral etoposide in patients in the pooled studies. The third and fifth columns contain information on the total number of patients and number of series the percentage depicted in the second and fourth columns is based on
| % all grades | Total patients with data/series with data | % Grade 3 and 4 | Total patients with data/series with data | |
|---|---|---|---|---|
| TOXICITY | ||||
| Asthenia/fatigue | 28.2 | 392 | NA | 0 |
| Neutropenia | 52.0 | 375/8 | 19.7 | 385/9 |
| Febrile neutropenia | 7.5% (1.7 gr 5) | 173/4 | ||
| Anemia | 42.4 | 375/8 | 5.5 | 398/9 |
| Thrombocytopenia | 9.9 | 375/8 | 3.7 | 428/10 |
| Peripheral Neuropathy | 10.3 | 107/2 | 0 | 107/2 |
| Nausea/GI toxicity | 58.8 | 308/8 | 6.2 | 289/7 |
| Mucositis | 31.8 | 267/6 | 2.2 | 184/3 |
| Alopecia | 59.0 | 273/8 | NA | |
| Anorexia | 25.0 | 54/2 | 2.9 | 171/3 |
| Transaminitis | 7.5% | 173/3 | 0 | 141/2 |
Discussion and Conclusion
Despite increasing options for the systemic treatment of patients with metastatic breast cancer, low dose etoposide remains a valid option for later line treatment of these patients, preferred by some clinicians for its ease of administration by the oral route that may favor quality of life and avoids clinic visits and drug infusions. The low, protracted mode of dosing of chemotherapeutic drugs, mostly with daily oral administration, often referred to as metronomic, is proposed to have an indirect effect on tumor progression through interference with neovascularization (19). This dosing produces different pharmacokinetic levels and clinical effects than the intravenous administration of etoposide used in other settings such as in regimens with cisplatin for the treatment of Small Cell Lung Cancer or Germ Cell Tumors (20, 21).
Etoposide is a podophyllotoxin derivative antineoplastic drug that works as an inhibitor of topoisomerase II (topo II). Inhibition of the alpha isoform of the enzyme by the drug results in stabilization of double stranded DNA cleavage sites induced by topo II and delays transition of cells through the S phase of the cell cycle and leads to cycle arrest in the G2 phase (22). Oral low dose protracted administration of etoposide was introduced in the 1990s based on data from few small phase II studies with a number of patients ranging up to few dozens in each (8–15). These have predated the introduction of more modern options such as capecitabine, vinorelbine and eribulin and included, in general, patients with few lines of previous chemotherapies in the metastatic setting (mostly 0 to 2). They confirmed the ease of administration and acceptable toxicity profile; although a low percentage of high grade toxicities and even rare fatalities from sepsis were also observed (14). More recently, a revival of the interest in oral etoposide has been seen in the literature with a few additional phase II studies and a retrospective series including now later line patients, given that other options are available (7, 17, 18).
This report pools all available studies on oral etoposide in metastatic breast cancer that used a metronomic mode of administration with daily doses in general ranging from 50 mg/m2 to a fixed daily dose of 100 mg in order to reach a more accurate estimation of these regimens efficacy and toxicity. This analysis confirms a modest PFS/TTP with oral etoposide of 3.6 months and OS of just below one year. A pooled RR rate estimation of about 18% and CBR of about 45% confirm the clinical impression that some patients derive a benefit from the drug. Nevertheless, it should be noted that since several of the studies have been performed in patients with fewer lines of treatment than current patient populations, who have mostly several lines of metastatic treatment before etoposide, a lower RR and CBR may be expected. Older studies performed in the 1990s used the 50 mg/m2 for 21 days of a 28-day cycle dosing, while the four studies done after 2000 used either a fixed dose of 50 or 100 mg or a dosing of 60 mg/m2 for 10 days of a 21-day cycle. Although comparisons between studies are difficult and there is variability of RR even in studies that used the same dose and schedule, it appears that no significant effect of dose exists. This may be due to the fact that the various dosing and schedules result in small overall difference in dose density received (e.g. total dose 600 mg/m2 in 21-days with the 60 mg/m2 for 10 days of a 21-day cycle and total dose 760 mg/m2 in 21-days with the 50 mg/m2 for 21 days of a 28-day cycle).
In the last decade several new chemotherapy options have been added to the later line metastatic breast cancer armamentarium including capecitabine, vinorelbine, gemcitabine and eribulin. With the caveats that inter-trials comparisons always entail, the current data suggest that oral etoposide remain a valid option with similar efficacy in metastatic breast cancer. For example a recent analysis of retrospective series of eribulin in pretreated metastatic breast cancer has disclosed a RR of 20%, a CBR of 46%, a pooled PFS OF 3.8 months and OS of 9.7 months, all very similar with the respective results for oral etoposide (23). In our series of metastatic breast cancer patients treated with vinorelbine in the first or later line setting, RR was 37% overall but only 12% as a second or later line treatment (24). The median TTP was six months for the whole series independently of line of treatment. A meta-analysis of twenty two studies of chemotherapy labeled metronomic in metastatic breast cancer patients was recently published (25). Treatments in the summarized studies were heterogeneous and included cyclophosphamide, methotrexate, vinorelbine and capecitabine among others alone or in combinations. No studies with oral etoposide were included. Pooled RR was 34.1% and the OS at 6 months 70% (25). Although these results appear to be somewhat better that the rates obtained with oral etoposide, patients were probably less heavily pre-treated, at least in some studies, and received combination with non-metronomic schedules in several of the included studies in this meta-analysis.
Oral etoposide in the doses and schedules used in the twelve pooled studies showed a manageable toxicity profile (Table 3). Although most patients experienced some toxicity of any grade, rates of grade 3 and 4 toxicities were low. Most common grade 3 and 4 toxicity was febrile neutropenia that was observed in almost 20% of patients for whom information was available and febrile neutropenia was present in 7.5% of patients. This is similar, for example, with the grade 3 and 4 neutropenia and febrile neutropenia rates observed with eribulin in heavily pretreated metastatic breast cancer patients (28.1% and 5.4% of patients respectively) in a similar pooled analysis (23).
There are limitations of the current analysis as already alluded to. First several of the included studies date from the nineties, they are small in size without comparison arms and their patient population is different from the patient population that would most probably be treated with oral etoposide today, as there are additional options, including oral targeted therapies. Nevertheless, the current analysis suggests that the overall efficacy of oral etoposide is probably not very different from other chemotherapy options available for later line metastatic breast cancer and given that it is cheaper than other alternatives, it represents a high benefit to cost option in the current financially conscious health systems environment. Additionally, oral etoposide could be a valid option for health systems with sparse resources.
The current analysis confirms that the older regimen of low dose oral etoposide given in a protracted manner has a modest benefit for patients with metastatic breast cancer, in general similar to other more recently introduced options and an acceptable adverse effect profile.
Thus, it may still be considered in this clinical scenario if active palliative treatment is warranted.
Footnotes
Ethics Committee Approval: Ethics committee approval was not requested for this study.
Informed Consent: Informed consent was not requested for this study.
Peer-review: Externally peer-reviewed.
Conflict of Interest: No conflict of interest was declared by the authors.
Financial Disclosure: The authors declared that this study has received no financial support.
References
- 1.Liedtke C, Kolberg HC. Systemic therapy of advanced/metastatic breast cancer-Current evidence and future concepts. Breast Care. 2016;11:275–281. doi: 10.1159/000447549. https://doi.org/10.1159/000447549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. https://doi.org/10.1186/1471-2288-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Br Med J. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. https://doi.org/10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neyeloff JL, Fuchs SC, Moreira LB. Meta-analyses and Forest plots using a microsoft excel spreadsheet: step-by-step guide focusing on descriptive data analysis. BMC Res Notes. 2012;5:52. doi: 10.1186/1756-0500-5-52. https://doi.org/10.1186/1756-0500-5-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martín M, Casado A, Lluch A, Adrover E, Diaz-Rubio E, García-Conde J. Preliminary results of a phase II trial of chronic oral etoposide in breast cancer. Cancer Treat Rev. 1993;19:47–52. doi: 10.1016/0305-7372(93)90047-u. https://doi.org/10.1016/0305-7372(93)90047-U. [DOI] [PubMed] [Google Scholar]
- 6.Erkisi M, Bilkay BC, Seyrek E, Hazar B, Burgut R. Refractory breast cancer: A comparison of two different chemotherapy regimens. J Chemother. 1997;9:442–445. doi: 10.1179/joc.1997.9.6.442. https://doi.org/10.1179/joc.1997.9.6.442. [DOI] [PubMed] [Google Scholar]
- 7.Valabrega G, Berrino G, Milani A, Aglietta M, Montemurro F. A retrospective analysis of the activity and safety of oral etoposide in heavily pretreated metastatic breast cancer patients. Breast J. 2015;21:241–245. doi: 10.1111/tbj.12398. https://doi.org/10.1111/tbj.12398. [DOI] [PubMed] [Google Scholar]
- 8.Saphner T, Weller EA, Tormey DC, Pandya KJ, Falkson CI, Stewart J, Robert NJ. 21-day oral etoposide for metastatic breast cancer: A phase II study and review of the literature. Am J Clin Oncol. 2000;23:258–262. doi: 10.1097/00000421-200006000-00010. https://doi.org/10.1097/00000421-200006000-00010. [DOI] [PubMed] [Google Scholar]
- 9.Pusztai L, Walters RS, Valero V, Boehnke-Michaud L, Buzdar AU, Hortobagyi GN. Daily oral etoposide inpatients with heavily pretreated metastatic breast cancer. Am J Clin Oncol. 1998;21:442–446. doi: 10.1097/00000421-199810000-00004. https://doi.org/10.1097/00000421-199810000-00004. [DOI] [PubMed] [Google Scholar]
- 10.Atienza DM, Vogel CL, Trock B, Swain SM. Phase II study of oral etoposide for patients with advanced breast cancer. Cancer. 1995;76:2485–2490. doi: 10.1002/1097-0142(19951215)76:12<2485::aid-cncr2820761212>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 11.Palombo H, Estapé J, Vi-olas N, Grau JJ, Ma-é JM, Daniels M, Mellado B. Chronic oral etoposide in advanced breast cancer. Cancer Chemother Pharmacol. 1994;34:527–529. doi: 10.1007/BF00686513. https://doi.org/10.1007/BF00686513. [DOI] [PubMed] [Google Scholar]
- 12.Martín M, Lluch A, Casado A, Santabárbara P, Adrover E, Valverde JJ, López-Martín JA, Rodriguez-Lescure A, Azagra P, García-Conde J, Diaz-Rubio E. Clinical activity of chronic oral etoposide in previously treated metastatic breast cancer. J Clin Oncol. 1994;12:986–991. doi: 10.1200/JCO.1994.12.5.986. https://doi.org/10.1200/JCO.1994.12.5.986. [DOI] [PubMed] [Google Scholar]
- 13.Bontenbal M, Planting AST, Verweij J, de Wit R, Kruit WHJ, Stoter G, Klijn JGM. Second-line chemotherapy with long-term low-dose oral etoposide in patients with advanced breast cancer. Breast Cancer Res Treat. 1995;34:185–189. doi: 10.1007/BF00665790. https://doi.org/10.1007/BF00665790. [DOI] [PubMed] [Google Scholar]
- 14.Calvert AH, Lind MJ, Millward MM, Cantwell BMJ, Gumbrell L, Proctor M, Simmons D, Chapman F, Robinson A, Charlton C, Balmanno K, Newell D. Long-term oral etoposide in metastatic breast cancer: clinical and pharmacokinetic results. Cancer Treat Rev. 1993;19:27–33. doi: 10.1016/0305-7372(93)90045-s. https://doi.org/10.1016/0305-7372(93)90045-S. [DOI] [PubMed] [Google Scholar]
- 15.Nešković-Konstantinović ZB, Bošnjak SM, Radulović SS, Mitrović L. Daily oral etoposide in metastatic breast cancer. Anti-cancer Drugs. 1996;7:543–547. doi: 10.1097/00001813-199607000-00009. https://doi.org/10.1097/00001813-199607000-00009. [DOI] [PubMed] [Google Scholar]
- 16.Jagodic M, Cufer T, Zakotnik B, Cervek J. Selection of candidates for oral etoposide salvage chemotherapy in heavily pretreated breast cancer patients. Anti-cancer Drugs. 2001;12:199–204. doi: 10.1097/00001813-200103000-00004. https://doi.org/10.1097/00001813-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Yuan P, Xu B, Wang J, Ma F, Fan Y, Li Q, Zhang P. Oral etoposide monotherapy is effective for metastatic breast cancer with heavy prior therapy. Chin Med J. 2012;125:775–779. [PubMed] [Google Scholar]
- 18.Yuan P, Di L, Zhang X, Yan M, Wan D, Li L, Zhang Y, Cai J, Dai H, Zhu Q, Hong R, Xu B. Efficacy of oral etoposide in pretreated metastatic breast cancer. Medicine. 2015;94:e774. doi: 10.1097/MD.0000000000000774. https://doi.org/10.1097/MD.0000000000000774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Digklia A, Voutsadakis IA. Combinations of VEGF pathway inhibitors with metronomic chemotherapy: Rational and current status. World J Exp Med. 2014;4:58–67. doi: 10.5493/wjem.v4.i4.58. https://doi.org/10.5493/wjem.v4.i4.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nichols C, Kollmannsberger C. First-line chemotherapy of disseminated germ cell tumors. Hematol Oncol Clin North Am. 2011;25:543–556. doi: 10.1016/j.hoc.2011.03.011. https://doi.org/10.1016/j.hoc.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Lara PN, Jr, Natale R, Crowley J, Lenz HJ, Redman MW, Carleton JE, Jett J, Langer CJ, Kuebler JP, Dakhil SR, Chansky K, Gandara DR. Phase III trial of irinotecan/cisplatin compared with etoposide/cisplatin in extensive-stage small-cell lung cancer: clinical and pharmacogenomics results from SWOG S0124. J Clin Oncol. 2009;27:2530–2535. doi: 10.1200/JCO.2008.20.1061. https://doi.org/10.1200/JCO.2008.20.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang J, Bogni A, Schuetz EG, Ratain M, Dolan ME, McLeod H, Gong L, Thorn C, Relling MV, Klein TE, Altman RB. Etoposide pathway. Pharmacogenet Genomics. 2009;19:552–553. doi: 10.1097/FPC.0b013e32832e0e7f. https://doi.org/10.1097/FPC.0b013e32832e0e7f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Voutsadakis IA. A systematic review and pooled analysis of retrospective series of eribulin in metastatic breast cancer. Anti-Cancer Drugs. 2017;28:557–564. doi: 10.1097/CAD.0000000000000493. https://doi.org/10.1097/CAD.0000000000000493. [DOI] [PubMed] [Google Scholar]
- 24.Stravodimou A, Zaman K, Voutsadakis IA. Vinorelbine with or without trastuzumab in metastatic breast cancer: A retrospective single institution series. ISRN Oncol. 2014;2014:289836. doi: 10.1155/2014/289836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y, Gu F, Liang J, Dai X, Wan C, Hong X, Zhang K, Liu L. The efficacy and toxicity profile of metronomic chemotherapy for metastatic breast cancer: A meta-analysis. PLoS ONE. 2017;12:e01773693. doi: 10.1371/journal.pone.0173693. https://doi.org/10.1371/journal.pone.0173693. [DOI] [PMC free article] [PubMed] [Google Scholar]