Abstract
Oxidative stress plays a pivotal role in the development of diabetes and hyperglycaemia. The protective effects of natural extracts against diabetes are mainly dependent on their antioxidant and hypoglycaemic properties. Broccoli (Brassica oleracea) exerts beneficial health effects in several diseases including diabetes; however, the mechanism has not been elucidated yet. The present study was carried out to evaluate the potential hypoglycaemic and antioxidant properties of aqueous broccoli extracts (BEs) in diabetic rats. Streptozotocin (STZ) drug was used as a diabetogenic agent in a single intraperitoneal injection dose of 50 mg/kg body weight. The blood glucose level for each rat was measured twice a week. After 8 weeks, all animals were fasted overnight and sacrificed; pancreatic tissues were homogenized and used for measuring oxidative DNA damage, biochemical assessment of glutathione (GSH), and total antioxidant capacity (TAC) as well as histopathological examination for pancreatic tissues was examined. Diabetic rats showed significantly higher levels of DNA damage, GSH depletion, and impaired TAC levels in comparison to non-diabetics (P<0.05). The treatment of diabetic rats with BE significantly reduced DNA damage and conserved GSH and TAC values (P<0.01). BE attenuated pancreatic histopathological changes in diabetic rats. The results of this study indicated that BE reduced the STZ mediated hyperglycaemia and the STZ-induced oxidative injury to pancreas tissue. The used in vivo model confirmed the efficacy of BE as an anti-diabetic herbal medicine and provided insights into the capacity of BE to be used for phytoremediation purposes for human type 2 diabetes.
Keywords: broccoli extract, oxidative stress, type 2 diabetes, hyperglycaemia, streptozotocin
INTRODUCTION
Broccoli (Brassica oleracea) is a member of the Brassicaceae family which is rich in potential health boosting components like vitamins, minerals, dietary fibre, flavonol glycosides, hydroxycinnamic acids, and sulphur-containing compounds, such as the glucosinolates. Broccoli, the “Crown Jewel of Nutrition” is a winter season vegetable crop found along the Mediterranean region which has been deemed as an anti-cancerous food by the American Cancer Society (1,2). Broccoli is also rich in vitamin C, a major antioxidant in Brassica vegetables (3,4). The amount of dietary fibre, vitamin C, and glucosinolates were reported as 4 g/100 g, 79 mg/100 g, and 61.7 mg/100 g in broccoli, respectively (5,6). Recent focus is on sulforaphane, a component present in cruciferous vegetables. It is a hydrolysis product of glucosinolate found in broccoli. The interest in sulforaphane is due to the conversion of carcinogens to inactive metabolites as it modulates the activities of phase II enzymes. In addition, researchers found that sulforaphane may have a positive influence in controlling diabetes (7,8). Most of the earlier studies were performed mainly on the proximate analysis, types of phenolic compounds, and cancer-preventing properties of functional components of broccoli. So far, there are no available data about the effect of broccoli extracts (BE) against STZ-induced diabetic rats.
Dietary habits are of great importance in human health. Diabetes mellitus (DM) is a serious global health problem, particularly increasing in the Middle East. This development is due to the extensive adaptation in lifestyle, including the tendency to a westernised diet, less physical activity, obesity and smoking (9). Diabetes mellitus is a group of disorders characterised by chronic hyperglycaemia due to a defect in the body’s ability to convert glucose (sugar) to energy. It is a common endocrine disorder that involves dysfunction of pancreatic β cell. The loss of β cell mass and the progressive decline in β cell function are an early profile of diabetes (10,11). To a greater extent, diabetes is preventable, and the threat can be reduced worldwide through information by providing awareness about the importance of health promotion activities and improving the quality of life (12,13). Epidemiological studies have shown that the consumption of fruits and vegetables could control the morbidity and mortality rates of certain types of diseases. This is due to the presence of bioactive components, namely fibres, polyphenol compounds, flavonoids, isoflavones, tocoferols, and ascorbic acid (14–16). The protective effect of Carica papaya on β cells in streptozotocin (STZ)-induced diabetic rats has been recently reported (10). The author concluded that Carica papaya L. leaf extract preserved the integrity of pancreatic cells, improved basal insulin secretion, and protected cultured cells from the adverse effects of STZ. Another study reported on the positive effects of garlic extracts in normal and STZ diabetic rats. The author observed that the administration of alcoholic extracts of garlic [0.25 g/kg body weight (BW)] reduced hyperglycaemia and associated weight loss of STZ-treated rats (17). These positive effects could be due to the dietary antioxidants which play a significant role in protecting against reactive oxygen species (ROS), which are associated with environmental pollution, UV radiation, and normal metabolic processes. Studies have also suggested that the accumulation of ROS can bring about complications of diabetes mellitus and other degenerative processes, such as DNA damage and mutation that may be related to cancer, heart disease, and aging (18–20). The present study was conducted to evaluate the possible antioxidant and hypoglycaemic effects of aqueous extracts of broccoli in STZ-induced diabetic rats.
MATERIALS AND METHODS
Broccoli collection and extract preparation
Broccoli was purchased from a local market and identified by an expert botanist. They were cleaned with tap water to remove the dirt adhering to them and were spread on tissue paper to absorb excess surface water. Broccoli was then frozen at −40°C for 12 h, freeze dried for four days (i.e. 96 h) at 22°C (i.e. drying started at −40°C and ended at 22°C) and 200 Pa. The dried broccoli was powdered by an electric grinder (Moulinex AR1043-UK0, Moulinex, Lyon, France) yielding 15.7 g powder per 100 g of fresh broccoli. The powder was extracted with distilled water (9.3 g dry solids/100 mL) at room temperature (22°C for 2 h). The extract was then centrifuged and filtered (Sanyo MSE Harrier 18/80, Sanyo, Tokyo, Japan) at 6,000 relative centrifugal force for 20 min at 4°C. The supernatant was collected and used for subsequent experiments.
Total polyphenols content of BE
Total polyphenols content of BE was determined according to the Folin-Ciocalteau method (21). Extracts (300 μL) were mixed with 3.6 mL of water, followed by the addition of 250 μL of Folin-Ciocalteau reagent and 750 μL of sodium carbonate solution. The mixture was allowed to react in a vortex mixer and then incubated for 2 h at room temperature (22±2°C) in the dark. The absorbance of the mixture was measured at 765 nm using a spectrophotometer (Thermo Fisher Scientific UK Ltd., Loughborough, UK). Total polyphenol content was determined from a calibration curve prepared with a standard gallic acid solution and was expressed as mg gallic acid equivalents (GAE) per gram of sample (mg GAE/g sample). The values were expressed as mean of three replicates±standard deviation (SD).
Evaluation of the free radical scavenging capacity of BE
The capacity of BE to scavenge 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical was measured by a spectrophotometric method (22). Briefly, 50 μL aliquots of BE, at different concentrations (10~100 μM), were mixed with 50 μL of a DPPH methanolic solution. Absorbance was measured at 517 nm after 30 min of reaction at room temperature. Controls contained all the reaction reagents except the BE or 2,6-di-tert-butyl-4-hydroxytoluene (BHT), the positive control. The free radical scavenging capacity of different samples was expressed as percentage DPPH inhibition, a higher percentage free radical scavenging activity value indicated a higher antioxidant activity.
Evaluation of the antioxidant activity of BE
A colorimetric method using 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) Antioxidant Assay Kit (Cat#AOX-1, Zen-Bio, Durham, NC, USA) was used. The assay is based on the incubation of BE at different concentrations (10~100 mM) with ABTS with a peroxidase (metmyoglobin) and hydrogen peroxide to produce the radical cation (ABTS+·), which has a relatively stable blue-green color that is measured at 405 nm. BE, as a potential antioxidant, inhibits the oxidation of ABTS to ABTS+· (causing suppression of the color production) to a degree that is proportional to its concentration. Trolox (a water-soluble tocopherol analogue) was used as a positive standard.
Animals and induction of experimental diabetes
Male adult Sprague-Dawley rats were used in this experiment. The rats were housed in individual polypropylene cages and were provided with a basal diet (standard laboratory chow diet from Oman Mills, Muscat, Oman) and normal tap water. They were given water and food ad libitum, each rat consumed 10~12 g diet/d. Rats were housed under standard conditions of temperature (22±2°C), humidity (60%), and a 12-h light/dark cycle. The protocol in this study was approved by the Sultan Qaboos University Animal Ethical Committee, and it was conducted in accordance to International laws and policies (EEC Council directives 86/609, OJL 358, 1 December, 12, 1987; NIH Guide for the Care and Use of Laboratory Animals, NIH Publication No. 85-23, 1985). STZ was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Rats were divided into 4 groups (n=5 in each group). ND, non-diabetic rats that received chow diet; D, STZ-induced diabetic rats that received chow diet; ND+BE, non-diabetic rats that received chow diet plus oral feeding of BE; D+BE, diabetic rats that received chow diet plus oral feeding of BE. The BE was administrated through oral gavages with an oral dose of 70.6 mg/kg BW of polyphenols in the BE per week. This polyphenol dose was calculated from the extract dose of 5 mL/week. Diabetes mellitus was induced by a single intraperitoneal injection of freshly prepared STZ at a dose of 50 mg/kg BW dissolved in 0.01 M citrate buffer, pH 4.5 (23). After one week of STZ injection, and overnight fast, blood was taken from the tail artery of rats to measure blood glucose. The blood glucose level was determined by the glucose oxidase method using a one touch basic plus glucometer (LifeScan Inc., Milpitas, CA, USA). Rats with fasting blood glucose levels higher than 5 mmol/L were selected for the diabetic groups. The experiments were carried out for 8 weeks, and the experimental groups were evaluated to assess the effect on fasting blood glucose and body weight. At the end of the experiment, all rats were euthanized, and the pancreas tissues were collected for biochemical assays and histopathological examination.
At the end of the experimental period, the animals were anesthetized with a lethal dose of a cocktail containing ketamine and xylazine. Thoracotomy was performed, and the pancreas was excised immediately. At the end of the experimental period, rats were weighed and sacrificed. Pancreas tissues were removed and weighed. One portion of the pancreas was used for histopathology, and the other portion was excised and homogenized (1 g/10 mL ice-cold potassium chloride, 150 mM). The homogenate was then used for determining the levels of reduced glutathione (GSH), total antioxidant capacity (TAC), oxidative DNA damage, and protein measurements.
Histopathological examination
After euthanasia, the pancreas from each rat was removed, weighed and soaked in 70% ethanol. After three days they were fixed in 10% formalin solution. The histopathological analysis was examined for the measurement of the islets of Langerhans. Samples preserved in 10% buffered formalin were grossed, dissected and placed in cassettes. Then, the samples were chemically processed using a histoprocessor (Thermo Scientific microm HM 325 model, Thermo Fisher Scientific UK Ltd.). After processing, the samples were embedded in wax and cut using a rotary microtome (Thermo Scientific STP120, Thermo Fisher Scientific UK Ltd.) at a thickness of 3 μm. Haematoxylin and eosin staining was done to evaluate tissues under conventional light microscope.
GSH measurement
Aliquots of the supernatant (20 μL) were mixed with 10 μL of monochlorobimane (25 mmol/L) and 10 μL of glutathione S-transferase reagent as provided by a commercial kit (catalogue # K251-100, Biovision, Milpitas, CA, USA). The volume was made up to 100 μL using a cell lysis buffer. After 20 min of incubation at 37°C, the samples were read in an enzyme-linked immunosorbent assay (ELISA) reader at 620 nm. The GSH content was determined by comparison with values from a standard curve using freshly prepared GSH and normalized to the protein content of the assayed pancreatic tissue homogenates.
TAC measurement
A colorimetric method using assay kit (catalogue # K274-100, Biovision) was used to measure the TAC. Aliquots of the supernatant (20 μL) were mixed with 10 μL of protein mask and 10 μL of Cu2+ reagent as provided by a commercial kit (catalogue # K251, Biovision). The volume was made up to 100 μL using an assay diluent. After 30 min of incubation at 37°C, the samples were read in an ELISA reader at 620 nm. The antioxidant capacity of the assayed samples was compared with that of the standard Trolox, a water soluble tocopherol analogue, which is widely used as a traditional standard for TAC measurement assays. The assay results were normalized to the protein content of the assayed pancreatic tissue homogenates.
Protein content analysis
Protein content of pancreas tissue homogenates was assayed using the Lowry’s method, and the protein content was expressed as mg/mL of sample (24).
DNA oxidative damage using 8-oxo-7,8-dihydro-20-deoxyguanosine (8-oxodGuo) assay
The pancreas tissues were homogenized in 50 mM phosphate buffer solution containing 0.1 M dithiothreitol and then centrifuged at 4°C for 20 min at 2,000 g. The pellets were used for 8-oxodGuo. The pancreas pellets were re-suspended, and the DNA was isolated using the method recommended by the European Standards Committee on Oxidative DNA Damage (25). The purified DNA (about 50 mg) was hydrolyzed with P1 nuclease (10 IU) and alkaline phosphatase (7 IU). The hydrolyzed mixture was filtered using a Micropure-EZ enzyme remover (EMD Millipore, Burlington, MA, USA), and 50 mL was injected into an high-performance liquid chromatography apparatus. The nucleosides were separated by a C18 reverse-phase column (5 mm, I.D. 0.46 cm×25 cm, Supelco Inc., Bellefonte, PA, USA). The 8-oxodGuo and 2dG in the DNA were detected using an ESA Coulochem II Electrochemical Detector (Esa Technology Inc., Santa Rosa, CA, USA) in line with a UV detector (26).
Statistical analysis
Statistical analysis was performed using GraphPad Prism (version 5.03, GraphPad Software Inc., San Diego, CA, USA). The results are expressed as mean±standard deviation (SD). The statistical analysis was performed using one way analysis of variance (ANOVA) followed by Tukey’s test, Student’s unpaired t-test for means comparisons, and a P value of less than 0.05 was considered significant.
RESULTS
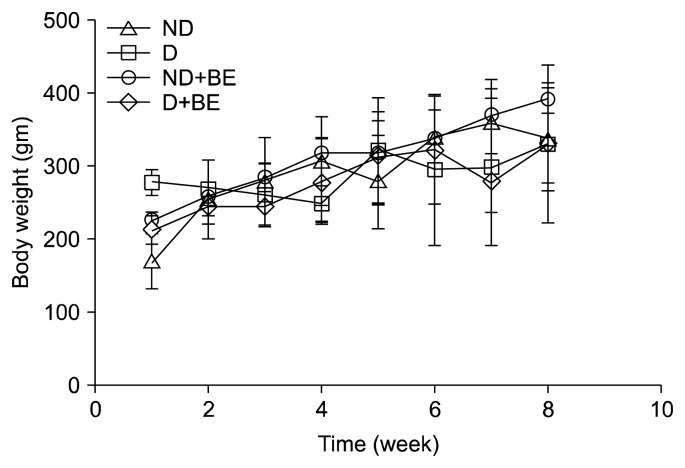
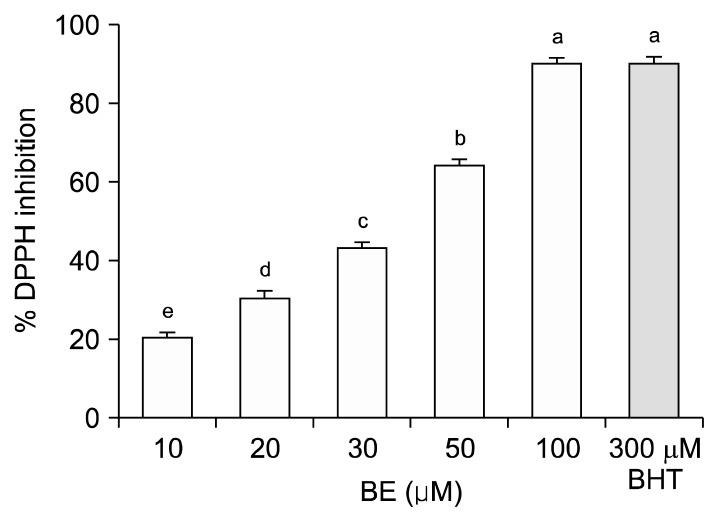
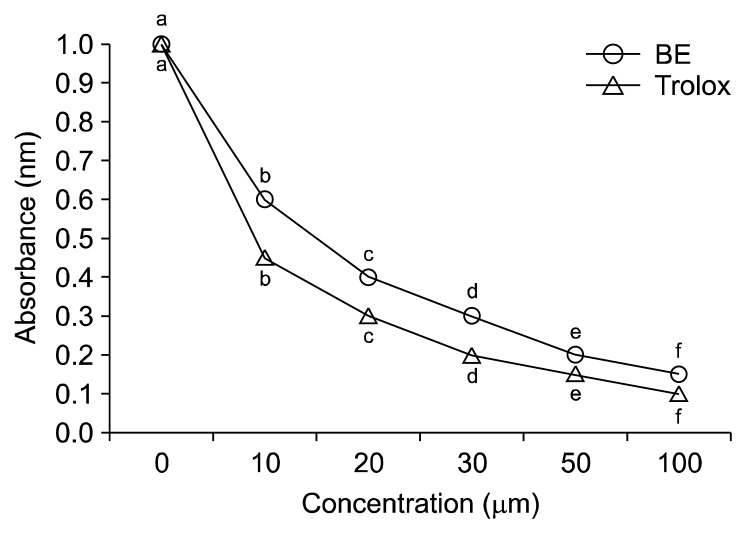
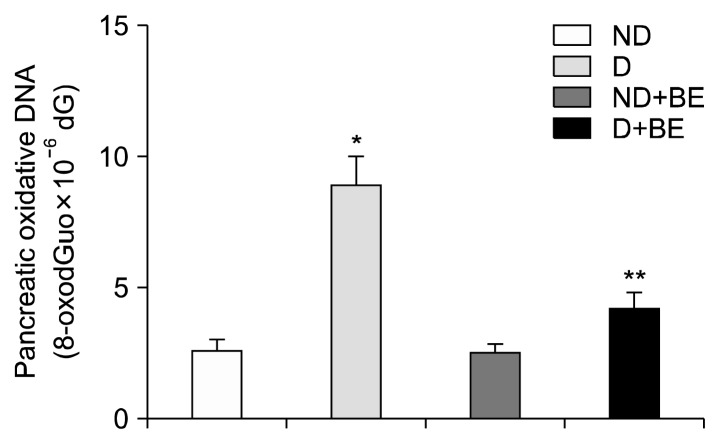
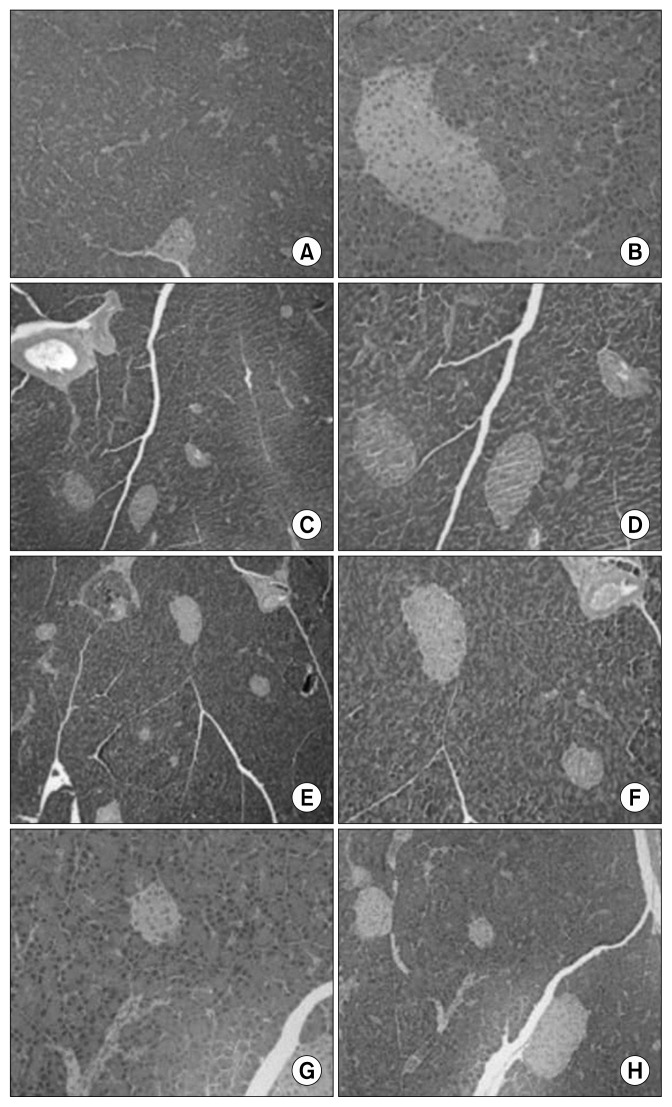
The total polyphenol content of fresh and freeze-dried BE was 33±0 to 34±1 mg GAE/g dry solids, respectively (data not shown). Fig. 1 depicts the changes in body weight in ND, D, ND+BE, and D+BE. Body weight was recorded weekly for the duration of the experiment. The initial body weight increased gradually throughout the experimental period for all the groups. The results showed that there was no significant difference in the body weight among all the groups. Table 1 represents the blood glucose levels and the pancreas weight during the experimental period along with GSH and TAC. The blood glucose level was significantly higher (P≤0.001) in the D group as compared to the ND rats (Table 1). However, the glucose levels were suppressed gradually by broccoli extract administration during the 8-week period in the D group. Pancreas weight (g) at sacrificial time is noted that the percentage occupied by islet tissue increased in proportion to the gain in weight for all groups. Fig. 2 and 3 depict the BE as an antioxidant. Fig. 2 shows the inhibition of DPPH formation to a degree that is proportional to broccoli extract concentration (10~100 μM) as compared to the BHT standard (P<0.05), whereas in Fig. 3, the broccoli extract inhibited the oxidation of ABTS to ABTS+· in a dose-dependent manner (P<0.05). Table 1 outlined the effects of BE on GSH and TAC in pancreatic tissues. The results showed that concomitant treatment of diabetic rats with BE significantly restored GSH and TAC to their basal levels (P<0.01). On the other hand, the STZ-induced group showed a significant depletion in GSH and TAC impairment as compared to the ND groups (P<0.05). The effects of BE administration on pancreatic DNA are illustrated in Fig. 4. The STZ-induced group showed a significant DNA damage as compared to the ND group. D+BE group significantly reduced the oxidative DNA damage as compared to the diabetic group (P<0.01). Fig. 5 represent islets of Langerhans from ND, D, ND+BE, and D+BE, respectively. Pancreas, the target tissue of diabetic etiology, had severe damage in the D group (Fig. 5C and 5D). The D group showed congested blood vessels within islets and acini. On the contrary, the ND group displayed a normal pancreatic architecture and histology (Fig. 5A and 5B). Sections are shown with a normal pancreatic tissue consisting of acini, duct, and islets of Langerhans. No inflammation, necrosis, or malignancy was observed. However, compared to the D group, histopathological examination of BE treated D group revealed islets of Langerhans with reduced size along with absence of vacuolation (Fig. 5E and 5F). No evidence of necrosis or malignancy is noted, and the rest of the pancreatic tissue is within normal limits for the ND+BE (Fig. 5G and 5H).
Fig. 1.
Effect of broccoli extract (BE) on body weights between different experimental groups. ND, non-diabetic rats that received chow diet; D, STZ-induced diabetic rats that received chow diet; ND+BE, non-diabetic rats that received chow diet plus oral feeding of BE; D+BE, diabetic rats that received chow diet plus oral feeding of BE.
Table 1.
Blood glucose, weight of pancreas, glutathione (GSH), and total antioxidant capacity (TAC) levels in the experimental groups
| Group | Blood glucose (mmol/L) | Pancreas weight (g) | GSH (nmol/mg protein) | TAC (nmol/mg protein) |
|---|---|---|---|---|
| ND | 4.64±0.43 | 0.243±0.04 | 28.0±4.1 | 266.7±32.0 |
| D | 21.00±4.11* | 0.216±0.01 | 10.0±3.5 | 159.4±24.0 |
| ND+BE | 4.22±0.54 | 0.236±0.02 | 27.9±2.4 | 258.3±12.1 |
| D+BE | 4.23±0.66 | 0.226±0.01 | 27.5±3.1** | 309.7±14.7** |
Results are the means±SD of five measurements.
Significantly higher as compared to ND group at *P≤0.001 and compared to D group at **P<0.01.
ND, non-diabetic rats that received chow diet; D, STZ-induced diabetic rats that received chow diet; ND+BE, non-diabetic rats that received chow diet plus oral feeding of broccoli extract; D+BE, diabetic rats that received chow diet plus oral feeding of broccoli extract.
Fig. 2.
Scavenging effect of broccoli extract (BE) and 2,6-ditert-butyl-4-hydroxytoluene (BHT) against 1,1-diphenyl-2-picrylhydrazyl (DPPH) free radical formation. Controls contained all the reaction reagents except BE or BHT. Results are means±SD of five measurements. Values with different letters (a–e) are significantly higher as compared to lower doses of the BE, at P<0.05.
Fig. 3.
Free radical scavenging activity of broccoli extract (BE) and Trolox (a water soluble tocopherol analogue as a positive standard) against 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) (ABTS) radical formation. Negative controls were 0 μM BE or 0 μM Trolox. Results are means±SD of five measurements. Values with different letters (a–f) are significantly inhibited the free radical scavenging activity at higher doses, P<0.05.
Fig. 4.
Effects of broccoli extract (BE) on pancreatic oxidative DNA damage. Results are means±SD of five measurements. *P<0.05 as compared to ND group and **P<0.01 as compared to D group. ND, non-diabetic rats that received chow diet; D, STZ-induced diabetic rats that received chow diet; ND+BE, non-diabetic rats that received chow diet plus oral feeding of BE; D+BE, diabetic rats that received chow diet plus oral feeding of BE.
Fig. 5.
Histopathological examination of pancreas of all the experimental groups. A and B, non-diabetic group; C and D, STZ-induced diabetic group; E and F, diabetic group that received chow diet plus oral feeding of broccoli extract; G and H, non-diabetic group that received chow diet plus oral feeding of broccoli extract.
The semi quantitative analysis of histology of pancreas tissues of rats treated with STZ with or without BE is summarized in Table 2. Values in the table are scores given by a histopathologist unaware of the treatments (0, no necrosis; 1, a few focal necrotic spots; 2, necrotic area was about one half; 3, necrotic spots were about two-thirds; 4, nearly the entire area was necrotic). Five rats were used in each group.
Table 2.
Semi quantitative analysis of histology of pancreas tissues of rats treated with streptozotocin with or without broccoli extract
| Group | % of necrosis | Score of necrosis |
|---|---|---|
| ND | 0 | 0 |
| D | 12±5.2 | 2±0 |
| ND+BE | 0 | 0 |
| D+BE | 0 | 0 |
Means±SD (five rats were used in each group).
Values in the table are scores given by a histopathologist unaware of the treatments.
0, no necrosis; 1, a few focal necrotic spots; 2, necrotic area was about one half; 3, necrotic spots were about two-thirds; 4, nearly the entire area was necrotic.
ND, non-diabetic rats that received chow diet; D, STZ-induced diabetic rats that received chow diet; ND+BE, non-diabetic rats that received chow diet plus oral feeding of broccoli extract; D+BE, diabetic rats that received chow diet plus oral feeding of broccoli extract.
DISCUSSION
The major findings of the present study were the total polyphenol content of both fresh and freeze-dried BE using water as solvent, suppression in blood glucose level in the treatment group, and measures of oxidative stress. A strong link between consumption of fruits and vegetables and their health benefits are due to their high nutritional value and functional components with antioxidant properties. Antioxidants are compounds which control and scavenge oxidative damage in foods and bio-molecules by slowing or inhibiting the oxidative process caused by ROS, thus enhancing the quality of the products (27). Studies have mentioned the positive and negative effects of processing techniques that might affect the phenolic composition and antioxidant activity of foods. The present study showed that the total polyphenol content varied from 334~3,166 mg GAE/100 g sample for fresh and freeze-dried broccoli when water was used as the extraction solvent at 22°C. This difference may be due to the release of phenolic compounds during cell wall rupture during freeze-drying and due to the increased solvent extraction efficiency (28,29). The total polyphenol content of commercial broccoli ranged from 48.2~157.8 mg/100 g fresh weight (30). A study reported that the total polyphenol content of aqueous extracts of red and white cabbages varied from 10.4~21.4 mg GAE/g dry weight (31). Previous investigations have reported that freeze drying is the most efficient drying technique to preserve bioactive components including antioxidant compounds in plants (32). The present study aimed to investigate the effects of freeze-dried broccoli on STZ-induced diabetes and to find an association between the reduction in the weights of animals and glucose levels in male Sprague-Dawley rats. The STZ dose used in the present study was 50 mg/kg BW (33,34) to produce hyperglycaemia in rats whereas other studies have used higher dose (70 mg/kg BW) of STZ (35). The present study selected a lower dose because at present, strains of rats may not tolerate and survive the dose used by previous investigators. The results of the present study depicted that STZ was potent in producing hyperglycaemia in experimental rats (36). The freeze-dried BE-treated diabetic rats had decreased glucose levels. The hypoglycaemic effect of freeze-dried BE may be due to the active component sulforaphane, a natural compound present in broccoli with many promising health benefits (37,38). The treated rats with STZ showed slight loss of their body weights which may be due to pancreatic damage (33). Previous studies have shown an association between hyperglycaemia and decreased body weight of diabetic animals. The present study concluded that STZ at 50 mg/kg BW induced diabetes and the rats showed higher glucose levels and reduced body weight. Studies have confirmed the synergistic effect of dietary antioxidants with cellular reductants in scavenging free-radicals and chelating transition metals that are catalysts in lipid peroxidation (39). In the present study, reduced GSH and TAC levels clearly support the presence of oxidative stress in diabetes rats. On the other hand, administration of broccoli extracts restored GSH and TAC. These effects may be due to its hypoglycaemic and free radical scavenging properties based on the fact that broccoli contains several compounds such as total polyphenol, flavonoids, and other components that can act as a potent antioxidants (40). In conclusion, the present findings illustrate that BE are promising in the control of diabetes by reducing blood glucose and oxidative stress. This may be due to the presence of active components that have antioxidant activity.
ACKNOWLEDGEMENTS
Ms. Sithara Suresh has received a Ph. D. scholarship from the Sultan Qaboos University. Authors would like to acknowledge the supports of the Sultan Qaboos University towards this research.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Lee SG, Kim JH, Son MJ, Lee EJ, Park WD, Kim JB, Lee SP, Lee IS. Influence of extraction method on quality and functionality of broccoli juice. Prev Nutr Food Sci. 2013;18:133–138. doi: 10.3746/pnf.2013.18.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El-Magd MMA. Evaluation of some broccoli cultivars growth, head yield and quality under different planting dates. J Appl Sci Res. 2013;9:5730–5736. [Google Scholar]
- 3.Ares AM, Nozal MJ, Bernal J. Extraction, chemical characterization and biological activity determination of broccoli health promoting compounds. J Chromatogr A. 2013;1313:78–95. doi: 10.1016/j.chroma.2013.07.051. [DOI] [PubMed] [Google Scholar]
- 4.Moreno DA, Carvajal M, López-Berenguer C, García-Viguera C. Chemical and biological characterisation of nutraceutical compounds of broccoli. J Pharm Biomed Anal. 2006;41:1508–1522. doi: 10.1016/j.jpba.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Campbell B, Han D, Triggs CM, Fraser AG, Ferguson LR. Brassicaceae: nutrient analysis and investigation of tolerability in people with Crohn’s disease in a New Zealand study. Funct Foods Health Dis. 2012;2:460–486. [Google Scholar]
- 6.Roe M, Church S, Pinchen H, Finglas P. [accessed Dec 2016];Nutrient analysis of fruit and vegetables. 2013 www.gov.uk/government/publications/nutrient-analysis-of-fruit-and-vegetables.
- 7.Xue M, Qian Q, Adaikalakoteswari A, Rabbani N, Babaei-Jadidi R, Thornalley PJ. Activation of NF-E2-related factor-2 reverses biochemical dysfunction of endothelial cells induced by hyperglycemia linked to vascular disease. Diabetes. 2008;57:2809–2817. doi: 10.2337/db06-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elbarbry F, Elrody N. Potential health benefits of sulforaphane: a review of the experimental, clinical and epidemiological evidences and underlying mechanisms. J Med Plants Res. 2011;5:473–484. [Google Scholar]
- 9.Al-Sinani S, Al-Shafaee M, Al-Mamari A, Woodhouse N, El-Shafie O, Hassan MO, Al-Yahyaee S, Albarwani S, Jaju D, Al-Hashmi K, Al-Abri M, Rizvi S, Bayoumi R. Impaired fasting glucose in Omani adults with no family history of type 2 diabetes. Sultan Qaboos Univ Med J. 2014;14:183–189. [PMC free article] [PubMed] [Google Scholar]
- 10.Miranda-Osorio PH, Castell-Rodriguez AE, Vargas-Mancilla J, Tovilla-Zarate CA, Ble-Castillo JL, Aguilar-Dominguez DE, Juarez-Rojop IE, Diaz-Zagoya JC. Protective action of Carica papaya on β-cells in streptozotocin-induced diabetic rats. Int J Environ Res Public Health. 2016;13:E446. doi: 10.3390/ijerph13050446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Russo GT, Giorda CB, Cercone S, Nicolucci A, Cucinotta D. Factors associated with beta-cell dysfunction in type 2 diabetes: the BETADECLINE study. PLoS One. 2014;9:e109702. doi: 10.1371/journal.pone.0109702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abdulhadi NMN, Al-Shafaee MA, Wahlström R, Hjelm K. Doctors’ and nurses’ views on patient care for type 2 diabetes: an interview study in primary health care in Oman. Prim Health Care Res Dev. 2013;14:258–269. doi: 10.1017/S146342361200062X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.WHO. Global Report on Diabetes. World Health Organization; Geneva, Switzerland: 2016. pp. 1–88. [Google Scholar]
- 14.Li Y, Guo C, Yang J, Wei J, Xu J, Cheng S. Evaluation of antioxidant properties of pomegranate peel extract in comparison with pomegranate pulp extract. Food Chem. 2006;96:254–260. doi: 10.1016/j.foodchem.2005.02.033. [DOI] [Google Scholar]
- 15.Lima GPP, Vianello F, Corrêa CR, Arnoux R, da Silva Campos RA, Borguini MG. Polyphenols in fruits and vegetables and its effect on human health. Food Nutr Sci. 2014;5:1065–1082. doi: 10.4236/fns.2014.511117. [DOI] [Google Scholar]
- 16.Tlili N, Mejri H, Yahia Y, Saadaoui E, Rejeb S, Khaldi A, Nasri N. Phytochemicals and antioxidant activities of Rhus tripartitum (Ucria) fruits depending on locality and different stages of maturity. Food Chem. 2014;160:98–103. doi: 10.1016/j.foodchem.2014.03.030. [DOI] [PubMed] [Google Scholar]
- 17.Bokaeian M, Nakhaee A, Moodi B, Farhangi A, Akbarzadeh A. Effects of garlic extract treatment in normal and streptozotocin diabetic rats infected with Candida albicans. Indian J Clin Biochem. 2010;25:182–187. doi: 10.1007/s12291-010-0033-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Susztak K, Raff AC, Schiffer M, Böttinger EP. Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes. 2006;55:225–233. doi: 10.2337/diabetes.55.01.06.db05-0894. [DOI] [PubMed] [Google Scholar]
- 19.Kotani K, Tsuzaki K, Taniguchi N, Sakane N. Correlation between reactive oxygen metabolites & atherosclerotic risk factors in patients with type 2 diabetes mellitus. Indian J Med Res. 2013;137:742–748. [PMC free article] [PubMed] [Google Scholar]
- 20.Alfadda AA, Sallam RM. Reactive oxygen species in health and disease. J Biomed Biotechnol. 2012;2012:936486. doi: 10.1155/2012/936486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Singleton L, Rossi A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic. 1965;16:144–158. [Google Scholar]
- 22.Lopez V, Akerreta S, Casanova E, García-Mina JM, Cavero RY, Calvo MI. In vitro antioxidant and anti-rhizopus activities of lamiaceae herbal extracts. Plant Foods Hum Nutr. 2007;62:151–155. doi: 10.1007/s11130-007-0056-6. [DOI] [PubMed] [Google Scholar]
- 23.Waly MI, Guizani N, Suresh S, Rahman MS. Ginger extract attenuates preliminary steps of streptozotocin-mediated oxidative stress in diabetic rats. Int J Nutr Pharmacol Neurol Dis. 2015;5:151–158. doi: 10.4103/2231-0738.167503. [DOI] [Google Scholar]
- 24.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 25.Gedik CM, Collins A ESCODD (European Standards Committee on Oxidative DNA Damage) Establishing the background level of base oxidation in human lymphocyte DNA: results of an interlaboratory validation study. FASEB J. 2005;19:82–84. doi: 10.1096/fj.04-1767fje. [DOI] [PubMed] [Google Scholar]
- 26.Lodovici M, Casalini C, Cariaggi R, Michelucci L, Dolara P. Levels of 8-hydroxydeoxyguanosine as a marker of DNA damage in human leukocytes. Free Radic Biol Med. 2000;28:13–17. doi: 10.1016/S0891-5849(99)00194-X. [DOI] [PubMed] [Google Scholar]
- 27.Shofian NM, Hamid AA, Osman A, Saari N, Anwar F, Dek MS, Hairuddin MR. Effect of freeze-drying on the antioxidant compounds and antioxidant activity of selected tropical fruits. Int J Mol Sci. 2011;12:4678–4692. doi: 10.3390/ijms12074678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Serna-Cock L, Vargas-Munoz DP, Aponte AA. Structural, physical, functional and nutraceutical changes of freeze-dried fruit. Afr J Biotechnol. 2015;14:442–450. doi: 10.5897/AJB2014.14189. [DOI] [Google Scholar]
- 29.Rabeta MS, Lin SP. Effects of different drying methods on the antioxidant activities of leaves and berries of Cayratia trifolia. Sains Malays. 2015;44:275–280. doi: 10.17576/jsm-2015-4402-16. [DOI] [Google Scholar]
- 30.Koh E, Wimalasiri KMS, Chassy AW, Mitchel AE. Content of ascorbic acid, quercetin, kaempferol and total phenolics in commercial broccoli. J Food Compos Anal. 2009;22:637–643. doi: 10.1016/j.jfca.2009.01.019. [DOI] [Google Scholar]
- 31.Gaafar AA, Aly HF, Salama ZA, Mahmoud KM. Characterizing the antioxidant and anticancer properties of secondary metabolites from red and white cabbages Brassica oleracea L. var. capitata. World J Pharm Res. 2014;3:171–186. [Google Scholar]
- 32.Hung PV, Duy TL. Effects of drying methods on bioactive compounds of vegetables and correlation between bioactive compounds and their antioxidants. Int Food Res J. 2012;19:327–332. [Google Scholar]
- 33.Nagarchi K, Ahmed S, Sabus A, Saheb SH. Effect of streptozotocin on glucose levels in albino Wister rats. J Pharm Sci Res. 2015;7:67–69. [Google Scholar]
- 34.Osicka TM, Yu Y, Panagiotopoulos S, Clavant SP, Kiriazis Z, Pike RN, Pratt LM, Russo LM, Kemp BE, Comper WD, Jerums G. Prevention of albuminuria by aminoguanidine or ramipril in streptozotocin-induced diabetic rats is associated with the normalization of glomerular protein kinase C. Diabetes. 2000;49:87–93. doi: 10.2337/diabetes.49.1.87. [DOI] [PubMed] [Google Scholar]
- 35.Gajdosík A, Gajdosíková A, Stefek M, Navarová J, Hozová R. Streptozotocin-induced experimental diabetes in male Wistar rats. Gen Physiol Biophys. 1999;18:54–62. [PubMed] [Google Scholar]
- 36.Kang N, Alexander G, Park JK, Maasch C, Buchwalow I, Luft FC, Haller H. Differential expression of protein kinase C isoforms in streptozotocin-induced diabetic rats. Kidney Int. 1999;56:1737–1750. doi: 10.1046/j.1523-1755.1999.00725.x. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Zhang Z, Sun W, Tan Y, Liu Y, Zheng Y, Liu Q, Cai L, Sun J. Sulforaphane attenuation of type 2 diabetes-induced aortic damage was associated with the upregulation of Nrf2 expression and function. Oxid Med Cell Longevity. 2014;2014:123963. doi: 10.1155/2014/123963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miao X, Bai Y, Sun W, Cui W, Xin Y, Wang Y, Tan Y, Miao L, Fu Y, Su G, Cai L. Sulforaphane prevention of diabetes-induced aortic damage was associated with the up-regulation of Nrf2 and its down-stream antioxidants. Nutr Metab. 2012;9:84. doi: 10.1186/1743-7075-9-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guizani N, Waly MI, Singh V, Rahman MS. Nabag (Zizyphus spina-christi) extract prevents aberrant crypt foci development in colons of azoxymethane-treated rats by abrogating oxidative stress and inducing apoptosis. Asian Pac J Cancer Prev. 2013;14:5031–5035. doi: 10.7314/APJCP.2013.14.9.5031. [DOI] [PubMed] [Google Scholar]
- 40.Tiveron AP, Melo PS, Bergamaschi KB, Vieira TM, Regitanod’Arce MA, Alencar SM. Antioxidant activity of Brazilian vegetables and its relation with phenolic composition. Int J Mol Sci. 2012;13:8943–8957. doi: 10.3390/ijms13078943. [DOI] [PMC free article] [PubMed] [Google Scholar]