Abstract
We evaluated the sleep enhancement activity of the medicinal herbs valerian (Valeriana officinalis), jujube (Ziziphus jujube), lotus seed (Nelumbo nucifera), Gastrodia elata, Polygonatum sibiricum, and baekbokryung (Poria cocos), which can relieve insomnia in a Drosophila model. Locomotor activity was measured in the Drosophila model to evaluate the sleep activity of Korean medicinal herbs traditionally used as sleep aids. The group treated with lotus seed extract showed less nocturnal activity. Treatment with 10 or 20 mg/mL of P. sibiricum significantly reduced nocturnal activity compared to the control group (P<0.05). The activity and sleep bouts of fruit flies were significantly decreased by a high-dose treatment (10 mg/mL) of lotus or P. sibiricum extracts at night. Caffeine-treated Drosophila showed increased nocturnal activity and decreased total sleep time (P<0.05). Flies receiving the 10 mg-doses of lotus seed or P. sibiricum extract showed significantly different nocturnal locomotor activity and total sleep time compared to caffeine-treated Drosophila. Lotus seed and P. sibiricum extracts are attractive and valuable sleep-potentiating nutraceuticals.
Keywords: Nelumbo nucifera, Polygonatum sibiricum, sleep, Drosophila melanogaster
INTRODUCTION
Sleep accounts for one-third of human life and is also an important part of animal life (1,2). Sleep is a tightly regulated and reversible quiescent state with defined behavior, homeostasis, and electrophysiological characteristics. It is a complex process affected by many factors, including genetic and environmental factors. Sleep is controlled by both homeostatic mechanisms and a circadian clock (3).
Sleep is essential to rejuvenate the body and promote health and performance, but many people do not get enough. Symptoms of sleep disturbances include difficulty falling asleep, difficulty sleeping, waking up too early, and unrecoverable or poor sleep quality (4). Hypnotic drugs are often prescribed for long periods to relieve insomnia in insomnia patients, despite recommendations for short-term use. Various side effects of long-term prescriptions make it difficult to effectively treat insomnia (5). These drugs can easily lead to dependency and addiction. In addition to these side effects, negative effects such as daytime fatigue, headaches, drowsiness, dizziness, depression, and nausea are also possible (6). As these safety concerns limit the usefulness of these common hypnotic agents, alternative treatments including herbal medicines may be an alternative for many patients with sleep disorders (7).
The therapeutic effect of medicinal herbs is often an integrative result of various bioactive compounds. Clinically, medicinal herbs are used for insomnia; they usually have no side effects and show excellent therapeutic effects. However, sleep enhancement activity has not yet been experimentally confirmed.
Drosophila melanogaster is an important model for analyzing the nervous system, including sleep. Drosophila and mammalian sleep have important similarities, such as sleep-related dopamine and γ-aminobutyric acid (GABA) signaling, a circadian rhythm, homeostatic factors, and similar neurological activity (8–10). Drosophila and mammals also exhibit similar neurological activity patterns when in different arousal states (10).
Herbal medicine represents one of the most frequently used complementary/alternative treatments for insomnia. In Korea, valerian (Valeriana officinalis), jujube (Ziziphus jujube), lotus (Nelumbo nucifera) seeds, Gastrodia elata, Polygonatum sibiricum, and baekbokryung (Poria cocos) are the most frequently used complementary and alternative treatments for sleep disorders. Therefore, we used a Drosophila model to evaluate the sleep enhancement activity of these medicinal herbs.
MATERIALS AND METHODS
Preparation of herbal extracts
Valerian, jujube, lotus seed powder, G. elata, P. sibiricum, and baekbokryung were purchased from the Kyungdong market (Seoul, Korea). Herbal medicines were sliced into 1- to 2-cm pieces, and water was added at a 10:1 ratio to the raw material (v/w). Each herb was extracted twice with water at 90±2°C for 3 h. After extraction, the extracted water was filtered through a sieve (Chunggye Sangsa, Gyeonggi, Korea) of 100 mesh (150 μm) and concentrated to 30°Brix. The concentrate was sterilized and spray-dried to provide an extract powder.
Fly stocks
Wild-type D. melanogaster Canton-S strain flies were obtained from the Bloomington Drosophila Stock Center at Department of Biology, Indiana University Bloomington (Bloomington, IN, USA). Fly stocks were maintained in incubators (25°C, 50~60% humidity) on standard food (11). Each sample of male flies was collected under CO2 anesthesia 2~5 days before treatment.
Locomotor activity assays
For the behavior assays, herbal extract powder was dissolved in distilled water and mixed into 5% sucrose-2% agar media (Hansol Tech Inc., Seoul, South Korea). The herbal extract was used for treatment at concentrations ranging from 0.25% to 1.0%.
Flies 1 to 4 days old were placed into individual 65-mm glass tubes in the Drosophila activity monitoring (DAM) system (TriKinetics Inc., Waltham, MA, USA) under CO2 anesthesia. A sleep episode was defined as 5-min bin of uninterrupted quiescence within the DAM system. Activity counts were summed across all wake bins at different concentrations. The flies were subjected to a 3-day acclimation period in the tubes, and recordings were done every 5~8 days under constant darkness at 25±1°C. Locomotor activity data were collected by DAM management software (TriKinetics Inc.) and visualized by Actogram J software (NIH, Bethesda, MD, USA) as previously described (11). Sleep analyses were performed at night in the control group and awake groups of 0.1% caffeine dietary intake. Sleep parameters, total activity, duration of sleep, and sleep bouts [no locomotor activity observed within a 5-min period (12)], were analyzed using R statistical software (R version 3.1.3, R foundation for Statistical Computing, Vienna, Austria).
Statistical analyses
Statistical analyses were performed using SPSS Statistics for Windows, version 12.0 (SPSS Inc., Chicago, IL, USA). Differences between groups were assessed with a one-way analysis of variance (ANOVA) and Tukey’s multiple tests (P<0.05). All data are represented as means±standard error of the mean (SEM).
RESULTS
Locomotor activities of Drosophila treated with Korean medicinal herbs
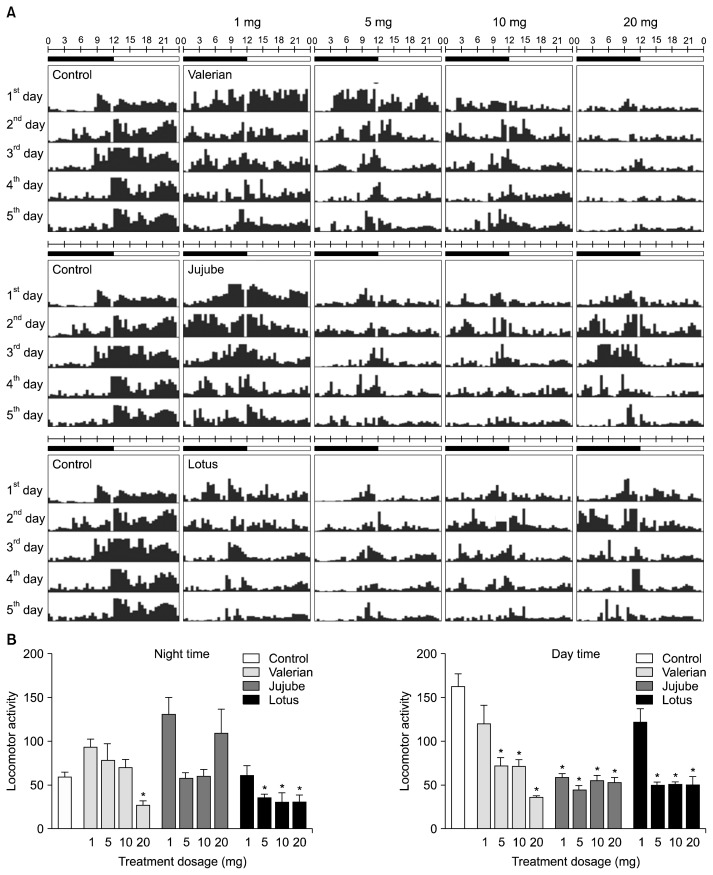
Locomotor activity was measured in the Drosophila model to evaluate the sleep activity of Korean medicinal herbs traditionally used as sleep aids. Fig. 1 shows the locomotor activity of flies treated with valerian, jujube, and lotus seed extract. The control group had a typical circadian rhythm, which was less active at night and more active during the day. Among the three herbs, the group treated with lotus seed extract showed less nocturnal movement. In particular, the 5-, 10-, and 20-mg treatments of lotus seed extract decreased nocturnal and diurnal locomotor activity compared to the other groups (Fig. 1).
Fig. 1.
Locomotor activity in valerian-, jujube-, and lotus-treated flies. Representative activity record (actogram) of a single fly recording from each treatment group. (A) Typical actograms of flies following doses of valerian (n=16), jujube (n=16), lotus (n=16), and the control (n=20). The average activity was measured at 30-min intervals for 5 days. The black and white bars above the actograms indicate night (22:00 to 10:00) and day (10:00 to 22:00). (B) Activity during subjective day and night. Values are the means±SEM for each group. *Significant differences at P<0.05 using Student’s t-test.
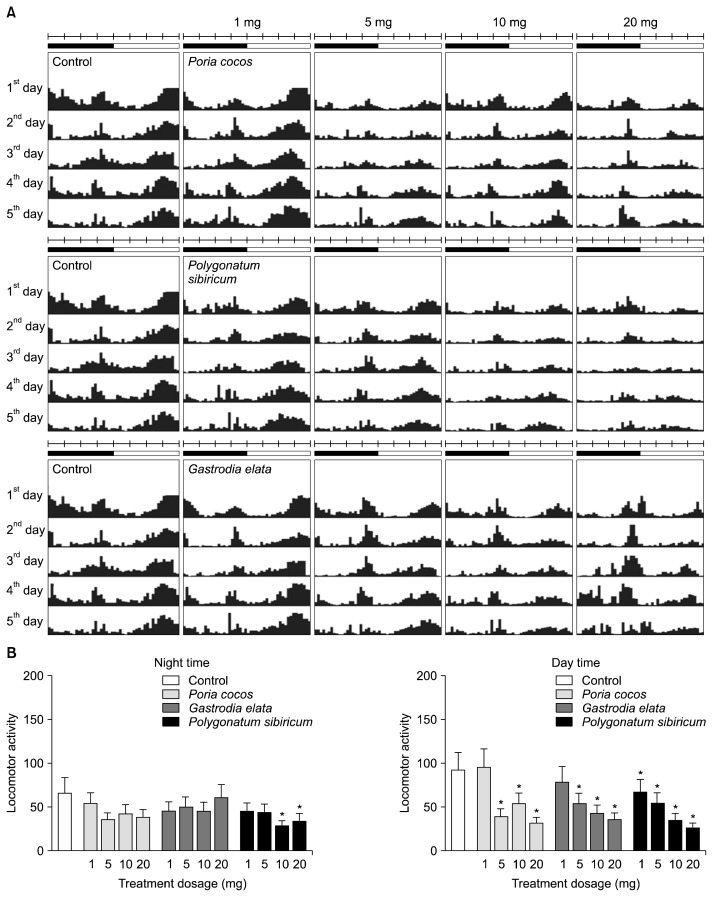
Behavioral changes were measured to determine the sleep activity induced by the other three herbs: G. elata, P. sibiricum, and P. cocos. As shown by the actogram in Fig. 2, nocturnal and diurnal movements tended to be low at high-concentration treatments. In particular, flies treated with P. sibiricum showed a lower level of locomotor activity than the others. The 10- and 20-mg treatments of P. sibiricum significantly reduced nocturnal locomotor activity compared to the control (P<0.05). P. sibiricum and G. elata showed a dose-dependent reduction in diurnal locomotor activity (Fig. 2B), although the 1-mg treatment of G. elata did not show significantly less locomotor activity than the control group. The above results showed that the lotus seed and P. sibiricum extracts exhibit sleep enhancement activity, as the locomotor activity of flies receiving those treatments changed compared to the control group.
Fig. 2.
Locomotor activity in Poria cocos-, Polygonatum sibiricum-, and Gastrodia elata-treated flies. Representative activity record (actogram) of a single fly from each treatment group. (A) Typical actograms of flies following doses of P. cocos (n=16), P. sibiricum (n=16), G. elata (n=16), and the control (n=20). Average activity was measured at 30-min intervals for 5 days. Black/white bars on top of the actograms indicate night (22:00 to 10:00) and day (10:00 to 22:00). (B) Activity during subjective day and night. Values are the means±SEM for each group. *Significant differences at P<0.05 using Student’s t-test.
Effects of lotus seed and P. sibiricum extracts on locomotor activity
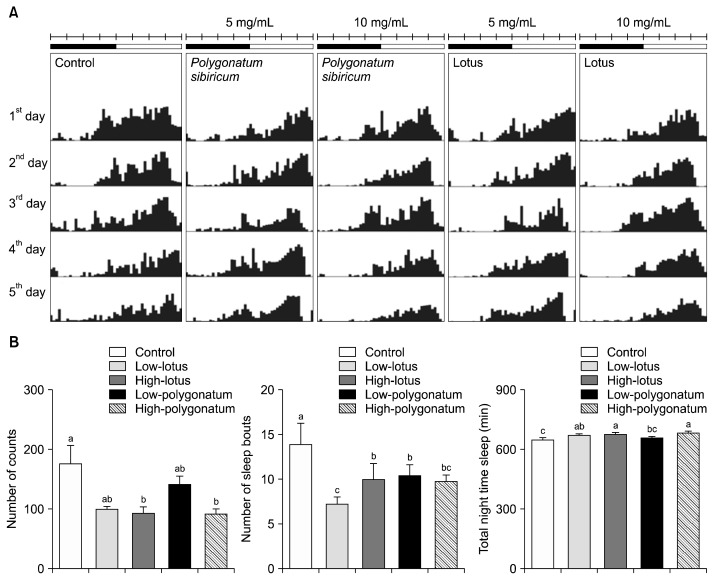
Fig. 3 shows nocturnal locomotor activity, sleep bouts, and total nocturnal sleep in the groups receiving lotus seed or P. sibiricum extracts. The locomotor activity of all groups was measured for 5 days using a DAM system (Fig. 3).
Fig. 3.
Effects of lotus seed and Polygonatum sibiricum extracts on the sleep behavior of fruit flies. (A) Subjective nocturnal activity, (B) number of sleep episodes, and (C) duration of subjective nocturnal sleep of the control group (sucrose-agar media group) and the groups receiving 5 mg/mL or 10 mg/mL of extract, as determined by the Drosophila Activity Monitoring (DAM) system. Values are the means±SEM for each group. Values not sharing common letters (a–c) are significantly different at P<0.05 using Tukey’s multiple comparisons test.
The nocturnal locomotor activity and individual sleep bout numbers were significantly decreased by high-dose treatment (10 mg/mL) of the extracts (Fig. 3). Low- and high-dose treatment groups (5 mg/mL) also showed a significant difference in numbers of sleep bouts (P<0.05). Flies that received high-dose treatment with lotus seed and P. sibiricum extracts had significantly higher total nighttime sleep than control flies (P<0.05).
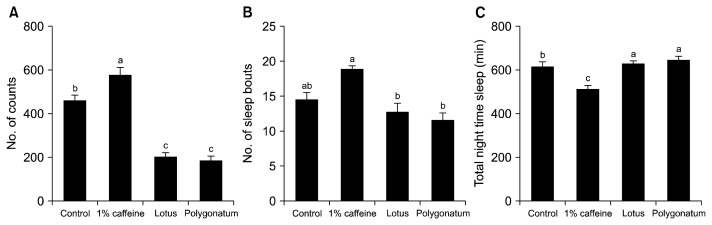
Effects of lotus seed and P. sibiricum extracts on a caffeine-induced arousal model
The effects of lotus seed or P. sibiricum extracts on 0.1% caffeine-induced Drosophila were investigated. Caffeine has been used to induce insomnia because it stimulates arousal behavioral activity and reduces sleep behavior. DAM was used to measure the locomotor activity of control flies and flies treated with caffeine, lotus seed extract, and P. sibiricum extract for 5 days (Fig. 4). Caffeine-treated Drosophila showed increased locomotor activity and decreased total nocturnal sleep (P<0.05). Flies treated with 10 mg lotus seed or P. sibiricum extracts showed significantly different locomotor activity and nocturnal sleep compared to Drosophila subjected to caffeine-induced arousal. However, there was no significant difference in locomotor activity, sleep episodes, or sleep time between flies treated with lotus seed and P. sibiricum extracts.
Fig. 4.
Effect of lotus seed and Polygonatum sibiricum extracts on fruit flies during caffeine-induced arousal. (A) Nocturnal activity, (B) sleep bout numbers, and (C) total nocturnal sleep of lotus seed and P. sibiricum extracts in flies receiving 0.1% caffeine, as measured by the Drosophila Activity Monitoring (DAM) system. Values are the means±SEM for each group. Values not sharing common letters (a–c) are significantly different at P<0.05 using Tukey’s multiple comparisons test.
DISCUSSION
Herbal/natural products are one of the most common forms of complementary and alternative medicine (13). They are readily available and are safe for long-term use (14). However, the mechanisms of action are not understood. The effects of these herbal medicines could not be compared from their results. No research has been conducted to investigate the sleep activities of Korean traditional herbs and the primary effects of their actions on patients with insomnia. In this study, the sleep activity of herbs that have traditionally been used as sleep-enhancing agents in Korea was measured in a Drosophila model.
The sleep activity induced by valerian, jujube, lotus seed powder, G. elata, P. sibiricum, and P. cocos was investigated. Due to multicomponent and multitarget actions, herbal medicines generally modulate several neurotransmitter systems, such as GABA, serotonin, and dopamine to treat psychiatric disorders (15). In search for the molecular mechanism underlying these traditional sleep-remedies, they were investigated for their ability to modulate the GABAA receptor. In traditional herbal medicine, valerian and jujube have been used as sedatives and hypnotic medicines to alleviate insomnia and anxiety (11,16). Conventional pharmacological treatments for insomnia include benzodiazepines/non-benzodiazepines (GABAA receptor agonists), antidepressants [serotonin (5-HT)2 receptor antagonists], and antihistamines (17). Jujube decreases monoaminergic system activity (18). Valerian extract and the constituent valerenic acid have been shown to modulate the GABAA receptor. Yuan et al. (19) reported that valerian extracts bound to the GABA receptor in vitro, and Dietz et al. (20) also reported another mechanism of sleep. Valerian extracts had high binding affinity for the 5-HT5A receptor. Sleep potentiating activity of valerian via GABA and 5-HT5A receptor has also been elucidated. Sleep effect of lotus was not reported, but sedative effects of lotus has been reported. Alkaloids of lotus showed a sedative-hypnotic effect by increasing the brain level of GABA, and by binding to the GABAA receptor (20). In our previous report (21), P. sibiricum contained GABA and tryptophan, which might be major contribute to the sleep promoting activity of the extract. The transcript level of GABAA-R2, GABAB-R1 receptor, and serotonin receptor mRNA were unchanged in the extract treated rat brain. These results confirmed that the extract possessed the sleep-promoting effect in a vertebrate model and that its GABA and tryptophan can elicit the same kind of pharmacological effects on the central nervous system. Lotus seed and P. sibiricum extracts showed higher sleep-potentiating activity than jujube and valerian (Fig. 1 and 2).
The lotus and P. sibiricum extract also showed sleep-potentiating activity in the caffeine-induced Drosophila arousal model (Fig. 4). Lotus is a perennial medicinal herb that has been widely used in Oriental medicine for centuries. Most parts, including the flowers, leaves, leaf stalks, seeds, and rhizomes, are used as both food and alternative medicine. Lotus seeds are rich in secondary metabolites such as flavonoids, alkaloids, saponins, tannins, and terpenoids, which have shown anxiolytic activity in various studies (22). Moreover, many studies have shown that alkaloids exhibit a variety of neuropharmacological activities, such as sedative, anticonvulsant, anti-depression, and neuroprotective effects (23,24). Thus, lotus seems to have the potential to exert therapeutic activity against central nervous system disorders such as anxiety, depression, and insomnia.
P. sibiricum, also known as Hwang Jeong in Korea, is a perennial medicinal herb belonging to the Liliaceae family. In Korea, P. sibiricum is widely consumed as tea. The plant has been reported to lower blood glucose and lipid levels and improve immune system activity and prevent aging (25). However, there is no scientific report on the sleep enhancement activity of water extracts of fresh P. sibiricum rhizomes. Various chemical components have been isolated from this species, including steroidal saponins, alkaloids, polysaccharides, flavonoids, and lignins (26,27). However, two major components of P. sibiricum, steroidal saponins and polysaccharides, exhibited extensive physiological activities and were intensively studied.
The rhizomes of P. sibiricum have been used as sleep enhancement therapy in folk remedies, but there is no scientific basis for this treatment. As shown in Fig. 3 and 4, the P. sibiricum rhizome has excellent sleep-potentiating activity. In our other results, the sleep potentiating activity of P. sibiricum rhizome extract was investigated in a vertebrate model by examining sleep profiles. The extract contains GABA and tryptophan, which can significantly contribute to sleep potentiation. The extract (160 mg/kg) significantly influenced sleep quality in pentobarbital-induced mice (21).
The above results suggest that both lotus seed and P. sibiricum extracts control sleep regulation. Further studies should be conducted to investigate the roles of lotus seed and P. sibiricum in sleep latency, quality, and quantity in a vertebrate model, and a molecular level analysis should be conducted to identify sleep mechanisms.
ACKNOWLEDGEMENTS
This research was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry (IPET) through the High Value-Added Food Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (116004-02-2-HD020).
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Siegel JM. Clues to the functions of mammalian sleep. Nature. 2005;437:1264–1271. doi: 10.1038/nature04285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahowald MW, Schenck CH. Insights from studying human sleep disorders. Nature. 2005;437:1279–1285. doi: 10.1038/nature04287. [DOI] [PubMed] [Google Scholar]
- 3.Cuesta M, Boudreau P, Boivin DB. Sleep Disorders Medicine. Springer; New York, NY, USA: 2017. Basic circadian timing and sleep-wake regulation; pp. 79–102. [DOI] [Google Scholar]
- 4.Emert SE, Tutek J, Lichstein KL. Associations between sleep disturbances, personality, and trait emotional intelligence. Personality and Individual Differences. 2017;107:195–200. doi: 10.1016/j.paid.2016.11.050. [DOI] [Google Scholar]
- 5.Sateia MJ, Buysse DJ, Krystal AD, Neubauer DN. Adverse effects of hypnotic medications. J Clin Sleep Med. 2017;13:839. doi: 10.5664/jcsm.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glass J, Lanctôt KL, Herrmann N, Sproule BA, Busto UE. Sedative hypnotics in older people with insomnia: meta-analysis of risks and benefits. BMJ. 2005;331:1169. doi: 10.1136/bmj.38623.768588.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pearson NJ, Johnson LL, Nahin RL. Insomnia, trouble sleeping, and complementary and alternative medicine: analysis of the 2002 National Health Interview Survey. Arch Intern Med. 2006;166:1775–1782. doi: 10.1001/archinte.166.16.1775. [DOI] [PubMed] [Google Scholar]
- 8.Dubowy C, Sehgal A. Circadian rhythms and sleep in Drosophila melanogaster. Genetics. 2017;205:1373–1397. doi: 10.1534/genetics.115.185157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung BY, Kilman VL, Keath JR, Pitman JL, Allada R. The GABAA receptor RDL acts in peptidergic PDF neurons to promote sleep in Drosophila. Curr Biol. 2009;19:386–390. doi: 10.1016/j.cub.2009.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Swinderen B, Nitz DA, Greenspan RJ. Uncoupling of brain activity from movement defines arousal states in Drosophila. Curr Biol. 2004;14:81–87. doi: 10.1016/j.cub.2003.12.057. [DOI] [PubMed] [Google Scholar]
- 11.Choi HS, Ko BS, Kim HD, Hong KB, Suh HJ. Effect of Valerian/Hop mixture on sleep-related behaviors in Drosophila melanogaster. Biol Pharm Bull. 2017;40:1101–1110. doi: 10.1248/bpb.b17-00262. [DOI] [PubMed] [Google Scholar]
- 12.Ko CH, Koon CM, Yu SL, Lee KY, Lau CB, Chan EH, Wing YK, Fung KP, Leung PC. Hypnotic effects of a novel anti-insomnia formula on Drosophila insomnia model. Chin J Integr Med. 2016;22:335–343. doi: 10.1007/s11655-014-1625-1. [DOI] [PubMed] [Google Scholar]
- 13.Singh A, Zhao K. Treatment of insomnia with traditional Chinese herbal medicine. Int Rev Neurobiol. 2017;135:97–115. doi: 10.1016/bs.irn.2017.02.006. [DOI] [PubMed] [Google Scholar]
- 14.Ernst E. When natural is not harmless. Int J Clin Pract. 2006;60:380–380. doi: 10.1111/j.1368-5031.2006.00924b.x. [DOI] [PubMed] [Google Scholar]
- 15.Sarris J, Panossian A, Schweitzer I, Stough C, Scholey A. Herbal medicine for depression, anxiety and insomnia: a review of psychopharmacology and clinical evidence. Eur Neuropsychopharmacol. 2011;21:841–860. doi: 10.1016/j.euroneuro.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 16.Zhao J, Li SP, Yang FQ, Li P, Wang YT. Simultaneous determination of saponins and fatty acids in Ziziphus jujuba (Suanzaoren) by high performance liquid chromatography-evaporative light scattering detection and pressurized liquid extraction. J Chromatogr A. 2006;1108:188–194. doi: 10.1016/j.chroma.2005.12.104. [DOI] [PubMed] [Google Scholar]
- 17.Fornaro M, Solmi M, Veronese N, De Berardis D, Buonaguro EF, Tomasetti C, Perna G, Preti A, Carta MG. The burden of mood-disorder/cerebrovascular disease comorbidity: essential neurobiology, psychopharmacology, and physical activity interventions. Int Rev Psychiatry. 2017;29:425–435. doi: 10.1080/09540261.2017.1299695. [DOI] [PubMed] [Google Scholar]
- 18.Hsieh MT, Chen HC, Kao HC, Shibuya T. Suanzaorentang, and anxiolytic Chinese medicine, affects the central adrenergic and serotonergic systems in rats. Proc Natl Sci Counc Repub China B. 1986;10:263–268. [PubMed] [Google Scholar]
- 19.Yuan CS, Mehendale S, Xiao Y, Aung HH, Xie JT, Ang-Lee MK. The gamma-aminobutyric acidergic effects of valerian and valerenic acid on rat brainstem neuronal activity. Anesth Analg. 2004;98:353–358. doi: 10.1213/01.ANE.0000096189.70405.A5. [DOI] [PubMed] [Google Scholar]
- 20.Dietz BM, Mahady GB, Pauli GF, Farnsworth NR. Valerian extract and valerenic acid are partial agonists of the 5-HT5a receptor in vitro. Brain Res Mol Brain Res. 2005;138:191–197. doi: 10.1016/j.molbrainres.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jo K, Kim HD, Kim S, Han SH, Choi HS, Suh HJ. Effect of the sleep-promoting by Polygonatum sibiricum rhizome extracts in vertebrate models. FASEB J. 2017;31:lb369. [Google Scholar]
- 22.Rajput MA, Khan RA. Phytochemical screening, acute toxicity, anxiolytic and antidepressant activities of the Nelumbo nucifera fruit. Metab Brain Dis. 2017;32:743–749. doi: 10.1007/s11011-017-9963-x. [DOI] [PubMed] [Google Scholar]
- 23.Martínez-Vázquez M, Estrada-Reyes R, Araujo Escalona AG, Ledesma Velázquez I, Martínez-Mota L, Moreno J, Heinze G. Antidepressant-like effects of an alkaloid extract of the aerial parts of Annona cherimolia in mice. J Ethnopharmacol. 2012;139:164–170. doi: 10.1016/j.jep.2011.10.033. [DOI] [PubMed] [Google Scholar]
- 24.Mukherjee PK, Saha K, Balasubramanian R, Pal M, Saha BP. Studies on psychopharmacological effects of Nelumbo nucifera Gaertn. rhizome extract. J Ethnopharmacol. 1996;54:63–67. doi: 10.1016/S0378-8741(96)01455-9. [DOI] [PubMed] [Google Scholar]
- 25.Liu L, Dong Q, Dong XT, Fang JN, Ding K. Structural investigation of two neutral polysaccharides isolated from rhizome of Polygonatum sibiricum. Carbohydr Polym. 2007;70:304–309. doi: 10.1016/j.carbpol.2007.04.012. [DOI] [Google Scholar]
- 26.Wang J, Lu CS, Liu DY, Xu YT, Zhu Y, Wu HH. Constituents from Polygonatum sibiricum and their inhibitions on the formation of advanced glycosylation end products. J Asian Nat Prod Res. 2016;18:697–704. doi: 10.1080/10286020.2015.1135905. [DOI] [PubMed] [Google Scholar]
- 27.Zhao X, Li J. Chemical constituents of the genus Polygonatum and their role in medicinal treatment. Nat Prod Commun. 2015;10:683–688. [PubMed] [Google Scholar]