Abstract
Saponins have been reported to possess several health beneficial activities including hypocholesterolemic, immune-stimulatory, and anticarcinogenic. The objectives of this study were to determine if soysaponins are radical scavengers and inducers of quinone reductase (QR) activity in Hepa1c1c7 murine hepatoma cell line. The antioxidant capacity of soyasaponin was evaluated using the 1,1′-diphenyl-2-picrylhydrazyl (DPPH) and 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) radical scavenging methods. Soyasaponin showed 75.7% radical scavenging activity in the DPPH assay and 81.4% in the ABTS method at 100 μg/mL concentration. Cellular proliferation was determined using the methylthiazolyldiphenyl-tetrazolium bromide colorimetric assay. Soyasaponin inhibited cell growth in a dose-dependent (0.1~100 μg/mL) manner, and growth inhibition was 30% and 39% at 100 μg/mL of saponin after 24 h and 48 h incubation, respectively. Soyasaponin showed QR induction in a dose-dependent manner. Ten, 50, and 100 μg/mL of soyasaponin resulted in a 1.6-, 2.2-, and 2.9-fold induction of QR, respectively. These results provide a basis for the potential of soysaponin as a chemopreventive agent.
Keywords: soyasaponin, antioxidant, cancer, quinone reductase, Hepa1c1c7 cell
INTRODUCTION
Saponins are triterpenoid or steroid glycosides naturally occurring plants. Relatively high concentrations of saponins have been reported in soybeans and soy products (1,2). Saponins are naturally occurring amphiphilic compounds present in many foods of plant origin. Legumes, in particular, are rich sources of dietary saponins (3,4). Saponins are complex glycosidic compounds primarily present in a diverse array of edible and inedible plants (5). Soybeans, however, are one of the major sources of glycosidic compounds found in the human food supply. Although soy contains other glycolipids (steryl glucosides and esterified steryl glucosides), saponins are one of the most investigated glycolipids (6). Linked to one or more sugar molecules, saponins consist of a steroid or triterpene group (the aglycone) and have characteristic surface activity (7). A detergent-like action has also been attributed to saponins. This arises from its water-soluble carbohydrate molecules being mixed with its fat-soluble saponin portion.
There has been much attention given to the health effects of soy consumption. Soybean saponins have been considered major active components contributing to the cholesterol-lowering effect of soy products (8). They were reported to inhibit tumor development in vitro and in vivo, especially in colon cancer models (9,10). Soysaponin I showed antihepatotoxic activity against carbon tetrachloride damage in primary cultured rat hepatocytes (11).
Saponins have been shown to provide antioxidant and cell-protective properties (12), immunopotentiating benefits (13) for both humoral and cellular responses (14), antiviral activity, and inhibitory actions against human immunodeficiency virus infection, offering potential for the treatment of retroviral infections (15). Other research points to the antibiotic, expectorant (16), and potential cancer protective benefits that saponins may provide. Further research supporting the cancer protective properties of saponins suggests that they may have cytotoxic and growth inhibitory effects on tumor cells (5), while providing antimutagenic activity (17,18).
Dietary saponins from soybeans and other sources have been shown to enhance immunity (12,19), are cytotoxic to Sarcoma 37 cells (20), inhibit DNA synthesis in tumor cells (21), decrease the growth of human epidermoid carcinoma cells (22) and human cervical carcinoma cells (23), and inhibit Epstein-Barr virus genome expression (24).
There are two categories of detoxification enzymes, phase I and II enzymes. Phase I enzymes modify the structures of foreign compounds via oxidation, reduction, or hydrolyzation reactions (25). Phase II enzymes can add, or conjugate endogenous compounds to the modified foreign compounds (25). Usually, phase II enzymes compete with phase I activating enzymes to limit the generation of electrophiles, thus reducing the risk of cancer initiation (26). Therefore, the maintenance of elevated levels of phase II enzymes in body tissues provides for a cancer chemopreventive defense against highly reactive electrophiles. Various synthetic organic compounds, such as β-naphthoflavone (BNF), tert-butylhydrquinone, butylated hydroxytoluene (BHT), and butylated hydroxyanisole, have been reported to be potent chemopreventive agents because they can induce phase II enzymes in cultured murine hepatoma cells (26).
The objectives of this study were to determine if soyasaponins are radical scavengers and inducers of phase II quinone reductase (QR) activity in Hepa1c1c7 murine hepatoma cell line.
MATERIALS AND METHODS
Materials
Hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), 1,1′-diphenyl-2-picrylhydrazyl (DPPH), 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium (ABTS), potassium persulfate, L-ascorbic acid, α-tocopherol, and BHT were obtained from Sigma Chemical Company (St. Louis, MO, USA).
Cell culture
Hepa1c1c7 cells (KCLB 22026) were obtained from the Korean Cell Line Bank (Seoul, Korea), and cell culture supplies were purchased from Sigma Chemical Company. Cells were maintained at 37°C with 5% CO2 in minimum essential medium (high glucose) (Sigma Chemical Company) supplemented with 10% fetal bovine serum (FBS), 2.2 g/L NaHCO3, penicillin (40 μg/mL), and streptomycin (100 μg/mL).
Preparation of soyasaponins
Semi-purified soyasaponins were prepared from a commercial preparation of soybean saponins (Wako Pure Chemical Industries, Ltd., Osaka, Japan), a fine yellow-brown powder. Nonsaponin constituents were extracted from the preparation by gently agitating the 3 g of saponins in 30 mL acetone at room temperature, and then centrifuging the mixture at 2,000 g for 30 min. This acetone extraction was repeated three times. The residue was then extracted in distilled water three times, and then washed in acetone to facilitate drying. The purified soyasaponin (3.3 mg) obtained was a white-yellowish powder, but the acetone and water extracts were yellow in color.
DPPH radical scavenging assay
The DPPH radical scavenging capacity was determined using the method of the Lee et al. (27) with slight modifications. The soyasaponin, ascorbic acid, α-tocopherol, or BHT was standardized to give a stock solution (25 mg/mL) and filtered through a 20 μm Whatman paper no 4. Aliquots (25 μL) were placed in a cuvette, and an ethanolic solution of DPPH (100 μM) was added to a final volume of 1 mL. The decrease in absorbance at 515 nm was determined continuously with data capturing at 30 s intervals using a UV-1601 PC spectrophotometer (Shimadzu Corporation, Kyoto, Japan). The degree of DPPH radical scavenging activity of the antioxidants was calculated as percentage of inhibition (% inhibition) using the following equation:
where Abscontrol is the absorbance at 0 min and Abssample is the absorbance of the sample at 5 min. An EC50 value was determined as the concentration that elicited a half-maximal response.
ABTS radical assay
The antioxidant scavenging capacity was determined using our modification of the technique of Pellegrini et al (28). Briefly, the ABTS radical cation was prepared by reacting 7 mM aqueous solution of ABTS with 2.45 mM potassium persulfate and diluted in ethanol to an absorbance of 0.70±0.20 at 734 nm. Then, 1 mL of this diluted solution was added to 100 μL of soyasaponin (final concentration of 3.1, 6.3, 12.5, 25, 50, and 100 μg/mL), ascorbic acid, α-tocopherol, and BHT. The absorbance was determined exactly 1 min after initial mixing. Appropriate solvent blanks were run for each assay.
Cell proliferation assay
The proliferation of cells was determined using the methylthiazolyldiphenyl-tetrazolium bromide (MTT) assay. To evaluate the effect of the soyasaponin on cell viability, the cells were seeded at a density of 1×104 cells per well containing 100 μL of culture medium in 96-well plates. After cultivation for 24 h, the medium was changed to fresh medium supplemented with the soyasaponin. The soyasaponin dissolved in medium was added at a final concentration of 0.1, 1, 10, or 100 μg/mL. After the cultivation for 24 or 48 h, cell viability was evaluated by the MTT assay. MTT (Sigman M5655) was dissolved in phosphate-buffered saline at 5 mg/mL and filtered to sterilize and remove a small amount of insoluble residue. Ten μL of the MTT stock solution (10 μL MTT+90 μL of phenol red-free and serum free medium) was added to all the wells of the 96-well plate culture. After the plate was incubated for 4 h at 37°C, dimethyl sulfoxide was added to the wells and mixed by pipetting to dissolve the dark blue formazan product. The absorbance of dissolved formazan in each well was measured with a microplate spectrophotometer at 570 nm with a reference wavelength of 690 nm. Cell viability was calculated by the following formula:
The wells without the soyasaponin and the cells (culture medium alone) were used as the blank.
Reduced nicotinamide adenine dinucleotide phosphate [NAD(P)H]:quinone reductase activity
Quinone reductase was measured using a spectrophotometric assay (29). Briefly, an aliquot of 300 μL cytosol was added to the 2.65 mL reaction mixture [final concentration: 25 mM Tris buffer, 0.7 mg bovine serum albumin (BSA), 0.01% Tween 20, 5 μM flavin adenine dinucleotide, 0.2 mM NAD(P)H]. The reaction was started by the addition of 10 μL 12 mM 2,6-dichlorophenol-indophenol (DPIP), and the product was measured at 600 nm over 90 s, using a Hitachi U-2000 spectrophotometer (Hitachi High Technologies, Dallas, TX, USA). Activity was measured in the presence and absence of dicumarol (10 μM). The dicumarol sensitive portion of the activity was taken as a measure of QR activity.
Protein analysis
Protein in cytosolic preparations was measured using a BioRad protein assay kit (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Samples were analyzed in triplicate with BSA as a standard.
Isolation of total RNA
Hepa1c1c7 cells were plated in 100-mm culture dishes at a density of 3×106 cells in 100 mL medium. After pre-incubation for 24 h, each plate was filled with fresh FBS-free medium that contained various concentrations of saponins. The cells were then incubated for a further 24 h at 37°C. Total RNA was isolated using TRIzol reagent (Life Science Technologies Ltd., Boone, NC, USA). The homogenized samples were incubated for 5 min at room temperature to allow the complete dissociation of nucleoprotein complexes. After the addition of 0.2 volumes of chloroform, samples were shaken vigorously for 15 s, incubated for 2~3 min, and centrifuged at 12,000 g for 15 min at 4°C. The total RNA remaining in the upper aqueous phase was precipitated by mixing with an equal volume of isopropanol. The mixtures were incubated for 10 min at 4°C and centrifuged at 12,000 g for 10 min at 4°C. The total RNA pellet was washed with 70% ethanol, dried, and dissolved in RNase-free water. The concentration and purity of total RNA were calculated by measuring the absorbance at 260 and 280 nm.
Semi-quantitative reverse transcription (RT)-polymerase chain reaction (PCR)
First-strand cDNA was synthesized with 1 μg of total RNAs and 1 μM of oligo (dT15) primer using Omniscript Reverse Transcriptase (Qiagen, Valencia, CA, USA). The primers used in this study are shown in Table 1. The PCR consisted of initial denaturation at 94°C for 3 min, 3-step cycling (30 cycles) at 94°C for 1 min, 60°C for 1 min, and 72°C for 1 min, and final extension at 72°C for 10 min. The amplified PCR products were loaded into 1.0% agarose gel. After ethidium bromide staining, the gel was illuminated on the UV transilluminator, and it was photographed using a Polaroide (Kodak, Needham, MA, USA). The densities of bands were measured by ImageJ version 1.34 software program (National Institutes of Health, Bethesda, MD, USA).
Table 1.
Sequences of primers used in this study
| Target gene | Primer | Sequence (5′→3′) |
|---|---|---|
| QR | Sense | TCG GAG AAC TTT CAG TAC CC |
| Antisense | TGC AGA GAG TAC ATG GAG CC | |
| GAPDH | Sense | GAC CCC TTC ATT GAC CTC AAC |
| Antisense | CAT ACC AGG AAA TGA GCT TG |
QR, quinone reductase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
Statistical analysis
Statistical analysis was performed using the SPSS Statistical Analysis System (version 17.0, SPSS Inc., Chicago, IL, USA). Data were analyzed using ANOVA and Duncan’s multiple test; P<0.05 was considered significant.
RESULTS AND DISCUSSION
Antioxidant activity
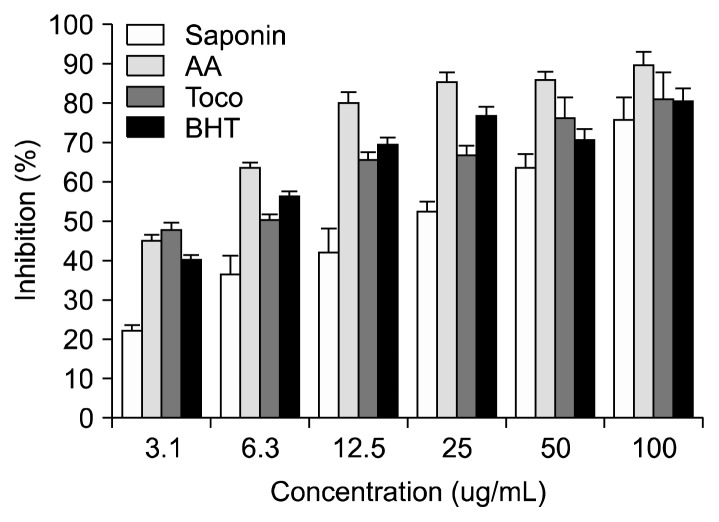
The antioxidant potential of soyasaponins was measured by different chemical assays: DPPH and ABTS assays. Fig. 1 shows the DPPH free radical scavenging activity of soyasaponins compared with known antioxidants of ascorbic acid, α-tocopherol, and BHT. The scavenging activity of ascorbic acid, α-tocopherol, and BHT, used as positive controls, were relatively more pronounced than that of soyasaponins. We found a direct dose-response relationship between soyasaponin concentration and antioxidant activity as determined from DPPH removal. The scavenging effects of soyasaponins and positive controls on the DPPH radical decreased in the order: ascorbic acid, BHT≈α-tocopherol, and soyasaponin, which were at the concentration of a 100 μg/mL. Free radical scavenging activities of these samples also increased with increasing concentrations.
Fig. 1.
Effects of soyasaponin and known antioxidants on DPPH-induced free radical scavenging activity. Data are mean±standard deviation (n=5). Saponin, soyasaponins; AA, ascorbic acid; Toco, α-tocopherol; BHT, butylated hydroxytoluene.
DPPH is a free radical that forms a stable molecule on accepting an electron or a hydrogen atom. In the DPPH assay, the antioxidants were able to reduce the stable radical DPPH to the yellow-colored diphenyl-picrylhydrazine (30). The method is based on the reduction of alcoholic DPPH solution in the presence of a hydrogen-donating antioxidant due to the formation of the non-radical form DPPH-H by the reaction (30). Fig. 2 illustrates the effects of soyasaponins on the suppression of the absorbance of the ABTS radical cation at 734 nm. The known antioxidants ascorbic acid, α-tocopherol, and BHT were used as positive controls. We also found a direct dose-response relationship between soyasaponin concentration and antioxidant activity as determined by the ABTS assay. Soyasaponins showed antioxidant potential, but a weaker radical scavenging power compared with ascorbic acid, α-tocopherol, and BHT. The scavenging effects of soyasaponins and the positive controls on the ABTS radical decreased in the order: ascorbic acid, BHT, α-tocopherol, and soyasaponin, which were at the concentration of a 50 μg/mL.
Fig. 2.
Effects of soyasaponin and known antioxidants on ABTS-induced free radical scavenging activity. Data are mean±standard deviation (n=5). Saponin, soyasaponins; AA, ascorbic acid; Toco, α-tocopherol; BHT, butylated hydroxytoluene.
Lee et al. (31) reported that 70.2 mM soyasaponin scavenged 50% of DPPH radicals comparable to the 50% DPPH radical scavenging activity of 52.1 mM α-tocopherol. Soyasaponin treatment decreased the lipopolysaccharide elevated levels of reactive oxygen species (ROS) and increased superoxide dismutase activity (32). Soyasaponins scavenged ROS and changed the NF-κB signaling pathways through increasing the regulation of an important anti-oxidation system (32).
Cell proliferation assay
Fig. 3 illustrates the effects of saponins at concentrations of 0.1~100 μg/mL on the growth of Hepa1c1c7 cells after 24 and 48 h of incubation. Soyasaponins inhibited the Hepa1c1c7 cell proliferation in a dose-dependent manner (n=5). At saponin concentrations of 0.1~100 μg/mL, the cell numbers were significantly reduced by 1.4~38% compared with the control. After 48 h of incubation, the cell numbers were decreased by 27% with 10 μg/mL soyasaponin and by 38% with 100 μg/mL soyasaponin.
Fig. 3.
Effect of soyasaponin on Hepa1c1c7 cell proliferation measured by MTT assay. Data are mean±standard deviation (n=4).
In soy and other saponin-containing plants, the saponin and glycoside contents vary considerably and depend on the plant part being extracted, plant species, environmental storage, and processing conditions (31,33). Zhang and Popovich (34) reported that soyasaponin inhibited HepG2 human hepatocellular carcinoma cell proliferation in a dose-response manner with 72 h LC50 values of 0.594±0.021 mg/mL. Soyasaponins have also been reported to protect against tert-butyl-hydroperoside damage in HepG2 cells and rat hepatocytes (11). Xiao et al. (35) showed that soyasaponins inhibit the proliferation of Hela human cervical carcinoma cell in dose- and time-dependent manners at the concentrations of 100~800 mg/L measured by the MTT assay. Soyasaponins decreased HT-29 human cancer cell growth in dose- and time-dependent manners by inducing differentiation and increasing alkaline phosphatase activity, suppressing protein kinase C and cyclooxygenase-2 activities (36,37). Soyasaponins effectively inhibited the growth of human hepatoma QGY-7703 and HCT-15 colon cancer cell proliferation by apoptosis and S-phase cell cycle arrest, respectively (38,39).
NAD(P)H:quinone reductase activity
Soyasaponin induced QR activity at all concentrations (Fig. 4). The QR activity was expressed based on the protein concentration of each treatment. The positive control (1 μM BNF) showed 106.5 nM DPIP/min, which was about a 2-fold induction of QR compared with the activity of the negative control (53.2 nM DPIP/min). The dose-dependent induction of QR was observed from 10 to 100 μg/mL soyasaponin, reaching maximum inductions of 2.2- and 2.9-fold with 50 and 100 μg/mL soyasaponin, respectively.
Fig. 4.
Effects of soyasaponin on catalytic activity of quinone reductase (QR) in Hepa1c1c7 cells treated for 24 h. Data are mean±standard deviation (n=5). The different letters (a-c) indicate significant differences at (P<0.05). DPIP, dichlorophenol-indophenol; BNF, β-naphthoflavone.
Fig. 5 shows the QR mRNA levels in Hepa1c1c7 quantified by RT-PCR with an endogenous standard (glyceraldehyde-3-phosphate dehydrogenase) after cells were exposed to saponins for 24 h. Saponins expressed dose-dependently the QR mRNA in Hepa1c1c7 cells and 50 and 100 μg/mL saponins for 2- and 4-fold increases. Phase II enzymes (such as glutathione transferase, NAD(P)H: quinone reductase, peroxide hydrolase, heme oxygenase, and uridine diphosphate-glucuronosyltransferase) are a series of enzymes that cause detoxification of reactive metabolites generated by phase I enzymes, typically cytochrome P450 (40). They play important roles in the detoxification of electrophiles, protecting against carcinogenesis and mutagenesis (40,41). QR is an important phase II enzyme in detoxification of quinones and measurement of the induction of QR provides a rapid and reliable indicator of the ability of compounds to induce antioxidant responsive element regulated enzymes (40). QR is generally thought to act as an antioxidant by preventing the formation of hydrogen peroxide from redox cycling of quinones and to not have direct effects on hydrogen peroxide, which was used to induce DNA in the present study.
Fig. 5.
Expression of quinone reductase (QR) mRNA in Hepa1c1c7 cells by RT-PCR analysis after the cells were treated with different concentrations of saponin for 24 h. Experiment was repeated three times with similar results. (A) Electrophoresis gel photo of PCR products. (B) The ratio of QR/glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in the cells treated with different concentrations of saponin by measuring densitometry. Data are mean±standard deviation (n=3). The different letters (a–c) indicate significant differences at (P<0.05).
ACKNOWLEDGEMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF-2016R1A2B4014977).
Footnotes
AUTHOR DISCLOSURE STATEMENT
The author declares no conflict of interest.
REFERENCES
- 1.Kerwin SM. Soy saponins and the anticancer effects of soybeans and soy-based foods. Curr Med Chem Anticancer Agents. 2004;4:263–272. doi: 10.2174/1568011043352993. [DOI] [PubMed] [Google Scholar]
- 2.Kao TH, Chen BH. Functional components in soybean cake and their effects on antioxidant activity. J Agric Food Chem. 2006;54:7544–7555. doi: 10.1021/jf061586x. [DOI] [PubMed] [Google Scholar]
- 3.Fenwick DE, Oakenfull D. Saponin content of food plants and some prepared foods. J Sci Food Agric. 1983;34:186–191. doi: 10.1002/jsfa.2740340212. [DOI] [PubMed] [Google Scholar]
- 4.Shi J, Arunasalam K, Yeung D, Kakuda Y, Mittal G, Jiang Y. Saponins from edible legumes: chemistry, processing, and health benefits. J Med Food. 2004;7:67–78. doi: 10.1089/109662004322984734. [DOI] [PubMed] [Google Scholar]
- 5.Oakenfull D, Sidhu GS. Could saponins be a useful treatment for hypercholesterolaemia? Eur J Clin Nutr. 1990;44:79–88. [PubMed] [Google Scholar]
- 6.Cooke HJ. Neurobiology of the intestinal mucosa. Gastroenterology. 1986;90:1057–1081. doi: 10.1016/0016-5085(86)90889-9. [DOI] [PubMed] [Google Scholar]
- 7.Sidhu GS, Oakenfull DG. A mechanism for the hypocholesterolaemic activity of saponins. Br J Nutr. 1986;55:643–649. doi: 10.1079/BJN19860070. [DOI] [PubMed] [Google Scholar]
- 8.Potter SM. Overview of proposed mechanisms for the hypocholesterolemic effect of soy. J Nutr. 1995;125:606S–611S. doi: 10.1093/jn/125.3_Suppl.606S. [DOI] [PubMed] [Google Scholar]
- 9.Rao AV, Sung MK. Saponins as anticarcinogens. J Nutr. 1995;125:717S–724S. doi: 10.1093/jn/125.3_Suppl.717S. [DOI] [PubMed] [Google Scholar]
- 10.Koratkar R, Rao AV. Effect of soya bean saponins on azoxymethane-induced preneoplastic lesions in the colon of mice. Nutr Cancer. 1997;27:206–209. doi: 10.1080/01635589709514526. [DOI] [PubMed] [Google Scholar]
- 11.Miyao H, Arao T, Udayama M, Kinjo J, Nohara T. Kaikasaponin III and soyasaponin I, major triterpene saponins of Abrus cantoniensis, act on GOT and GPT: influence on transaminase elevation of rat liver cells concomitantly exposed to CCl4 for one hour. Planta Med. 1998;64:5–7. doi: 10.1055/s-2006-957355. [DOI] [PubMed] [Google Scholar]
- 12.Maharaj I, Froh KJ, Campbell JB. Immune responses of mice to inactivated rabies vaccine administered orally: potentiation by Quillaja saponin. Can J Microbiol. 1986;32:414–420. doi: 10.1139/m86-078. [DOI] [PubMed] [Google Scholar]
- 13.Nakashima H, Okubo K, Honda Y, Tamura T, Matsuda S, Yamamoto N. Inhibitory effect of glycosides like saponin from soybean on the infectivity of HIV in vitro. AIDS. 1989;10:655–658. doi: 10.1097/00002030-198910000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Oakenfull D. Saponins in food–a review. Food Chem. 1981;7:19–40. doi: 10.1016/0308-8146(81)90019-4. [DOI] [Google Scholar]
- 15.Hilf R, Goldenberg H, Michel I, Orlando RA, Archer FL. Enzymes, nucleic acids, and lipids in human breast cancer and normal breast tissue. Cancer Res. 1970;30:1874–1882. [PubMed] [Google Scholar]
- 16.Perkins RG, Kummerow FA. Major lipid classes in plasma membrane isolated from liver of rats fed a hepatocarcinogen. Biochim Biophys Acta. 1976;424:469–480. doi: 10.1016/0005-2760(76)90036-9. [DOI] [PubMed] [Google Scholar]
- 17.Sindambiwe JB, Calomme M, Geerts S, Pieters L, Vlietinck AJ, Vanden Berghe DA. Evaluation of biological activities of triterpenoid saponins from Maesa lanceolata. J Nat Prod. 1998;61:585–590. doi: 10.1021/np9705165. [DOI] [PubMed] [Google Scholar]
- 18.Berhow MA, Wagner ED, Vaughn SF, Plewa MJ. Characterization and antimutagenic activity of soybean saponins. Mutat Res. 2000;448:11–22. doi: 10.1016/S0027-5107(99)00225-0. [DOI] [PubMed] [Google Scholar]
- 19.Bomford R. Studies on the cellular site of action of the adjuvant activity of saponin for sheep erythrocytes. Int Arch Allergy Appl Immunol. 1982;67:127–131. doi: 10.1159/000233002. [DOI] [PubMed] [Google Scholar]
- 20.Huang HP, Cheng CF, Lin WQ, Yang GX, Song JY, Ren GY. Antitumor activity of total saponins from Dolichos falactus Klein. Acta Pharmacol Sin. 1982;3:286–288. [PubMed] [Google Scholar]
- 21.Zhang YD, Shen JP, Song J, Wang YL, Shao YN, Li CF, Zhou SH, Li YF, Li DX. Effects of Astragalus saponin 1 on cAMP and cGMP levels in plasma and DNA synthesis in the regenerating liver. Yao Xue Xue Bao. 1984;19:619–621. [PubMed] [Google Scholar]
- 22.Aswal BS, Bhakuni DS, Goel AK, Kar K, Mehrotra BN, Mukherjee KC. Screening of Indian plants for biological activity: Part X. Indian J Exp Biol. 1984;22:312–332. [PubMed] [Google Scholar]
- 23.Sati OP, Pant G, Nohara T, Sato A. Cytotoxic saponins from asparagus and agave. Pharmazie. 1985;40:586. [PubMed] [Google Scholar]
- 24.Tokuda H, Konoshima T, Kozuka M, Kimura T. Inhibitory effects of 12-O-tetradecanoylphorbol-13-acetate and teleocidin B induced epstein-barr virus by saponin and its related compounds. Cancer Lett. 1988;40:309–317. doi: 10.1016/0304-3835(88)90090-0. [DOI] [PubMed] [Google Scholar]
- 25.Ingelman-Sundberg M. Genetic susceptibility to adverse effects of drugs and environmental toxicants: the role of the CYP family of enzymes. Mutat Res. 2001;482:11–19. doi: 10.1016/S0027-5107(01)00205-6. [DOI] [PubMed] [Google Scholar]
- 26.Wilkinson J, 4th, Clapper ML. Detoxication enzymes and chemoprevention. Proc Soc Exp Biol Med. 1997;216:192–200. doi: 10.3181/00379727-216-44169. [DOI] [PubMed] [Google Scholar]
- 27.Lee CH, Yang L, Xu JZ, Yeung SYV, Huang Y, Chen ZY. Relative antioxidant activity of soybean isoflavones and their glycosides. Food Chem. 2005;90:735–741. doi: 10.1016/j.foodchem.2004.04.034. [DOI] [Google Scholar]
- 28.Fellegrini N, Ke R, Yang M, Rice-Evans C. Screening of dietary carotenoids and carotenoid-rich fruit extracts for antioxidant activities applying 2,2′-azinobis(3-ethylenebenzothiazoline-6-sulfonic acid radical cation decolorization assay. Methods Enzymol. 1999;299:379–389. doi: 10.1016/S0076-6879(99)99037-7. [DOI] [Google Scholar]
- 29.Ernster L, Atallah AS, Hochstein P. DT diaphorase and the cytotoxicity and mutagenicity of quinone-derived oxygen radicals. Prog Clin Biol Res. 1986;209A:353–363. [PubMed] [Google Scholar]
- 30.Gülçin İ, Mshvildadze V, Gepdiremen A, Elias R. Screening of antiradical and antioxidant activity of monodes-mosides and crude extract from Leontice smirnowii tuber. Phytomedicine. 2006;13:343–351. doi: 10.1016/j.phymed.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 31.Lee IA, Park YJ, Yeo HK, Han MJ, Kim DH. Soyasaponin I attenuates TNBS-induced colitis in mice by inhibiting NF-κB pathway. J Agric Food Chem. 2010;58:10929–10934. doi: 10.1021/jf102296y. [DOI] [PubMed] [Google Scholar]
- 32.Zha L, Chen J, Sun S, Mao L, Chu X, Deng H, Cai J, Li X, Liu Z, Cao W. Soyasaponins can blunt inflammation by inhibiting the reactive oxygen species-mediated activation of PI3K/Akt/NF-κB pathway. PLoS One. 2014;9:e107655. doi: 10.1371/journal.pone.0107655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Güçlü-Ustündağ O, Mazza G. Saponins: properties, applications and processing. Crit Rev Food Sci Nutr. 2007;47:231–258. doi: 10.1080/10408390600698197. [DOI] [PubMed] [Google Scholar]
- 34.Zhang W, Popovich DG. Effect of soyasapogenol A and soyasapogenol B concentrated extracts on Hep-G2 cell proliferation and apoptosis. J Agric Food Chem. 2008;56:2603–2608. doi: 10.1021/jf0731550. [DOI] [PubMed] [Google Scholar]
- 35.Xiao JX, Huang GQ, Zhang SH. Soyasaponins inhibit the proliferation of Hela cells by inducing apoptosis. Exp Toxicol Pathol. 2007;59:35–42. doi: 10.1016/j.etp.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 36.Oh YJ, Sung MK. Soybean saponins inhibit cell proliferation by suppressing PKC activation and induce differentiation of HT-29 human colon adenocarcinoma cells. Nutr Cancer. 2001;39:132–138. doi: 10.1207/S15327914nc391_18. [DOI] [PubMed] [Google Scholar]
- 37.Kim HY, Yu R, Kim JS, Kim YK, Sung MK. Antiproliferative crude soy saponin extract modulates the expression of IκBα, protein kinase C, and cyclooxygenase-2 in human colon cancer cells. Cancer Lett. 2004;210:1–6. doi: 10.1016/j.canlet.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 38.Huang J, Zhang M, Li Z, Niu W, Zhang SH. Study on the inhibitory effects of soybean saponins on human hepatoma cell. Acta Nutr Sin. 2004;26:477–481. [Google Scholar]
- 39.Ellington AA, Berhow MA, Singletary KW. Inhibition of Akt signaling and enhanced ERK1/2 activity are involved in induction of macroautophagy by triterpenoid B-group soyasaponins in colon cancer cells. Carcinogenesis. 2006;27:298–306. doi: 10.1093/carcin/bgi214. [DOI] [PubMed] [Google Scholar]
- 40.Zhao Q, Yang XL, Holtzclaw WD, Talalay P. Unexpected genetic and structural relationships of a long-forgotten flavoenzyme to NAD(P)H : quinone reductase (DT-diaphorase) Proc Natl Acad Sci USA. 1997;94:1669–1674. doi: 10.1073/pnas.94.5.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwak MK, Egner PA, Dolan PM, Ramos-Gomez M, Groopman JD, Itoh K, Yamamoto M, Kensler TW. Role of phase 2 enzyme induction in chemoprotection by dithiolethiones. Mutat Res. 2001;480–481:305–315. doi: 10.1016/S0027-5107(01)00190-7. [DOI] [PubMed] [Google Scholar]