Abstract
This study was conducted to investigate whether regular dietary intake of L-arginine could affect the occurrence of metabolic syndrome (MetS). Eligible adult men and women (n=1,237), who participated in the Tehran Lipid and Glucose Study, were followed for a median of 6.3 years. Dietary intakes of L-arginine and serum nitrate and nitrite (NOx) concentration were assessed at baseline (2006~2008), and demographics, anthropometrics, and biochemical variables were evaluated at baseline and follow-up examinations. The occurrence of MetS was assessed in relation to total L-arginine, intakes of L-arginine from animal and plant sources, with adjustment of potential confounding variables. Participants who had higher intake of L-arginine also had higher serum NOx at baseline (35.0 vs. 30.5 μmol/L, P<0.05). After 6 years of follow-up, higher intakes of L-arginine from animal sources were accompanied with increased risk of MetS [odd ratios (OR)=1.49, 95% confidence interval (95% CI)=1.02~2.18]. Compared to the lowest, the highest intakes of L-arginine from plant sources were related to significantly reduced risk of MetS (OR=0.58, 95% CI=0.32~0.99). In conclusion, our findings suggest a potentially protective effect of plant derived L-arginine intakes against development of MetS and its phenotypes; moreover, higher intakes of L-arginine from animal sources could be a dietary risk factor for development of metabolic disorders.
Keywords: L-arginine, nitric oxide, metabolic syndrome
INTRODUCTION
L-Arginine, a conditionally essential amino acid, is the main substrate of nitric oxide (NO) synthase (NOS) family enzymes and is responsible for production of the endothelium-derived relaxing factor NO (1,2). Mean dietary intake of L-arginine is reported as 4~6 g/d (3,4). Amounts of L-arginine in different protein sources range from 3~15%, and soy protein, peanuts, walnuts, and fish are relatively rich in L-arginine whereas cereal proteins are poor sources (3~4% of total amino acids) (5). No recommended dietary allowance has yet been defined for L-arginine intake and differences in dietary patterns between populations may be responsible for differences in the mean intakes and plasma levels of L-arginine worldwide (5–8).
Several clinical studies indicated beneficial outcomes of L-arginine supplementation on diabetes, insulin resistance, hypertension, and vascular dysfunction (9–12). Data on the effects of L-arginine supplementation on improvement of NO production are inconsistent (13–18).
The L-arginine-NO pathway is involved in many physiological processes and there is a growing body of evidence indicating that an impaired L-arginine-NO pathway may be an important determinant for development of cardiometabolic disorders, namely vascular dysfunction, cardiovascular disease, chronic kidney disease, endocrine disorders, insulin resistance, type 2 diabetes, and metabolic syndrome (MetS) (8,19–22). Due to the importance of NO in regulation of oxidative stress and inflammatory processes, an impaired L-arginine-NO pathway is considered a main risk for development of MetS (8). Dietary intakes of L-arginine in two cohort studies had no significant association with the incidence of hypertension, acute coronary heart disease (CHD), or cardiovascular mortality (23,24). We recently, in a 5-year follow-up of 2,284 adults, showed that higher intake of animal-derived L-arginine may be a risk factor for development of CHD events (25).
In this study, we aimed to evaluate the association of dietary total L-arginine intakes and two different sources of L-arginine, both animal and plant sources, and the occurrence of MetS after a median 6.3 years of follow-up in a national representative population.
SUBJECTS AND METHODS
Study population
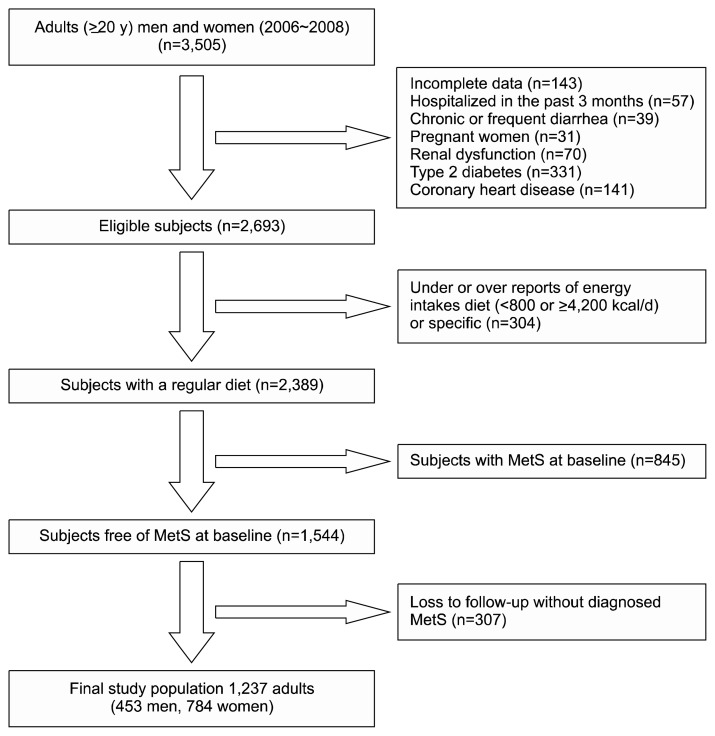
This study was conducted within the framework of the Tehran Lipid and Glucose Study (TLGS) (26). According to exclusion criteria, 1,237 adults (453 men, 784 women), aged 20~84 years, were included in the final analyses (Fig. 1). Written informed consents were obtained from all participants and the study protocol was approved (ethics committee number: 57ECRIES94/02/15) by the ethics research council of the Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences. The study protocol was conducted according to the principles of the Declaration of Helsinki.
Fig. 1.
Flowchart of the study population. MetS, metabolic syndrome.
Details of demographics, anthropometrics, and biochemical measures were reported elsewhere (26). Baseline measurements were conducted in 2006~2008; second and third examinations were carried out in 2009~2011, and 2012~2014, respectively.
Laboratory assays
Fasting blood samples were taken after 12~14 h from all study participants at baseline and again at the second and third follow-up examinations. Fasting blood glucose (FBG) was measured by the enzymatic colorimetric method using glucose oxidase. Enzymatic colorimetric analysis with glycerol phosphate oxidase was used to measure triglyceride (TG) levels. High-density lipoprotein cholesterol (HDL-C) was measured after precipitation of the apolipoprotein B containing lipoproteins with phosphotungstic acid. Analyses were performed using Pars Azmoon kits (Pars Azmoon Inc., Tehran, Iran) and a Selectra 2 auto-analyzer (Vital Scientific, Spankeren, Netherlands). Inter- and intra-assay coefficients of variation of all assays were <5%.
Serum creatinine levels were assayed using the kinetic colorimetric Jaffe method. Serum nitrate and nitrite (NOx) concentration was measured by a rapid, simple spectrophotometric method (27–30).
Fasting serum insulin was measured in a subgroup of participants (n=1,141) at baseline and again in the second examination by the electrochemiluminescence immunoasaay, using Roche Diagnostics kits and the Roche/Hitachi Cobas e-411 analyzer (Roche Diagnostics GmbH, Mannheim, Germany). The intra- and inter-assay coefficients of variation for insulin were 1.2 and 3.5 %, respectively. Homeostatic model assessment of insulin resistance (HOMA-IR) was also determined, as a simple and validated alternative tool for assessment of insulin resistance in epidemiological studies, by the following formula: fasting insulin (μU/mL)×fasting glucose (mmol/L)/22.5 (31,32).
Dietary intakes
A validated (33) 168-item food frequency questionnaire (FFQ) was used to assess in typical food intakes, and to estimate total L-arginine intakes and L-arginine from animal and plant sources, over the previous year. Trained dietitians, with at least 5 years of experience in the TLGS survey, asked participants to designate their intake frequency for each food item consumed during the past year on a daily, weekly, or monthly basis. Portion sizes of consumed foods reported in household measures were then converted to grams (34). We used the US Department of Agriculture Food Composition Table to analyze foods and beverages for their energy and nutrient content.
Definitions
Metabolic syndrome components were defined according to diagnostic criteria proposed by National Cholesterol Education Program–Adult Treatment Panel III (35), and the latest cutoff points of waist circumference for Iranian adults (36). Participants were considered to have metabolic syndrome at baseline if they have at least 3 of the metabolic abnormalities: 1) Hyperglycaemia as FBG ≥100 mg/dL (5.6 mmol/L) or drug treatment of impaired fasting glucose, 2) Hypertriglyceridemia as serum triglycerides ≥150 mg/dL (1.69 mmol/L) or drug treatment, 3) Low HDL-C as serum HDL-C <40 mg/dL (1.04 mmol/L) for men, and <50 mg/dL (1.29 mmol/L) for women or drug treatment, 4) Hypertension as blood pressure ≥130/85 mmHg or drug treatment for hypertension, and 5) Abdominal obesity as waist circumference ≥95 cm for both genders; names of MetS phenotypes, represented by any three or more combinations of the five MetS components, were defined in combination of following letters: W, elevated waist circumference; G, elevated blood glucose; T, elevated triglyceride levels; B, elevated blood pressure; H, low-HDL-C. Diabetes was defined as fasting serum glucose ≥126, 2 h serum glucose ≥200, or anti-diabetic medications (37). According to the World Health Organization classification, menopause was defined as the absence of spontaneous menstrual bleeding for over 12 months, for which no other pathologic or physiologic cause could be determined (38). Coronary heart disease was defined as definite myocardial infarction (MI) [with diagnostic electrocardiogram (ECG) and biomarkers], probable MI (positive ECG findings plus cardiac symptoms or signs plus missing biomarkers or positive ECG findings plus equivocal biomarkers), unstable angina (new cardiac symptoms or changing symptom patterns and positive ECG findings with normal biomarkers), angiographic proven CHD, and death from CHD (39).
Statistical analysis
Log-transformed variables with non-normal distribution (serum NOx and TG) were used in the analyses. Mean [±standard deviation (SD)] values and the proportions of baseline characteristics of the participants with and without the MetS, were compared, using the independent sample t test or chi-square test, respectively.
Dietary intake of L-arginine was adjusted for total energy intake, based on the residuals method (40). Dietary intakes of L-arginine were categorized into quartiles. The percentages of participants according to MetS phenotypes and L-arginine from animal and plant sources (quartile 4 vs. quartiles 1, 2, and 3) were analyzed using the chi-square test.
A univariate analyse was performed for each potential confounder including age (y), sex (male/female), MetS score (summing up of MetS risk factors including abdominal obesity, low-HDL-C, hyper-triglyceridemia, hypertension, and disglycemia), serum creatinine, menopause status (yes/no), using of medications (yes/no), smoking (yes/no), energy intakes (kcal/d), and dietary intakes of protein (g/d), carbohydrates (g/d), total fats (g/d), and fibre (g/d). Variables with PE (P for entry) <0.2 in the univariate analyses were selected for the multivariable models; PE determines which variables should be included in the final multivariable model.
To investigate the association of L-arginine intakes and changes of serum insulin and HOMA-IR, linear regression models were used with adjustment of the above mentioned confounding variables. Multivariable logistic regression models with adjustment for potential confounders were used to determine the incidence of MetS across quartiles of total L-arginine, and intakes of L-arginine from animal and plant sources.
All statistical analyses were conducted using SPSS (version 16.0, SPSS Inc., Chicago, IL, USA), and P-values <0.05 were considered significant.
RESULTS
General characteristics of the participants
Mean age of participants (36.9% men) was 41.7±14.6 years. The cumulative incidence of MetS phenotype was 29.2% (34.6% in men, 26.1% in women) after a median follow-up of 6.3 years.
Baseline characteristics of the study population are presented in Table 1. Participants with diagnosed MetS were more likely to be older, and had higher body mass index, waist circumference, triglyceride levels, systolic blood pressure, diastolic blood pressure, serum creatinine, and lower HDL-C, at baseline. Dietary L-arginine intakes did not differ between subjects with and without MetS, but a higher serum NOx concentrations (27.6 vs. 25.5 μmol/L, P=0.001) was observed in subjects with MetS.
Table 1.
Baseline characteristics of the participants (n=1,237)
| MetS− | MetS+ | P | |
|---|---|---|---|
| Age at baseline (y) | 39.8±14.4 | 46.4±14.1 | 0.001 |
| Men (%) | 34.1 | 43.7 | 0.001 |
| Smoking (%) | 6.9 | 11.1 | 0.011 |
| Body mass index (kg/m2) | 25.3±3.9 | 28.0±3.5 | 0.001 |
| Waist circumference (cm) | 83.8±10.9 | 93.1±9.6 | 0.001 |
| Fasting blood glucose (mg/dL) | 87.9±16.9 | 94.1±22.0 | 0.001 |
| Serum triglycerides1) (mg/dL) | 97.5 (94.6~100) | 136 (129~141) | 0.001 |
| HDL-C (mg/dL) | 46.7±10.7 | 41.2±9.0 | 0.001 |
| Systolic blood pressure (mm Hg) | 108±14.4 | 115±15.2 | 0.001 |
| Diastolic blood pressure (mm Hg) | 69.2±9.05 | 73.6±8.8 | 0.001 |
| Serum creatinine | 1.03±0.16 | 1.05±0.15 | 0.044 |
| Serum NOx1) (μmol/L) | 25.5 (24.5~26.3) | 27.6 (26.0~29.1) | 0.001 |
| Total L-arginine (g/d) | 4.1±1.5 | 4.0±1.5 | 0.55 |
| L-arginine from animal sources (g/d) | 1.7±0.94 | 1.9±1.0 | 0.12 |
| L-arginine from plant sources (g/d) | 2.3±1.8 | 2.1±1.8 | 0.18 |
MetS, metabolic syndrome; HDL-C, high-density lipoprotein cholesterol; NOx, nitrate and nitrite.
Data are mean±SD.
Data are geometric mean (95% confidence interval).
Dietary information
Mean dietary intakes of protein and L-arginine were 78.1 ±28.1 g/d and 4.08±1.46 g/d, respectively. Mean (SD) intake of L-arginine from animal and plant sources was 1.82±0.96 and 2.26±1.81 g/d, respectively. Lowest and highest categories of L-arginine intake, defined as the 10th and 90th percentile, were <2.42 and ≥6.11 g/d, respectively. Mean dietary intake of L-arginine to total protein ratio was 0.052±0.02, with 50.7±33.1% of total L-arginine intakes being from animal sources. At baseline, serum NOx concentration was 30.5, 31.4, 32.6, and 35.0 μmol/L in the first, second, third, and fourth quartile categories of total L-arginine intake.
Table 2 shows dietary intakes of the participants across quartiles for total L-arginine intakes, indicating no significant difference in L-arginine intakes from animal sources across total L-arginine quartiles, whereas the relative amount of plant sources of L-arginine increased across increasing intakes of total L-arginine (4.37 vs. 0.64 g/d, P<0.01). Dietary intakes of energy, protein and fibre also increased across quartile categories of total L-arginine (P<0.01).
Table 2.
Dietary intakes of the participants across quartiles of total L-arginine intakes
| Q1 | Q2 | Q3 | Q4 | |
|---|---|---|---|---|
| Total arginine (g/d) | ||||
| Range | <3.03 | 3.03~3.84 | 3.84~4.91 | ≥4.91 |
| Median | 2.55 | 3.45 | 4.34 | 5.72 |
| Arginine from animal sources (g/d) | 1.82±0.95 | 1.83±0.93 | 1.89±1.00 | 1.73±0.97 |
| Arginine from vegetable sources (g/d) | 0.64±1.02 | 1.60±0.96 | 2.46±1.05 | 4.37±1.59** |
| Energy intake (kcal/d) | 1,533±362 | 2,050±384 | 2,508±463 | 3,118±565** |
| Carbohydrate (% energy) | 57.3±7.7 | 57.2±7.1 | 57.8±6.8 | 56.9±7.4 |
| Protein (% energy) | 12.8±2.1 | 13.2±2.2 | 13.6±2.2 | 14.7±2.6** |
| Total fats (% energy) | 32.0±7.8 | 31.9±7.5 | 31.1±6.5 | 30.9±6.6 |
| Total fibre (g/d) | 34.2±1.2 | 35.2±0.9 | 40.1±1.0 | 40.3±1.2** |
Data are mean±SD.
P<0.01 (analysis of variance or analysis of covariance with adjustment of total energy intake was used).
Metabolic syndrome and dietary L-arginine
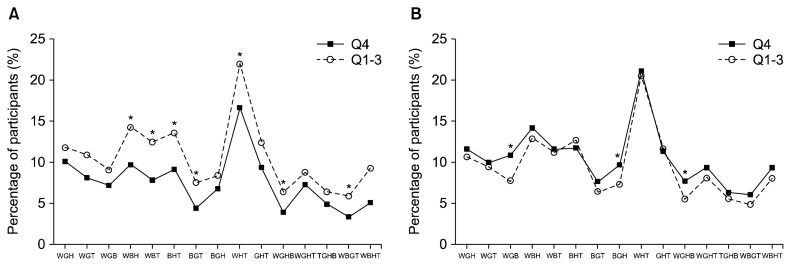
The prevalence of MetS phenotypes across quartiles of L-arginine from plant sources are illustrated in Fig. 2A. Subjects in the highest compared to the lowest quartiles, had a significantly lower prevalence of WBH (9.7 vs. 14.3 %, P=0.002), WBT (7.8 vs. 12.5, P=0.002), BHT (9.2 vs. 13.6, P=0.005), BGT (4.4 vs. 7.6, P=0.008), WHT (16.7 vs. 22.0, P=0.007), WGHB (3.9 vs. 6.4, P=0.031), and WBGT (3.4 vs. 5.9, P=0.02).
Fig. 2.
The prevalence of metabolic syndrome (MetS) phenotypes across quartiles of L-arginine from plant sources (A) and animal sources (B). W, elevated waist circumference; G, elevated blood glucose; T, elevated triglyceride levels; B, elevated blood pressure; H, low-high-density lipoprotein cholesterol.
The prevalence of MetS phenotypes across quartiles of L-arginine from animal sources are illustrated in Fig. 2B. Subjects in the highest compared to the lowest quartiles, had a significant higher prevalence of WGB (10.9 vs. 7.8 %, P<0.05), BGH (9.7 vs. 7.4%, P<0.05), and WGHB (7.7 vs. 5.5%, P<0.05).
There was an inverse association for intakes of L-arginine from plant sources and changes of serum insulin [β=−2.59, 95% confidence interval (95% CI)=−5.32 and −0.12] and HOMA-IR (β=−2.82, 95% CI=−4.49, and −1.13); total intakes of L-arginine and L-arginine from animal sources had no significant association between changes of serum insulin and HOMA-IR (data are not shown).
The occurrence of MetS across quartiles of L-arginine intakes is presented in Table 3. After adjustment of all potential confounding variables, a non-significant decreasing risk of MetS was observed in the highest, compared to the lowest, quartile of L-arginine intakes [odd ratios (OR)=0.83, 95% CI=0.38~1.31]. Higher intakes of L-arginine from animal sources were accompanied with increased risk of MetS (OR=1.49, 95% CI=1.02~2.18), whereas from plant sources, compared to the lowest intakes of L-arginine, the highest were related to a significantly reduced risk of MetS (OR=0.58, 95% CI=0.32~0.99).
Table 3.
The occurrence of MetS across quartiles of total L-arginine, intakes of L-arginine from animal and plant sources (n=1,237)
| Q1 | Q2 | Q3 | Q4 | |
|---|---|---|---|---|
| Total L-arginine (g/d) | <3.03 | 3.03~3.84 | 3.84~4.91 | ≥4.91 |
| Model 1 | 1 | 1.1 (0.76~1.53) | 0.99 (0.69~1.41) | 0.96 (0.67~1.36) |
| Model 2 | 1 | 1.22 (0.84~1.76) | 1.01 (0.69~1.47) | 0.93 (0.64~1.36) |
| Model 3 | 1 | 1.21 (0.83~1.74) | 0.98 (0.68~1.44) | 0.83 (0.38~1.31) |
| L-arginine from animal sources (g/d) | <1.19 | 1.19~1.63 | 1.63~2.19 | ≥2.19 |
| Model 1 | 1 | 0.88 (0.61~1.27) | 1.09 (0.77~1.56) | 1.28 (0.91~1.82) |
| Model 2 | 1 | 0.85 (0.59~1.24) | 1.08 (0.75~1.55) | 1.28 (0.89~1.83) |
| Model 3 | 1 | 0.88 (0.59~1.31) | 1.22 (0.83~1.80) | 1.49 (1.02~2.18) |
| L-arginine from plant sources (g/d) | <1.14 | 1.14~2.08 | 2.08~3.31 | ≥3.31 |
| Model 1 | 1 | 1.15 (0.81~1.62) | 0.96 (0.67~1.37) | 0.82 (0.56~1.18) |
| Model 2 | 1 | 1.11 (0.78~1.58) | 0.93 (0.65~1.33) | 0.81 (0.57~1.17) |
| Model 3 | 1 | 1.12 (0.76~1.65) | 0.85 (0.55~1.32) | 0.58 (0.32~0.99) |
Data are odds ratio (95% confidence interval).
Model 1, crude model; Model 2, adjusted for age and sex; Model 3, additional adjustment for baseline metabolic syndrome (MetS) score (summing up of MetS risk factors including abdominal obesity, low-high-density lipoprotein cholesterol, hyper-triglyceridemia, hypertension, and disglycemia), serum creatinine, smoking, use of medication, menopause status, energy intakes, fiber, fats, and protein intakes.
Median of total L-arginine intakes was 2.54, 3.46, 4.34, and 5.74, in the Q1, Q2, Q3, and Q4, respectively.
DISCUSSION
Our findings showed a non-significant decreasing trend in the occurrence of MetS in subjects who had a regular L-arginine intakes ≥4.91 g/d. We also assessed the risk of MetS across animal and plant sources of L-arginine; our findings showed a protective effect of plant sources of L-arginine against the development of MetS whereas higher intakes of L-arginine from animal sources were accompanied with increased occurrence of MetS. Plant sources of L-arginine were also inversely related to changes of serum insulin and insulin resistance index during the follow-up. The prevalence of main MetS phenotypes was also lower in the highest compared to the lowest L-arginine intakes from plant sources.
The possible modulatory effects of L-arginine supplementation on NO-mediated pathways is currently considered as an effective strategy for prevention and treatment of MetS and its phenotypes including abdominal obesity, type 2 diabetes and dyslipidemia (41) whereas limited studies have examined the association of regular dietary intakes of L-arginine and the risk of cardio-metabolic disorders. L-arginine intake below the median range (3.8 g/d) was associated with higher levels of C reactive protein (CRP) and highest level of L-arginine intake (>7.5 g/d) were related to 30% less likely to have a CRP above 3.0 mg/L; moreover, a lower prevalence of elevated SBP and LDL-C was also observed in subjects who consumed >7.5 g/d L-arginine (4). Findings of a population-based cohort did not support the hypothesis that dietary arginine intake may lower the risk of coronary heart disease mortality (24). Similarly, in a 10-year follow-up of participants of Kuopio Ischemic Heart Disease Risk Factor Study, total L-arginine intake had no association with blood pressure or the risk of acute coronary events (23). In this study, the portion of animal derived L-arginine was higher than plant derived and there was an increasing trend in animal derived L-arginine across quartiles of total intakes of L-arginine (23).
It seems that animal and plant sources of L-arginine may induce different physiological effects in the body; it has been suggested that utilization of plant derived L-arginine is better than animal derived because higher ratio of lysine to L-arginine in animal proteins and competition of lysine with L-arginine for the same plasma membrane transport mechanism (42). It should also be noted that the different effects of animal- and plant-sources of L-arginine on metabolic disorders, observed in our study, may be due to different composition of animal and plant proteins or other nutrients such as fatty acids in animal-based foods or fibre and phytochemicals in plant-based foods. To statistically modify these dietary potential confounders, we therefore adjusted dietary intakes of protein, fibre and fats in logistic regression models.
The strengths of the current study were a population-based prospective setting, and use of a validated FFQ to assess regular dietary intake.
This study had several limitations. First, lack of data on serum levels of L-arginine was an important limitation of this study; however, an acceptable correlation has been reported between dietary L-arginine intakes and serum L-arginine, in previous studies. Moreover, due to some inherent limitations of observational studies including selection bias, information bias in measuring exposure or outcome, and non-differential misclassification should be considered in interpretation of the findings. Inherent limitation of FFQ such as under or over estimations of dietary intakes was also another issue in our study. Dietary information was also assessed only at baseline examinations and possible changes in dietary patterns during the follow-up examinations have not been considered; however previous observations in our population indicated stability of major dietary patterns over the time.
In conclusion, our findings suggested a potential protective effect of plant derived L-arginine intakes against development of MetS and its phenotypes; moreover, higher intakes of L-arginine from animal sources could be a dietary risk factor for development of metabolic disorders. Considering the limited epidemiological studies available in relation to long-term effects of dietary L-arginine intakes on cardiometabolic outcomes including MetS, type 2 diabetes, hypertension and cardiovascular events, further cohort studies are required to clarify the possible association.
ACKNOWLEDGEMENTS
We thank the Tehran Lipid and Glucose Study participants and the field investigators of the Tehran Lipid and Glucose Study for their cooperation and assistance in physical examinations, biochemical evaluation and database management. We thank Ms N Shiva for critical editing of the English grammar and syntax of the manuscript.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Litvinova L, Atochin DN, Fattakhov N, Vasilenko M, Zatolokin P, Kirienkova E. Nitric oxide and mitochondria in metabolic syndrome. Front Physiol. 2015;6:20. doi: 10.3389/fphys.2015.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghasemi A, Zahediasl S. Is nitric oxide a hormone? Iran Biomed J. 2011;15:59–65. [PMC free article] [PubMed] [Google Scholar]
- 3.King DE, Mainous AG, 3rd, Geesey ME. Variation in L-arginine intake follow demographics and lifestyle factors that may impact cardiovascular disease risk. Nutr Res. 2008;28:21–24. doi: 10.1016/j.nutres.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wells BJ, Mainous AG, 3rd, Everett CJ. Association between dietary arginine and C-reactive protein. Nutrition. 2005;21:125–130. doi: 10.1016/j.nut.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 5.Böger RH. The pharmacodynamics of L-arginine. Altern Ther Health Med. 2014;20:48–54. [PubMed] [Google Scholar]
- 6.Morris SM., Jr Arginine: beyond protein. Am J Clin Nutr. 2006;83:508S–512S. doi: 10.1093/ajcn/83.2.508S. [DOI] [PubMed] [Google Scholar]
- 7.Tousoulis D, Antoniades C, Tentolouris C, Goumas G, Stefanadis C, Toutouzas P. L-arginine in cardiovascular disease: dream or reality? Vasc Med. 2002;7:203–211. doi: 10.1191/1358863x02vm434ra. [DOI] [PubMed] [Google Scholar]
- 8.Assumpção CR, Brunini TMC, Matsuura C, Resende AC, Mendes-Ribeiro AC. Impact of the L-arginine-nitric oxide pathway and oxidative stress on the pathogenesis of the metabolic syndrome. Open Biochem J. 2008;2:108–115. doi: 10.2174/1874091X00802010108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lucotti P, Setola E, Monti LD, Galluccio E, Costa S, Sandoli EP, Fermo I, Rabaiotti G, Gatti R, Piatti P. Beneficial effects of a long-term oral L-arginine treatment added to a hypocaloric diet and exercise training program in obese, insulin-resistant type 2 diabetic patients. Am J Physiol Endocrinol Metab. 2006;291:E906–E912. doi: 10.1152/ajpendo.00002.2006. [DOI] [PubMed] [Google Scholar]
- 10.Monti LD, Casiraghi MC, Setola E, Galluccio E, Pagani MA, Quaglia L, Bosi E, Piatti P. L-arginine enriched biscuits improve endothelial function and glucose metabolism: a pilot study in healthy subjects and a cross-over study in subjects with impaired glucose tolerance and metabolic syndrome. Metabolism. 2013;62:255–264. doi: 10.1016/j.metabol.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 11.Piatti PM, Monti LD, Valsecchi G, Magni F, Setola E, Marchesi F, Galli-Kienle M, Pozza G, Alberti KG. Long-term oral L-arginine administration improves peripheral and hepatic insulin sensitivity in type 2 diabetic patients. Diabetes Care. 2001;24:875–880. doi: 10.2337/diacare.24.5.875. [DOI] [PubMed] [Google Scholar]
- 12.Dong JY, Qin LQ, Zhang Z, Zhao Y, Wang J, Arigoni F, Zhang W. Effect of oral L-arginine supplementation on blood pressure: a meta-analysis of randomized, double-blind, placebo-controlled trials. Am Heart J. 2011;162:959–965. doi: 10.1016/j.ahj.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Mariotti F, Petzke KJ, Bonnet D, Szezepanski I, Bos C, Huneau JF, Fouillet H. Kinetics of the utilization of dietary arginine for nitric oxide and urea synthesis: insight into the arginine-nitric oxide metabolic system in humans. Am J Clin Nutr. 2013;97:972–979. doi: 10.3945/ajcn.112.048025. [DOI] [PubMed] [Google Scholar]
- 14.Alvares TS, Conte-Junior CA, Silva JT, Paschoalin VM. Acute L-arginine supplementation does not increase nitric oxide production in healthy subjects. Nutr Metab. 2012;9:54. doi: 10.1186/1743-7075-9-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Petrella E, Pignatti L, Neri I, Facchinetti F. The L-arginine/ nitric oxide pathway is impaired in overweight/obese pregnant women. Pregnancy Hypertens. 2014;4:150–155. doi: 10.1016/j.preghy.2014.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Lee J, Ryu H, Ferrante RJ, Morris SM, Jr, Ratan RR. Translational control of inducible nitric oxide synthase expression by arginine can explain the arginine paradox. Proc Natl Acad Sci USA. 2003;100:4843–4848. doi: 10.1073/pnas.0735876100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu G, Meininger CJ. Nitric oxide and vascular insulin resistance. Biofactors. 2009;35:21–27. doi: 10.1002/biof.3. [DOI] [PubMed] [Google Scholar]
- 18.Luiking YC, Engelen MP, Deutz NE. Regulation of nitric oxide production in health and disease. Curr Opin Clin Nutr Metab Care. 2010;13:97–104. doi: 10.1097/MCO.0b013e328332f99d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajapakse NW, Nanayakkara S, Kaye DM. Pathogenesis and treatment of the cardiorenal syndrome: implications of L-arginine-nitric oxide pathway impairment. Pharmacol Ther. 2015;154:1–12. doi: 10.1016/j.pharmthera.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Virarkar M, Alappat L, Bradford PG, Awad AB. L-arginine and nitric oxide in CNS function and neurodegenerative diseases. Crit Rev Food Sci Nutr. 2013;53:1157–1167. doi: 10.1080/10408398.2011.573885. [DOI] [PubMed] [Google Scholar]
- 21.Hoang HH, Padgham SV, Meininger CJ. L-arginine, tetrahydrobiopterin, nitric oxide and diabetes. Curr Opin Clin Nutr Metab Care. 2013;16:76–82. doi: 10.1097/MCO.0b013e32835ad1ef. [DOI] [PubMed] [Google Scholar]
- 22.Rajapakse NW, Mattson DL. Role of L-arginine in nitric oxide production in health and hypertension. Clin Exp Pharmacol Physiol. 2009;36:249–255. doi: 10.1111/j.1440-1681.2008.05123.x. [DOI] [PubMed] [Google Scholar]
- 23.Venho B, Voutilainen S, Valkonen VP, Virtanen J, Lakka TA, Rissanen TH, Ovaskainen ML, Laitinen M, Salonen JT. Arginine intake, blood pressure, and the incidence of acute coronary events in men: the Kuopio Ischaemic Heart Disease Risk Factor Study. Am J Clin Nutr. 2002;76:359–364. doi: 10.1093/ajcn/76.2.359. [DOI] [PubMed] [Google Scholar]
- 24.Oomen CM, van Erk MJ, Feskens EJ, Kok FJ, Kromhout D. Arginine intake and risk of coronary heart disease mortality in elderly men. Arterioscler Thromb Vasc Biol. 2000;20:2134–2139. doi: 10.1161/01.ATV.20.9.2134. [DOI] [PubMed] [Google Scholar]
- 25.Bahadoran Z, Mirmiran P, Tahmasebinejad Z, Azizi F. Dietary L-arginine intake and the incidence of coronary heart disease: Tehran lipid and glucose study. Nutr Metab. 2016;13:23. doi: 10.1186/s12986-016-0084-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Azizi F, Rahmani M, Emami H, Mirmiran P, Hajipour R, Madjid M, Ghanbili J, Ghanbarian A, Mehrabi Y, Saadat N, Salehi P, Mortazavi N, Heydarian P, Sarbazi N, Allahverdian S, Saadati N, Ainy E, Moeini S. Cardiovascular risk factors in an Iranian urban population: Tehran lipid and glucose study (phase 1) Soz Praventivmed. 2002;47:408–426. doi: 10.1007/s000380200008. [DOI] [PubMed] [Google Scholar]
- 27.Miranda KM, Espey MG, Wink DA. A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 2001;5:62–71. doi: 10.1006/niox.2000.0319. [DOI] [PubMed] [Google Scholar]
- 28.Ghasemi A, Hedayati M, Biabani H. Protein precipitation methods evaluated for determination of serum nitric oxide end products by the Griess assay. J Med Sci Res. 2007;2:29–32. [Google Scholar]
- 29.Ghasemi A, Zahediasl S. Preanalytical and analytical considerations for measuring nitric oxide metabolites in serum or plasma using the Griess method. Clin Lab. 2012;58:615–624. [PubMed] [Google Scholar]
- 30.Ghasemi A, Zahediasl S, Azizi F. Elevated nitric oxide metabolites are associated with obesity in women. Arch Iran Med. 2013;16:521–525. [PubMed] [Google Scholar]
- 31.Borai A, Livingstone C, Kaddam I, Ferns G. Selection of the appropriate method for the assessment of insulin resistance. BMC Med Res Methodol. 2011;11:158. doi: 10.1186/1471-2288-11-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Muniyappa R, Lee S, Chen H, Quon MJ. Current approaches for assessing insulin sensitivity and resistance in vivo: advantages, limitations, and appropriate usage. Am J Physiol Endocrinol Metab. 2008;294:E15–E26. doi: 10.1152/ajpendo.00645.2007. [DOI] [PubMed] [Google Scholar]
- 33.Mirmiran P, Esfahani FH, Mehrabi Y, Hedayati M, Azizi F. Reliability and relative validity of an FFQ for nutrients in the Tehran lipid and glucose study. Public Health Nutr. 2010;13:654–662. doi: 10.1017/S1368980009991698. [DOI] [PubMed] [Google Scholar]
- 34.Hosseini-Esfahani F, Jessri M, Mirmiran P, Bastan S, Azizi F. Adherence to dietary recommendations and risk of metabolic syndrome: Tehran Lipid and Glucose Study. Metabolism. 2010;59:1833–1842. doi: 10.1016/j.metabol.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 35.Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC, Jr, Spertus JA, Costa F. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute scientific statement. Curr Opin Cardiol. 2006;21:1–6. doi: 10.1097/01.hco.0000200416.65370.a0. [DOI] [PubMed] [Google Scholar]
- 36.Azizi F, Hadaegh F, Khalili D, Esteghamati A, Hosseinpanah F, Delavari A, Larijani B, Mirmiran P, Zabetian A, Mehrabi Y, Kelishadi R, Aghajani H. Appropriate definition of metabolic syndrome among Iranian adults: report of the Iranian National Committee of Obesity. Arch Iran Med. 2010;13:426–428. [PubMed] [Google Scholar]
- 37.American Diabetes Association. Standards of medical care in diabetes–2014. Diabetes Care. 2014;37:S14–S80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 38.Ramezani Tehrani F, Bahri M, Gholami R, Hashemi S, Nakhoda K, Azizi F. Secular trend of menopausal age and related factors among Tehrani women born from 1930 to 1960; Tehran Lipid and Glucose Study. Arch Iran Med. 2014;17:406–410. [PubMed] [Google Scholar]
- 39.Nejat A, Mirbolouk M, Mohebi R, Hasheminia M, Tohidi M, Saadat N, Azizi F, Hadaegh F. Changes in lipid measures and incident coronary heart disease: Tehran Lipid & Glucose Study. Clin Biochem. 2014;47:1239–1244. doi: 10.1016/j.clinbiochem.2014.03.004. [DOI] [PubMed] [Google Scholar]
- 40.Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. 1997;65:1220S–1228S. doi: 10.1093/ajcn/65.4.1220S. [DOI] [PubMed] [Google Scholar]
- 41.Jobgen WS, Fried SK, Fu WJ, Meininger CJ, Wu G. Regulatory role for the arginine-nitric oxide pathway in metabolism of energy substrates. J Nutr Biochem. 2006;17:571–588. doi: 10.1016/j.jnutbio.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 42.Smulders RA, Aarsen M, Teerlink T, De Vries PM, Van Kamp GJ, Donker AJ, Stehouwer CD. Haemodynamic and biochemical responses to L-arginine and L-lysine infusions in normal subjects: L-arginine-induced vasodilatation cannot be explained by non-specific effects of cationic amino acids. Clin Sci. 1997;92:367–374. doi: 10.1042/cs0920367. [DOI] [PubMed] [Google Scholar]