Abstract
Butein is reported to have many biological effects, including anti-fibrogenic, anti-cancer, and anti-inflammatory activities. This study investigated the effects of butein on adipocyte differentiation and the Nrf2/heme oxygenase-1 (HO-1) pathway’s involvement in its anti adipogenic mechanism. Butein treatment reduced protein expression of key adipogenic transcription factors such as CCAAT-enhancer-binding protein α (C/EBPα) and peroxisome proliferator-activated receptor γ (PPARγ). At a concentration of 5, 10, and 25 μM butein, PPARγ was decreased by 78.8, 68.3, and 31.4% and C/EBPα by 87.3, 71.7, and 42.1%, respectively. Butein also increased Nrf2 and HO-1 protein expression in a dose-dependent manner. Treatment with zinc protoporphyrin, a specific HO-1 inhibitor, abolished the inhibitory effects of butein on adipogenic transcription factor protein expression. Therefore, butein inhibits adipogenesis, at least partially, through upregulation of the Nrf-2/HO-1 signaling pathway in 3T3-L1 adipocytes.
Keywords: butein, adipogenesis, obesity, heme oxygenase, 3T3-L1 cells
INTRODUCTION
Obesity has emerged as a critical health problem due to its close association with metabolic diseases such as diabetes and cardiovascular disease (1). Obesity is induced by increased adipocyte size and number (2). Adipocyte differentiation from preadipocytes is a complicated process involving modifications to cellular morphology, gene expression, and hormone sensitivity (3). This procedure is controlled by complicated interactions between adipogenic factors (4). In this system, peroxisome proliferator-activator receptor-γ (PPARγ) and CCAAT/enhancer-binding protein α (C/EBPα) act as the key regulators of adipogenesis (5). These transcription factors are significant for lipid homeostasis as they regulate target genes associated with fat accumulation, fatty acid transport, and lipolysis (6).
Oxidative stress is considered one of the major factors involved in obesity-related disease. Nuclear factor erythroid-derived factor 2-related factor 2 (Nrf2) is a major regulator of antioxidant and detoxification gene expression in response to oxidative stress (7). The role of Nrf2 is to activate cytoprotective enzymes and expression of antioxidant genes such as glutathione S-transferases, NAD(H)P oxidoreductase 1, and heme oxygenase-1 (HO-1) in response to oxidative stress (8). HO-1 is recognized to play a critical role in antioxidant protective processes. This enzyme catalyzes the rate-limiting stage of free heme degradation into iron, carbon monoxide, and biliverdin. These metabolites are associated with antioxidant activities (9). In addition, some studies have reported that HO-1 plays a critical role in energy metabolism beyond oxidative stress (10). Therefore, Nrf2 and its downstream enzyme, HO-1, may be a potential target in treating obesity-related diseases.
Chalcones, a subclass of flavonoids, possess an open C-ring structure. It has been reported that chalcone and its derivatives have numerous biological activities (12). 3,4,2′,4′-Tetrahydroxychalcone (butein) is a chalcone derivative from various natural plants and it is known as a major biological compound of Toxicodendron vernicifluum and Caragana jubata (13). These plants traditionally have been used for the treatment of pain, parasitic, and thrombotic diseases. Butein has attracted much attention because it has been reported to have various biological activities including antioxidant (13), anti-fibrogenic (14), anti-inflammatory (15), and anti-tumor activities (16). A recent study reported that butein inhibited adipocyte differentiation at the cellular level (17). As the Nrf2/HO-1 pathway is a critical regulator of cellular defense against oxidative stress, targeting this pathway represents an efficient strategy to prevent and treat a variety of chronic diseases. In addition, attenuating oxidative stress may be the key in the treatment or prevention of obesity-related diseases. Therefore, this study aimed to investigate whether the butein-upregulated Nrf2/HO-1 pathway is involved in the expression of adipocyte-specific transcription factors such as PPARγ and C/EBPα.
MATERIALS AND METHODS
Materials
Butein was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Dulbecco’s modified Eagle’s medium (DMEM) and phosphate-buffered saline (PBS) were purchased from HyClone (Logan, UT, USA). Bovine calf serum (BS), fetal bovine serum (FBS), and penicillin-streptomycin were obtained from GIBCO (BRL Life Technologies, Grand Island, NY, USA). Insulin (INS), isobutyl methylxanthine (IBMX), dexamethasone (DEX), and dimethyl sulfoxide were purchased from Sigma-Aldrich Co.. Primary antibodies against β-actin, C/EBPα, PPARγ, Nrf2, HO-1, and proliferating cell nuclear antigen (PCNA) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Cell culture and treatment
Mouse fibroblast 3T3-L1 cells (ATCC No. CL-173) were obtained from American Type Culture Collection (Manassas, VA, USA). Briefly, 3T3-L1 preadipocytes were incubated in DMEM containing 10% BS and 100 U/mL penicillin-streptomycin. Two days after confluence (day 0), the cells were treated with DMEM containing 10% FBS, 871 nM INS, 0.5 mM IBMX, and 1 μM DEX for 48 h (day 2). On day 2, the differentiation medium was replaced with 10% FBS/DMEM containing 871 nM INS and incubated for 48 h (day 4), followed by culturing with 10% FBS/DMEM.
Oil red O staining
Intracellular lipid accumulation was measured using Oil red O staining. 3T3-L1 adipocytes were treated with control or samples (5, 10, and 25 μM) for 6 days. In brief, cells were washed twice with cold PBS and fixed in 10% neutral formalin at room temperature, and then washed with PBS. The lipid droplets were stained with 0.3% Oil red O. The cells were washed with distilled water and the staining dye extracted with isopropyl alcohol. Absorbance was then measured at 490 nm.
Protein extraction and Western blot analysis
For the Western blot analysis, cells were washed with cold PBS and lysed by using a PRO-PREP protein extraction solution (iNtRON Biotechnology, Gyeonggi, Korea). The protein concentration of each sample was determined by spectrophotometry using Take3 Micro-Volume Plates (BioTek Instruments Inc., Winooski, VT, USA). Samples were loaded onto a polyacrylamide gel and subjected to sodium dodecyl sulfide-polyacrylamide gel electrophoresis, followed by electrophoretic transfer to nitrocellulose membrane. The nitrocellulose membranes were blocked with 5% skim milk in Tris-buffered saline (TBS), and then incubated with primary antibodies against Nrf2, HO-1, C/EBPα, PPARγ, PCNA, and β-actin at room temperature. Membranes were then washed with TBS and incubated with horseradish peroxidase-conjugated secondary antibody at room temperature. The specific protein bands were visualized on X-ray film via chemiluminescence using enhanced chemiluminescence (ECL) reagent (Pierce Biotechnology Inc., Rockford, IL, USA). The density of specific bands was calculated using Image J (NIH, Bethesda, MD, USA).
Statistical analysis
The results were expressed as mean±standard error (SE) and were representative of three or more independent experiments. Statistical comparisons were determined by one-way analysis of variance using SAS version 9.0 (SAS Institute Inc., Cary, NC, USA). The level of significance was established at a P-value of <0.05.
RESULTS AND DISCUSSION
Inhibitory effect of butein on lipid accumulation and adipogenic transcription factors
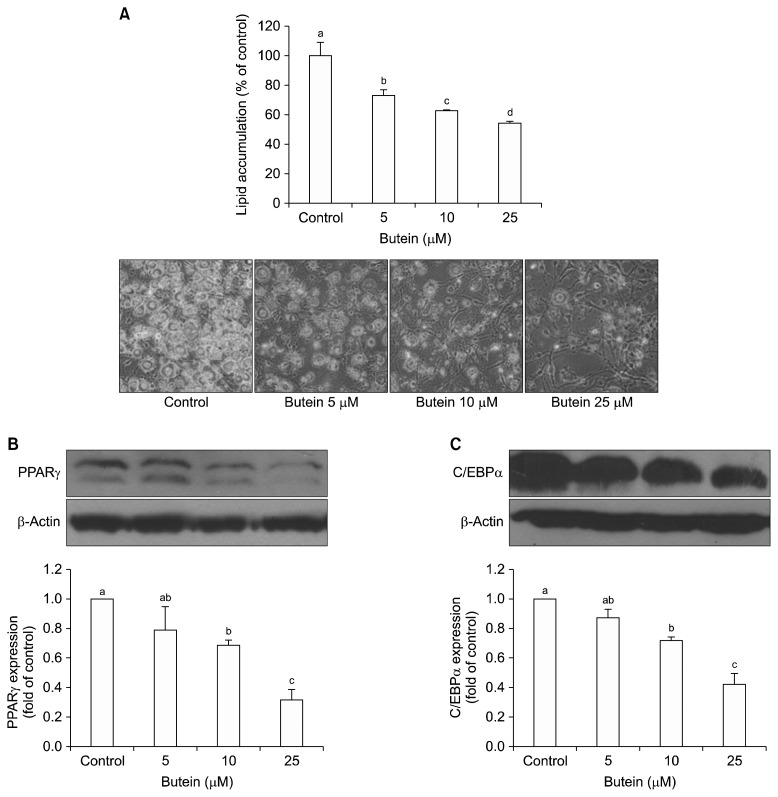
Butein did not show any cytotoxicity at concentrations up to 25 μM (data not shown). To explore the anti-adipogenic effects of butein, 3T3-L1 preadipocytes were treated with butein for 6 days. To measure lipid accumulation after differentiation, the differentiated adipocytes were stained with Oil red O. Treatment with 5, 10, and 25 μM butein significantly decreased lipid accumulation by 73.0 %, 62.6%, and 54.2%, respectively, compared with that in fully differentiated adipocytes (Fig. 1A).
Fig. 1.
Effect of butein on adipocyte lipid accumulation (A) and peroxisome proliferator-activated receptor γ (PPARγ) (B), and CCAATenhancer-binding protein α (C/EBPα) (C). Each value is expressed as the mean±SE (n=3) as determined by ANOVA, Duncan’s new multiple range test. Values with different letters (a–d) are significantly different (P<0.05).
Differentiation of 3T3-L1 preadipocytes to adipocytes requires a network of transcription factors, such as PPARγ and C/EBPα (18). PPARγ is a nuclear receptor that regulates various cellular processes including differentiation, immune response, and cell survival. It is a dominant regulator of adipocyte differentiation, acting through activation of adipose-specific genes (19). Additionally, induction of C/EBPα is followed by transcriptional activation of many genes involved with an adipocyte phenotype (20). When preadipocytes are stimulated by differentiation hormones, transcription factors such as PPARγ and C/EBPα are upregulated and work cooperatively to enhance the expression of several other adipogenic marker genes (21).
To determine how butein inhibits the differentiation of preadipocytes into mature adipocytes, this study investigated its effects on the protein expression levels of key adipogenic transcription factors such as PPARγ and C/EBPα. As shown in Fig. 1B and 1C, butein significantly suppressed PPARγ and C/EBPα expression levels compared to those in differentiated adipocytes.
Effects of butein on Nrf-2 translocation and HO-1 expression
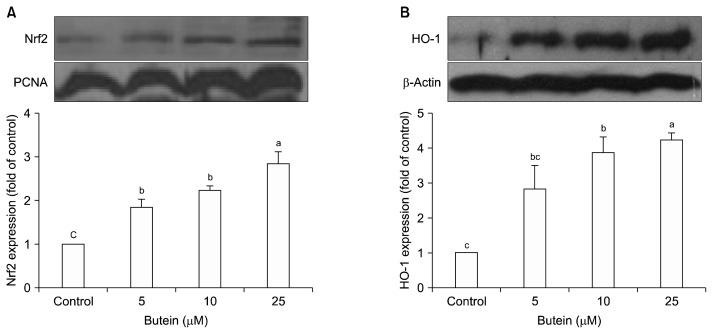
Nrf2 is one of the key antioxidant regulators against oxidative stress (22). Under basal conditions, Nrf2 remains in the cytoplasm and binds to Kelch-like ECH-associated protein 1 (Keap1). However, in response to oxidative stress, Nrf2 is released from Keap1 and subsequently translocated into the nucleus (23). Nrf2 then binds to antioxidant-reactive element sites in gene promoters coding for phase II enzymes (24). Although much is known about the role of the Nrf2 pathway, such as its antioxidant and cytoprotective effects, little is known about its interaction with other signaling pathways, such as adipogenesis signaling pathways. To determine the involvement of Nrf2/HO-1 in adipogenesis, the nuclear accumulation of Nrf2 and expression levels of its downstream protein, HO-1, were measured. Because translocation of Nrf2 occurs early when challenged with adipogenic hormones (25), 3T3-L1 cells were treated with butein for 3 days, and then nuclear fractions were extracted for western blotting. The results showed that treatment with butein significantly increased Nrf2 translocation to the nucleus (Fig. 2A) and HO-1 protein expression level (Fig. 2B) in a dose-dependent manner.
Fig. 2.
Effect of butein on Nrf2 translocation in to the nucleus (A) and heme oxygenase-1 (HO-1) expression (B). Each value is expressed as the mean±SE (n=3) as determined by ANOVA, Duncan’s new multiple range test. Values marked with different letters (a–c) are significantly different (P<0.05).
Effect of butein induced HO-1 on lipid accumulation during adipocyte differentiation
Recent studies have shown that an increase in antioxidant genes is therapeutically significant for adipocyte differentiation (26). Interestingly, emerging evidence indicates that HO-1 is closely related to metabolic diseases such as obesity (9). Therefore, this study evaluated the role of butein-induced HO-1 during adipocyte differentiation by measuring lipid accumulation and adipogenic transcription factor expression levels.
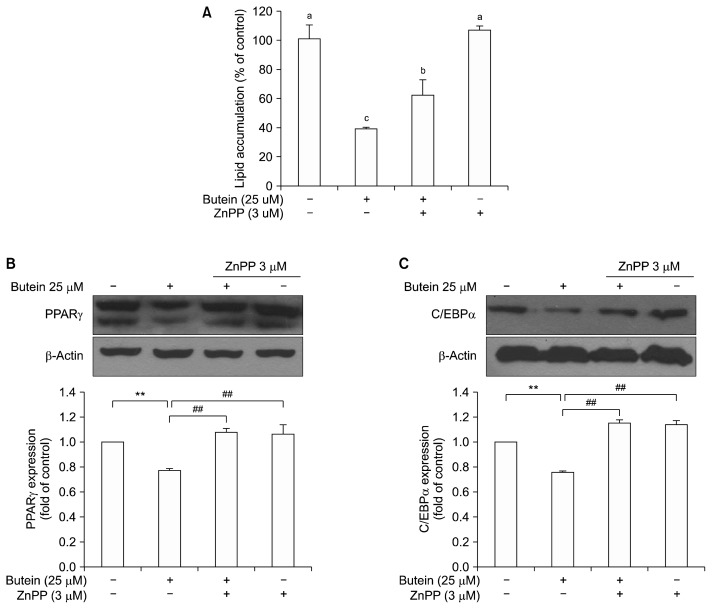
3T3-L1 adipocytes were co-treated with HO-1, zinc protoporphyrin (ZnPP) inhibitor to test whether this treatment could affect the adipogenesis. Fig. 3 shows the influence of an HO-1 inhibitor on butein-induced adipogenesis suppression. ZnPP is well known as a strong inhibitor of heme oxygenase-1 (26). Butein-induced inhibitory lipid accumulation was abolished when 3T3-L1 adipocytes were co-treated with ZnPP (Fig. 3A). Reduced PPARγ and C/EBPα expression (Fig. 3B and 3C) was also restored when 3T3-L1 adipocytes were treated with ZnPP. These results suggest that butein-induced HO-1 can attenuate lipid accumulation through key adipogenic transcription factors such as PPARγ and C/EBPα. These results indicated that HO-1 plays a key role in the butein-mediated induction of adipogenic key transcription factors.
Fig. 3.
Effect of butein involved with heme oxygenase-1 on lipid accumulation (A) and on peroxisome proliferator-activated receptor γ (PPARγ) (B), and CCAAT-enhancer-binding protein α (C/EBPα) (C) expression. Each value is expressed as the mean±SE (n=3) as determined by ANOVA (**P<0.05), Duncan’s new multiple range test (##P<0.05). ZnPP, zinc protoporphyrin.
To elucidate the molecular mechanism producing the anti-adipogenic effect involved with Nrf2/HO-1 pathway of butein, this study examined the adipogenic key transcription factors with treatment of HO-1 inhibitor. Overall, these results indicate that butein not only inhibits lipid accumulation through the inhibition of key adipogenic regulators, but it also upregulates the Nrf2/HO-1 antioxidant pathway. Butein is able to increase HO-1 expression through the activation of Nrf2 in 3T3-L1 adipocytes and reveal an antiadipogenic effect of butein. This study also demonstrated that butein’s effects were associated, at least partially, with HO-1 induction. Although further studies are warranted, these results suggest that butein has desirable properties for the treatment of obesity and related diseases.
ACKNOWLEDGEMENTS
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (project number: 2017R1D 1A1B03036360), Republic of Korea.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Hursting SD, Dunlap SM. Obesity, metabolic dysregulation, and cancer: a growing concern and an inflammatory (and microenvironmental) issue. Ann NY Acad Sci. 2012;1271:82–87. doi: 10.1111/j.1749-6632.2012.06737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kopelman PG. Obesity as a medical problem. Nature. 2000;404:635–643. doi: 10.1038/35007508. [DOI] [PubMed] [Google Scholar]
- 3.Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes Dev. 2000;14:1293–1307. [PubMed] [Google Scholar]
- 4.Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Madsen MS, Siersbæk R, Boergesen M, Nielsen R, Mandrup S. Peroxisome proliferator-activated receptor γ and C/ EBPα synergistically activate key metabolic adipocyte genes by assisted loading. Mol Cell Biol. 2014;34:939–954. doi: 10.1128/MCB.01344-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park UH, Jeong JC, Jang JS, Sung MR, Youn H, Lee SJ, Kim EJ, Um SJ. Negative regulation of adipogenesis by kaempferol, a component of Rhizoma Polygonati falcatum in 3T3-L1 cells. Biol Pharm Bull. 2012;35:1525–1533. doi: 10.1248/bpb.b12-00254. [DOI] [PubMed] [Google Scholar]
- 7.Pi J, Leung L, Xue P, Wang W, Hou Y, Liu D, Yehuda-Shnaidman E, Lee C, Lau J, Kurtz TW, Chan JY. Deficiency in the nuclear factor E2-related factor-2 transcription factor results in impaired adipogenesis and protects against diet-induced obesity. J Biol Chem. 2010;285:9292–9300. doi: 10.1074/jbc.M109.093955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kwak MK, Wakabayashi N, Kensler TW. Chemoprevention through the Keap1-Nrf2 signaling pathway by phase 2 enzyme inducers. Mutat Res. 2004;555:133–148. doi: 10.1016/j.mrfmmm.2004.06.041. [DOI] [PubMed] [Google Scholar]
- 9.Ndisang JF. Role of heme oxygenase in inflammation, insulin-signalling, diabetes and obesity. Mediators Inflamm. 2010;2010:359732. doi: 10.1155/2010/359732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka H, Nakamura S, Onda K, Tazaki T, Hirano T. Sofalcone, an anti-ulcer chalcone derivative, suppresses inflammatory crosstalk between macrophages and adipocytes and adipocyte differentiation: implication of heme-oxygenase-1 induction. Biochem Biophys Res Commun. 2009;381:566–571. doi: 10.1016/j.bbrc.2009.02.086. [DOI] [PubMed] [Google Scholar]
- 11.Cheng ZJ, Kuo SC, Chan SC, Ko FN, Teng CM. Antioxidant properties of butein isolated from Dalbergia odorifera. Biochim Biophys Acta. 1998;1392:291–299. doi: 10.1016/S0005-2760(98)00043-5. [DOI] [PubMed] [Google Scholar]
- 12.Lee SH, Nan JX, Zhao YZ, Woo SW, Park EJ, Kang TH, Seo GS, Kim YC, Sohn DH. The chalcone butein from Rhus verniciflua shows antifibrogenic activity. Planta Med. 2003;69:990–994. doi: 10.1055/s-2003-45143. [DOI] [PubMed] [Google Scholar]
- 13.Lee SH, Seo GS, Sohn DH. Inhibition of lipopolysaccharide-induced expression of inducible nitric oxide synthase by butein in RAW 264.7 cells. Biochem Biophys Res Commun. 2004;323:125–132. doi: 10.1016/j.bbrc.2004.08.063. [DOI] [PubMed] [Google Scholar]
- 14.Cioce M, Canino C, Pulito C, Muti P, Strano S, Blandino G. Butein impairs the protumorigenic activity of malignant pleural mesothelioma cells. Cell Cycle. 2012;11:132–140. doi: 10.4161/cc.11.1.18474. [DOI] [PubMed] [Google Scholar]
- 15.Song NJ, Yoon HJ, Kim KH, Jung SR, Jang WS, Seo CR, Lee YM, Kweon DH, Hong JW, Lee JS, Park KM, Lee KR, Park KW. Butein is a novel anti-adipogenic compound. J Lipid Res. 2013;54:1385–1396. doi: 10.1194/jlr.M035576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ilavenil S, Arasu MV, Lee JC, Kim DH, Roh SG, Park HS, Choi GJ, Mayakrishnan V, Choi KC. Trigonelline attenuates the adipocyte differentiation and lipid accumulation in 3T3-L1 cells. Phytomedicine. 2014;21:758–765. doi: 10.1016/j.phymed.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Kim J, Lee YJ, Kim JM, Lee SY, Bae MA, Ahn JH, Han DC, Kwon BM. PPARγ agonists induce adipocyte differentiation by modulating the expression of Lipin-1, which acts as a PPARγ phosphatase. Int J Biochem Cell Biol. 2016;81:57–66. doi: 10.1016/j.biocel.2016.10.018. [DOI] [PubMed] [Google Scholar]
- 18.Vidal-Puig AJ, Considine RV, Jimenez-Liñan M, Werman A, Pories WJ, Caro JF, Flier JS. Peroxisome proliferator-activated receptor gene expression in human tissues. Effects of obesity, weight loss, and regulation by insulin and gluco-corticoids. J Clin Invest. 1997;99:2416–2422. doi: 10.1172/JCI119424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Darlington GJ, Ross SE, MacDougald OA. The role of C/EBP genes in adipocyte differentiation. J Biol Chem. 1998;273:30057–30060. doi: 10.1074/jbc.273.46.30057. [DOI] [PubMed] [Google Scholar]
- 20.Tang QQ, Zhang JW, Daniel Lane M. Sequential gene promoter interactions of C/EBPβ, C/EBPα, and PPARγ during adipogenesis. Biochem Biophys Res Commun. 2004;319:235–239. doi: 10.1016/j.bbrc.2004.04.176. [DOI] [PubMed] [Google Scholar]
- 21.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun. 1997;236:313–322. doi: 10.1006/bbrc.1997.6943. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi M, Yamamoto M. Molecular mechanisms activating the Nrf2-Keap1 pathway of antioxidant gene regulation. Antioxid Redox Signal. 2005;7:385–394. doi: 10.1089/ars.2005.7.385. [DOI] [PubMed] [Google Scholar]
- 23.Yu X, Kensler T. Nrf2 as a target for cancer chemoprevention. Mutat Res. 2005;591:93–102. doi: 10.1016/j.mrfmmm.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 24.Hou Y, Xue P, Bai Y, Liu D, Woods CG, Yarborough K, Fu J, Zhang Q, Sun G, Collins S, Chan JY, Yamamoto M, Andersen ME, Pi J. Nuclear factor erythroid-derived factor 2-related factor 2 regulates transcription of CCAAT/enhancer-binding protein β during adipogenesis. Free Radic Biol Med. 2012;52:462–472. doi: 10.1016/j.freeradbiomed.2011.10.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imhoff BR, Hansen JM. Differential redox potential profiles during adipogenesis and osteogenesis. Cell Mol Biol Lett. 2011;16:149–161. doi: 10.2478/s11658-010-0042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Regehly M, Greish K, Rancan F, Maeda H, Böhm F, Röder B. Water-soluble polymer conjugates of ZnPP for photodynamic tumor therapy. Bioconjug Chem. 2007;18:494–499. doi: 10.1021/bc060158u. [DOI] [PubMed] [Google Scholar]