Abstract
Rhizoid of Laminaria japonica was hydrolyzed with proteases and carbohydrases to obtain antioxidant materials. Oxygen radical absorbance capacity (ORAC) of the enzymatic extracts was evaluated and the Protamex extract (PE) exhibited the highest ORAC value. PE also potently scavenged 2,2-diphenyl-1-picrylhydrazyl radical, 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic) acid cation radical, and hydrogen peroxide (H2O2) and had good reducing power. PE inhibited hydroxyl radical-induced DNA scission by measuring the conversion of supercoiled pBR322 plasmid DNA to the open circular form. The cytoprotective effect of PE against H2O2-induced hepatic cell damage was also investigated. PE showed a dose-dependent cytoprotective effect in cultured hepatocytes by inhibiting intracellular reactive oxygen species scavenging activity. In addition, PE up-regulated the expression of heme oxygenase-1, which is a cytoprotective enzyme, by activating translocation of nuclear factor-erythroid 2-related factor 2. Taken together, the enzymatic extract of rhizoid of L. japonica, particularly PE, may be useful for antioxidant additives.
Keywords: rhizoid, Laminaria japonica, antioxidant, enzymatic extraction, cytoprotective
INTRODUCTION
Dietary antioxidants from natural bioresources can prevent oxidative stress-induced cellular damage and/or preserve food systems by retarding lipid peroxidation. Accumulating evidence demonstrate that reactive oxygen species (ROS), including hydroxyl radical, superoxide anion, and hydrogen peroxide (H2O2), are involved in the development of human diseases through inflicting structural changes of fatty acids, proteins, and DNA as well as cellular components (1,2). Endogenous antioxidant molecules play key roles in preventing cellular damage from oxidative stress caused by ROS. However, the capacity of the antioxidant defense system may be altered with the progression of aging and other factors, including environmental pollutants, high fat diets, and pathological conditions (3). Thus, supplementation of exogenous antioxidants has been recognized as an alternative way to promote human health through suppressing ROS.
Laminaria japonica is widely cultured and consumed in Korea, China, and Japan. It has been used as folk remedy in Korea to promote health. Recently, L. japonica has attracted much attention as a biologically active material or nutraceutical due to its versatile bioactivities, including antibacterial, anti-inflammatory, antioxidant, and immunomodulatory activities (4–7). Polysaccharides and sulfated polysaccharides are representative bioactive molecules in L. japonica. In addition, enzymatic extract and fermented L. japonica also have antioxidant activities both in vitro and in vivo (8,9). All the observed bioactivities and molecules are from the edible parts of L. japonica. There is scanty information regarding the bioactivities of rhizoid of L. japonica because it is normally discarded as a processing waste. There have been several attempts in the production of value-added products from under-utilized biomass. One of the well-characterized approaches is enzymatic hydrolysis without any nutritional changes and byproduct production (10–12). Due to enzyme specificity, an enzymatic reaction allows the production of various products with different structure, size, and composition and the products may show versatile bioactivities.
Therefore, the objective of this study was to extract potential antioxidants from rhizoid of L. japonica through enzymatic hydrolysis using proteases and carbohydrases. The antioxidant and cytoprotective effects of the extracts were then investigated.
MATERIALS AND METHODS
Materials
Rhizoid of L. japonica was obtained from a local market (Yeosu, Korea) and stored at −20°C until use. 2,2-diphenyl-1-picrylhydrazyl (DPPH), peroxidase, 2,2′-azino-bis (3-ethylbenzthiazoline-6-sulphonic) acid (ABTS), 2′,7′-di-chlorodihydrofluorescin diacetate (DCFH-DA), and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). For enzyme-assisted extraction, carbohydrases [Amyloglucosidase 300 LTM (AMG): 260 U/mL, Celluclast® 1.5 L FG: 700 U/g, Dextrozyme, Promozyme: 400 pullulanase unit Novo/mL, and Viscozyme L: 100 fungal β-glucanase unit/mL], and proteases (Alcalase 2.4 L FG: 2.4 U/g, Flavourzyme 500 MG: 500 U/g, Neutrase 0.8 L: 0.8 U/g, and Protamex: 1.5 U/g) were purchased from Novozyme (Bagsværd, Denmark). Pepsin (1:10,000 U) was purchased from Junsei Chemical Co., Ltd. (Tokyo, Japan). All other chemicals used in this study were of analytical grade.
Proximate composition
Association of Official Analytical Chemists methods were applied to determine proximate compositions (official method No. 950.36 for protein, No. 935.38 for lipid, No. 930.22 for ash, and No. 926.5 for moisture) in rhizoid of L. japonica (13). Carbohydrate content was calculated by subtracting the sum of moisture, protein, ash, and lipid contents from 100.
Amino acid analysis
A 50 mg sample was hydrolyzed with 6.0 N HCl in a vacuum-sealed ampoule at 110°C for 24 h. After removing HCl from the hydrolyzed sample on a rotary evaporator, the sample was resuspended in 10 mL of 0.2 M sodium citrate buffer (pH 2.2). Amino acids were determined with a Biochrom 20 amino acid analyzer (Biochrom Ltd., Cambridge, UK) using a single ion-exchange resin column (4.0×150 mm) with ninhydrin as the color reactant.
Preparation of enzymatic extracts from rhizoid of L. japonica
The pulverized powder of rhizoid of L. japonica was subjected to extraction. Briefly, 60 mL distilled water was added to 1 g of rhizoid of L. japonica followed by the addition of 20 mg of each enzyme. The enzyme-assisted extraction was performed for 8 h at an optimum temperature and pH for each enzyme: AMG, 60°C and pH 4.5; Celluclast®, 50°C and pH 4.5; Dextrozyme, 60°C and pH 4.5; Promozyme, 60°C and pH 5.0; Viscozyme, 50°C and pH 4.5; Alcalase, 50°C and pH 7.0; Flavourzyme, 50°C and pH 7.0; Neutrase, 50°C and pH 7.0; Protamex, 50°C and pH 7.0; and pepsin, 37°C and pH 2.0. Extracts were clarified by centrifugation at 5,000 g for 20 min to remove the unhydrolyzed residue. Finally, the enzyme-assisted extracts of rhizoid of L. japonica were obtained after filtration of the supernatants. The enzyme-assisted extracts were lyophilized and stored at −20 °C until use (14).
Oxygen radical absorbance capacity (ORAC) assay
ORAC values of the enzymatic extracts were measured according to the method of Zulueta et al. (15) with slight modifications. Briefly, the enzymatic extract was dissolved in sodium phosphate buffer (75 mM, pH 7.0) and 50 μL of enzymatic extract was mixed with 50 μL of fluorescein (78 nM) in a 96-well microplate followed by incubation at 37°C for 15 min. Fluorescence was recorded every 5 min for 60 min after addition of 25 μL of 2,2′-azobis(2-amidinopropane) dihydrochloride (221 mM) at excitation wavelength of 485 nm and emission wavelength of 582 nm. A standard curve was prepared using Trolox (1~20 μM). ORAC values were expressed as μM Trolox equivalent (TE)/mg sample.
DPPH scavenging activity
DPPH scavenging activity was measured according to a published method (14). Briefly, 70 μL of enzymatic extract was mixed with 70 μL of DPPH solution (1.5×10−4 M in MeOH) and the mixture was allowed to stand for 30 min in a dark room. The absorbance was measured at 517 nm.
ABTS+ radical scavenging activity
ABTS+ radical stock solution was prepared by incubating 7 mM ABTS and 2.4 mM potassium persulfate for 16 h in the dark (16). Prior to the experiment, the stock solution was diluted to a working solution to reach an absorbance of 1.50±0.05 at 414 nm. A 50 μL enzymatic extract was mixed with 150 μL of the working solution and incubated at room temperature for 10 min. Absorbance was then measured at a wavelength of 414 nm.
H2O2 scavenging activity
H2O2 scavenging activity of the enzymatic extract was determined according to the method of Müller (17). Briefly, 100 μL of enzymatic extract and sodium phosphate buffer (0.1 M, pH 5.0) was mixed with 20 μL of H2O2 (10 mM) in each well of a 96-well plate and incubated at 37°C for 5 min. After adding 30 μL of ABTS (1.25 mM) and peroxidase (1 U/mL) to each well, the mixture was incubated at 37°C for 10 min. The absorbance was then measured at a wavelength of 405 nm.
Reducing power
A 200 μL enzymatic extract was mixed with 300 μL of sodium phosphate buffer (0.1 M, pH 6.6) and 500 μL of potassium ferricyanide (1% w/v) followed by incubation at 50°C for 20 min. After adding 500 μL of trichloroacetic acid, 100 μL of the upper layer was transferred to a test tube followed by the addition of 100 μL of distilled water and 20 μL of FeCl3 (0.1% w/v). The absorbance was then measured at a wavelength of 700 nm.
Protection of enzymatic extracts against oxidative stress-induced DNA damage in vitro
The protection ability of the enzymatic extract against hydroxyl radical-induced DNA damage in vitro was investigated according to the method of Yeung et al. (18) with slight modifications. Briefly, a reaction mixture containing 0.5 μg of pBR322 DNA, 2 mM FeSO4, and enzymatic extract (62.5~500 μg/mL) was prepared in an Eppendorf tube with final volume of 12 μL. The mixture was incubated at 37°C for 30 min after adding 4 μL of H2O2 (10 mM). The mixture was subjected to 0.8% agarose gel electrophoresis. DNA bands were visualized by staining with ethidium bromide.
Cell culture
Human hepatocytes (Chang liver cells, ATCC® CCL-13TM, American Type Culture Collection, Manassas, VA, USA) were cultured in DMEM supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. Cultures were maintained at 37°C in a humidified incubator with 5% CO2.
Cytotoxic and protective effect of enzymatic extract against H2O2-induced hepatocyte damage
Cytotoxicity of the enzymatic extract was evaluated using the MTT assay. Hepatocytes (1×104 cells/well) were seeded into a 96-well cell culture plate for 24 h followed by incubation with the enzymatic extract for another 24 h. Then, 100 μL of MTT (1 mg/mL) was added into each well of the 96-well plate after aspiring the cultured medium. The plate was incubated at 37°C for 4 h. Then 100 μL of DMSO was added into each well of the 96-well plate in order to dissolve the formazan crystals. The absorbance was measured at a wavelength of 540 nm. To evaluate the protective effect of the enzymatic extract against H2O2-induced hepatocyte damage, hepatocytes were pretreated with the enzymatic extract (0.0625~0.5 mg/mL) for 2 h. After washing three times with phosphate buffered saline, cells were exposed to 650 μM H2O2. After 24 h of incubation at 37°C, the MTT assay was performed as described above.
Intracellular ROS determination
Intracellular formation of ROS was determined according to the method of Lee et al. (19) with slight modifications. Briefly, hepatocytes (1×104 cells/well) were seeded into a 96-well black cell culture plate. After growing to confluency, hepatocytes were labelled with 20 μM of DCFH-DA in Hank’s balanced salt solution (HBSS) for 30 min and then treated with the enzymatic extract for 2 h. After washing hepatocytes with HBSS three times, 650 μM H2O2 was added into each well. After 30 min of incubation at 37°C, fluorescence was recorded at an excitation wavelength of 485 nm and emission wavelength of 535 nm. The percentage of fluorescence intensity (ROS formation) was compared to that of the control group without the enzymatic extract which was arbitrarily assigned a value of 100%.
Western blot analysis
Whole cell lysates were prepared using radioimmunoprecipitation assay buffer (Sigma Chemical Co., St. Louis, MO, USA) containing protease inhibitor. Proteins were separated by 10% SDS-PAGE and transferred onto nitrocellulose membrane. After blocking with 5% skim milk, the nitrocellulose membrane was incubated with primary antibody [1:1,000 for heme oxygenase-1 (HO-1) and 1: 500 for nuclear factor-erythroid 2-related factor 2 (Nrf2)] overnight at 4°C. The membrane was washed and incubated with the horseradish peroxidase-secondary antibody for 2 h and the protein band was visualized using an enhanced chemiluminescence western blotting detection kit (Pierce Biotechnology, Rockford, IL, USA). Protein levels were normalized to the level of β-actin or lamin B.
Statistics
All results were expressed as mean±standard deviation (SD) (n=3). Differences between means of each group were assessed by one-way analysis of variance followed by Duncan’s test using Predictive Analytics SoftWare Statistics 19.0 software (SPSS Inc., Chicago, IL, USA). A P-value of less than 0.05 was considered statistically significant.
RESULTS AND DISCUSSION
Proximate composition and enzymatic extraction of rhizoid of L. japonica
Before analysis of proximate composition, rhizoid of L. japonica was air-dried and protected against direct sunlight. The carbohydrate content in rhizoid of L. japonica was the highest (58.03±0.25 g/100 g), followed by ash content (20.33±0.05 g/100 g) and crude protein content (15.46±0.23 g/100 g). The high amount of ash in rhizoid of L. japonica was attributed to its absorption of inorganic salt in seawater (20). Protein and carbohydrate contents in this study were similar to those in the edible parts of different seaweed species previously reported (21,22).
Enzymatic extraction using proteases and carbohydrases can enhance the extraction yield of bioactive compounds (including polyphenols, peptides, and water soluble polysaccharides) from seaweeds. These biomaterials have shown potential health benefits with antioxidant, anticoagulant, anti-inflammatory, and antidiabetic effects (22–26). Therefore, bioactive materials were extracted from rhizoid of L. japonica through the enzymatic reaction using five proteases and carbohydrases. The yields of enzymatic extraction using proteases were: 10.19±0.25 % for Alcalase, 9.87±0.81% for Flavourzyme, 7.62±0.29 % for Neutrase, 12.10±1.04% for Protamex, and 22.52± 1.25% for pepsin. The yields of enzymatic extraction using carbohydrases were: 10.23±0.38% for AMG, 10.07±0.83% for Celluclast®, 6.94±0.25% for Dextrozyme, 12.94±0.61% for Promozyme, and 10.47±0.89% for Viscozyme. Although the crude carbohydrate content in rhizoid of L. japonica was higher than the other contents, yields of carbohydrase extracts were not much higher than those of protease extracts. In particular, the yield of the pepsin extract was significantly higher than that of the other extracts.
Antioxidant activities of enzymatic extracts
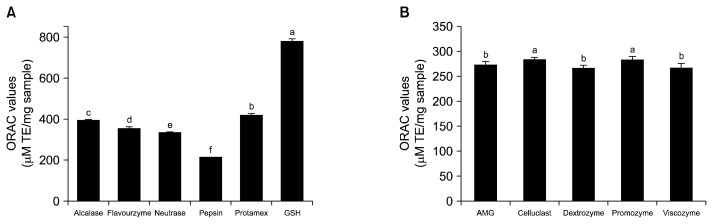
Antioxidant ability of a wide variety of foods have been tested using the ORAC assay (27,28). As shown in Fig. 1, ORAC values of protease and carbohydrase extracts varied. ORAC values for carbohydrase extracts ranged from 267.49±5.30 to 285.09±4.80 μM TE/mg sample while those of protease extracts ranged from 212.75±1.32 to 421.98±3.11 μM TE/mg sample. Although the pepsin extract showed the highest yield, it had the lowest ORAC value. Overall, protease extracts had higher ORAC values than carbohydrase extracts. The Protamex extract (PE) had the highest ORAC value of 421.98±3.11 μM TE/mg sample. All extracts showed lower ORAC values compared to glutathione as a positive control. Extraction without enzymes were also carried out and they showed weak ORAC values (data not shown). Therefore, the PE was selected for further analysis of antioxidant capacities.
Fig. 1.
Oxygen radical absorbance capacity (ORAC) values of (A) protease and (B) carbohydrase extracts from rhizoid of L. japonica. Each value is expressed as mean±SD (n=3). Bars with different letters (a–f) are significantly different by Duncan’s test (P<0.05).
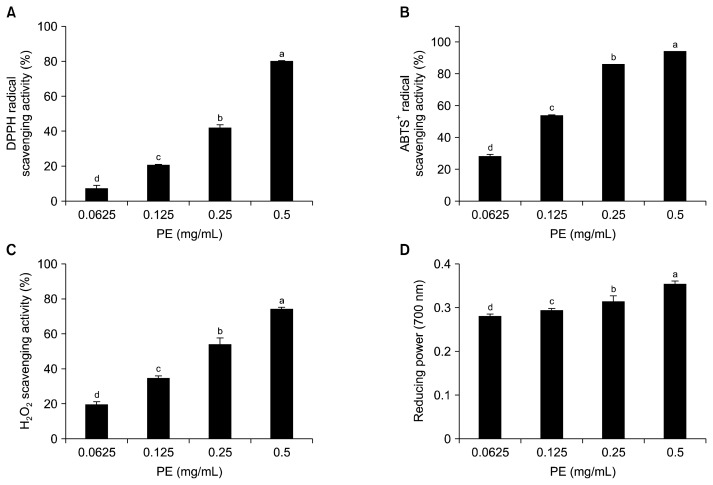
Various antioxidant assays including DPPH scavenging, ABTS+ radical scavenging, H2O2 scavenging, and reducing power have been used to reflect the hydrogen and/or electron donating capacity of antioxidant compounds (29). Therefore, antioxidant capacities of PE which showed the highest ORAC value were tested using the above mentioned antioxidant assays. As shown in Fig. 2, PE exhibited dose-dependent antioxidant capacities. At a concentration of 500 μg/mL, the DPPH scavenging, ABTS+ radical scavenging, and H2O2 scavenging capacities were 80.38±0.44%, 94.57±0.05%, and 74.10±1.26%, respectively. PE also showed potent reducing power (Fig. 2D). Previous work has demonstrated that enzymatic extracts of the edible part of L. japonica exhibit potent antioxidant abilities, including DPPH scavenging, H2O2 scavenging, and reducing power (8). However, this is the first study reporting that enzymatic extracts from the rhizoid of L. japonica have potent antioxidant activities. The activities were higher than those of enzymatic extracts from L. japonica (8).
Fig. 2.
(A) DPPH radical scavenging activity, (B) ABTS+ radical scavenging activity, (C) H2O2 scavenging activity, and (D) reducing power of Protamex extract (PE). Each value is expressed as mean±SD (n=3). Bars with different letters (a–d) are significantly different (P<0.05) by Duncan’s test.
Protection of PE against DNA strand breaks
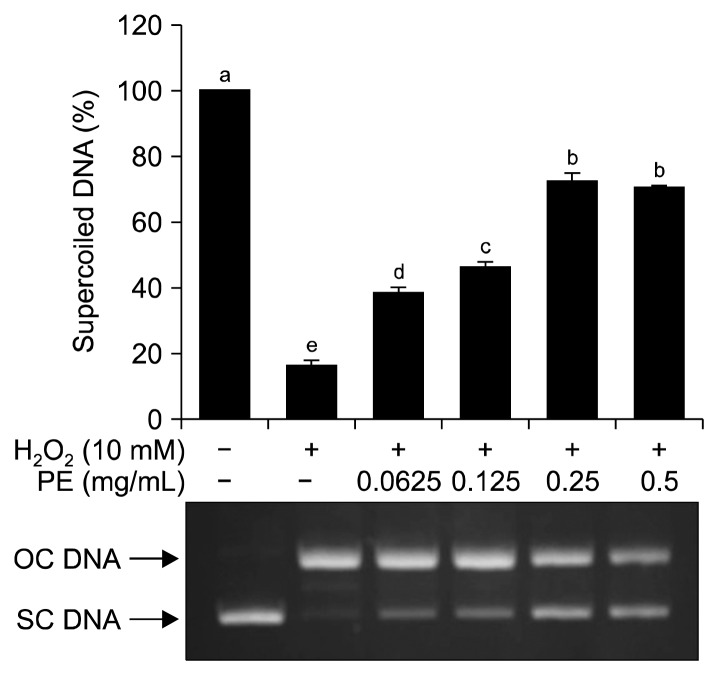
It is well known that the imbalance between antioxidant defense system and oxidants is involved in the development of some human diseases including cancer and neurodegenerative diseases (30). Overproduction of ROS that causes oxidative DNA damage is responsible for the development of these diseases (31). In this study, the ability of PE to protect against hydroxyl radical-induced DNA damage was further investigated. The supercoiled DNA form will become the open circular DNA form when exposed to hydroxyl radicals derived from the Fenton’s reaction (18). As shown in Fig. 3, supercoiled DNA was completely converted to open circular DNA after incubation with Fenton’s reagent. However, addition of PE to Fenton’s reagent resulted in decreased conversion of supercoiled DNA to open circular DNA in a dose-dependent manner.
Fig. 3.
Protective effect of Protamex extract (PE) against hydroxyl radical-induced DNA damage. Each value is expressed as mean±SD (n=3). Bars with different letters (a–e) are significantly different (P<0.05) by Duncan’s test. OC, open circular; SC, supercoiled.
Cytoprotective effect of PE against H2O2-induced hepatic cell damage
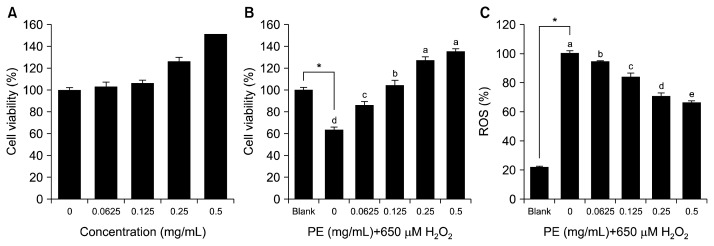
Oxidative stress results in the development of liver diseases because the liver is a major organ attacked by ROS (32). A number of risk factors such as alcohol, environmental pollutants, and drugs may be involved in oxidative liver damage, leading to severe liver diseases (32). Interventions using dietary antioxidants and/or various antioxidative therapies have been proposed to prevent and treat liver diseases (33,34). In this study, 650 μM H2O2, which had been previously determined as the concentration for hepatic cell damage, was applied to promote oxidative stress in hepatocytes. Before determining the cytoprotective effect of PE, non-cytotoxic concentrations of PE were determined using the MTT assay. Treatment with PE stimulated hepatic cell proliferation in a dose-dependent manner. A 1.5-fold increase in cell viability was observed in the group treated with 0.5 mg/mL of PE compared to the non-treated group (Fig. 4A). As shown in Fig. 4B, H2O2 treatment alone significantly decreased cell viability up to 63.50% compared to the blank group (without PE or H2O2). However, pretreatment with PE at 0.5 mg/mL dramatically increased cell viability up to 134.92%, indicating the cytoprotective ability of PE against H2O2-induced hepatic cell damage. As shown in Fig. 4C, pretreatment with PE significantly quenched intracellular ROS in a dose-dependent manner. The intracellular ROS scavenging ability of PE might be attributed to its cytoprotective effect.
Fig. 4.
(A) Cytotoxicity of Protamex extract (PE) in cultured hepatocytes, (B) cytoprotective ability of PE against hydrogen peroxide-induced hepatic cell damage, and (C) inhibition activity of PE against intracellular ROS generation in cultured hepatocytes. Each value is expressed as mean±SD (n=3). Bars with different letters (a–e) are significantly different (P<0.05) by Duncan’s test.
Effect of PE on HO-1 expression and Nrf2 translocation
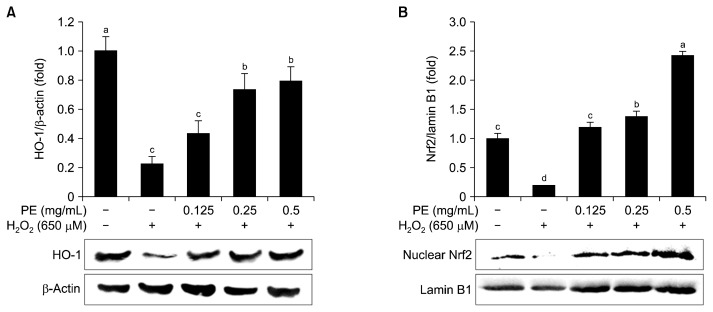
HO-1 is a phase II detoxifying enzyme that catalyzes the rate-limiting step in heme catabolism, producing carbon monoxide, birlirubin, and ferrous ion (35). Many studies have reported that induction of HO-1 by chemopreventive compounds plays an important role in the cellular adaptive survival response to oxidative stress and other toxic insults (36,37). To elucidate the cytoprotective effect of PE against H2O2-induced hepatic cell damage, HO-1 induction was determined. As shown in Fig. 5A, pretreatment with PE significantly increased the induction of HO-1 compared to H2O2 treatment alone. Many studies have demonstrated that phenolic compounds, bioactive peptides, and mucopolysaccharides have hepatic cell protective effects through up-regulating HO-1 induction (38–41).
Fig. 5.
(A) Effect of Protamex extract (PE) on heme oxygenase-1 (HO-1) expression, (B) nuclear translocation of Nrf2 in cultured hepatocytes against H2O2-induced hepatotoxicity. Whole cell lysate was used for HO-1 analysis and nuclear fraction was used for Nrf2 analysis by western blotting. Each value is expressed as mean±SD (n=3). Bars with different letters (a–d) are significantly different (P<0.05) by Duncan’s test.
Nrf2 is a transcription factor required for the expression of antioxidant/phase II detoxifying enzymes such as HO-1. Translocation of Nrf2 into the nucleus by dietary antioxidants is strongly involved in their cytoprotective effect against oxidative stress-mediated cell injury (42, 43). Thus, Nrf2 translocation into the nucleus was evaluated after PE treatment. As shown in Fig. 5B, PE treatment increased Nrf2 translocation into the nucleus compared to H2O2 treatment alone, indicating that PE up-regulated HO-1 expression through activating Nrf2 translocation.
Amino acid composition
Amino acid compositions of proteins and its peptides are major factors affecting their functionality and bioactivity. Amino acid compositions of rhizoid of L. japonica and its PE are summarized in Table 1. Aspartic acid (Asp), glutamic acid (Glu), and histidine (His) were the most abundant amino acids in rhizoid of L. japonica whereas PE contained higher amounts of Asp, Glu, and leucine (Leu). It is known that hydrophobic amino acids including His, proline, tyrosine, lysine, phenylalanine, cysteine, and methionine can enhance the potency of antioxidant peptides through hydrogen and/or electron donating capacity (3, 44). In addition, acidic amino acids such as Asp and Glu exhibit antioxidant activity through metal chelating action (45). These residues constitute about 55.9 g/100 g of the amino acids in PE, suggesting that the observed antioxidant and cytoprotective effects of PE might be attributed to its high antioxidant amino acids.
Table 1.
Amino acid compositions of rhizoid of L. japonica and its Protamex extract (PE) (unit: g/100 g)
| Amino acid | Rhizoid of L. japonica | PE |
|---|---|---|
| Aspartic acid | 15.2±0.2 | 14.2±0.2 |
| Threonine | 6.4±0.1 | 5.9±0.1 |
| Serine | 5.9±0.1 | 5.0±0.0 |
| Glutamic acid | 12.1±0.1 | 13.5±0.1 |
| Proline | 4.5±0.1 | 6.0±0.1 |
| Glycine | 4.4±0.1 | 5.9±0.1 |
| Alanine | 4.9±0.1 | 6.9±0.1 |
| Cysteine | 0.7±0.0 | 3.0±0.0 |
| Valine | 5.7±0.1 | 6.9±0.1 |
| Methionine | 1.9±0.0 | 2.0±0.0 |
| Isoleucine | 3.8±0.1 | 4.6±0.1 |
| Leucine | 6.2±0.1 | 7.3±0.1 |
| Tyrosine | 3.8±0.0 | 2.8±0.0 |
| Phenylalanine | 5.1±0.1 | 4.2±0.1 |
| Histidine | 9.3±0.1 | 6.6±0.1 |
| Lysine | 6.2±0.1 | 3.6±0.0 |
| Arginine | 3.8±0.1 | 1.5±0.0 |
In conclusion, the antioxidant and cytoprotective effects of enzymatic extract by Protamex hydrolysis from rhizoid of L. japonica were investigated. PE exhibited potent antioxidant capacities through ORAC, DPPH, ABTS+ radical, and H2O2 scavenging activities. PE showed a cytoprotective effect against H2O2-induced oxidative damage in cultured hepatocytes through intracellular ROS scavenging action. In addition, PE up-regulated HO-1 expression, a phase II detoxifying enzyme, through activating nuclear translocation of Nrf2 that may play a key role in preventing H2O2-induced hepatic cell damage.
ACKNOWLEDGEMENTS
This study was financially supported by Chonnam National University, 2016.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Halliwell B. Free radicals, antioxidants, and human disease: curiosity, cause, or consequence? Lancet. 1994;344:721–724. doi: 10.1016/S0140-6736(94)92211-X. [DOI] [PubMed] [Google Scholar]
- 2.Aruoma OI. Nutrition and health aspects of free radicals and antioxidants. Food Chem Toxicol. 1994;32:671–683. doi: 10.1016/0278-6915(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 3.Samaranayaka AGP, Li-Chan ECY. Food-derived peptidic antioxidants: a review of their production, assessment, and potential applications. J Funct Foods. 2011;3:229–254. doi: 10.1016/j.jff.2011.05.006. [DOI] [Google Scholar]
- 4.Liu M, Liu Y, Cao MJ, Liu GM, Chen Q, Sun L, Chen H. Antibacterial activity and mechanisms of depolymerized fucoidans isolated from Laminaria japonica. Carbohydr Polym. 2017;172:294–305. doi: 10.1016/j.carbpol.2017.05.060. [DOI] [PubMed] [Google Scholar]
- 5.Victor Lin HT, Lu WJ, Tsai GJ, Chou CT, Hsiao HI, Hwang PA. Enhanced anti-inflammatory activity of brown seaweed Laminaria japonica by fermentation using Bacillus subtilis. Proc Biochem. 2016;51:1945–1953. doi: 10.1016/j.procbio.2016.08.024. [DOI] [Google Scholar]
- 6.Cui C, Lu J, Sun-Waterhouse D, Mu L, Sun W, Zhao M, Zhao H. Polysaccharides from Laminaria japonica: structural characteristics and antioxidant activity. LWT-Food Sci Technol. 2016;73:602–608. doi: 10.1016/j.lwt.2016.07.005. [DOI] [Google Scholar]
- 7.Fang Q, Wang JF, Zha XQ, Cui SH, Cao L, Luo JP. Immunomodulatory activity on macrophage of a purified polysaccharide extracted from Laminaria japonica. Carbohydr Polym. 2015;134:66–73. doi: 10.1016/j.carbpol.2015.07.070. [DOI] [PubMed] [Google Scholar]
- 8.Sevevirathne M, Lee KH, Ahn CB, Park PJ, Je JY. Evaluation of antioxidant, anti-Alzheimer’s and anti-inflammatory activities of enzymatic hydrolysates from edible brown seaweed (Laminaria japonica) J Food Biochem. 2012;36:207–216. doi: 10.1111/j.1745-4514.2010.00527.x. [DOI] [Google Scholar]
- 9.Cha JY, Senevirathne M, Lee BJ, Kang YM, Kim YM, Kim JS, Cho YS, Jung WK, Ahn CB, Je JY. Fermented sea tangle (Laminaria japonica) attenuates ethanol-induced oxidative stress in Sprague-Dawley rats. J Food Biochem. 2013;37:80–87. doi: 10.1111/j.1745-4514.2011.00603.x. [DOI] [Google Scholar]
- 10.Ahn CB, Kim JG, Je JY. Purification and antioxidant properties of octapeptide from salmon byproduct protein hydrolysate by gastrointestinal digestion. Food Chem. 2014;147:78–83. doi: 10.1016/j.foodchem.2013.09.136. [DOI] [PubMed] [Google Scholar]
- 11.Harnedy PA, FitzGerald RJ. Bioactive peptides from marine processing waste and shellfish: a review. J Funct Foods. 2012;4:6–24. doi: 10.1016/j.jff.2011.09.001. [DOI] [Google Scholar]
- 12.Park SY, Kim YS, Ahn CB, Je JY. Partial purification and identification of three antioxidant peptides with hepatoprotective effects from blue mussel (Mytilus edulis) hydrolysate by peptic hydrolysis. J Funct Foods. 2016;20:88–95. doi: 10.1016/j.jff.2015.10.023. [DOI] [Google Scholar]
- 13.AOAC. Official Methods of Analysis AOAC International. 17th ed. Association of Official Analytical Chemists; Gauthsbrug, MD, USA: 2000. Official methods of analysis. [Google Scholar]
- 14.Ahn CB, Park PJ, Je JY. Preparation and biological evaluation of enzyme-assisted extracts from edible seaweed (Enteromorpha prolifera) as antioxidant, anti-acetylcholinesterase and inhibition of lipopolysaccharide-induced nitric oxide production in murine macrophages. Int J Food Sci Nutr. 2012;63:187–193. doi: 10.3109/09637486.2011.616486. [DOI] [PubMed] [Google Scholar]
- 15.Zulueta A, Esteve MJ, Frigola A. ORAC and TEAC assays comparison to measure the antioxidant capacity of food products. Food Chem. 2009;114:310–316. doi: 10.1016/j.foodchem.2008.09.033. [DOI] [Google Scholar]
- 16.Park MK, Kim CH. Extraction of polyphenols from apple peel using cellulase and pectinase and estimation of antioxidant activity. J Korean Soc Food Sci Nutr. 2009;38:535–540. doi: 10.3746/jkfn.2009.38.5.535. [DOI] [Google Scholar]
- 17.Müller HE. Detection of hydrogen peroxide produced by microorganism on an ABTS peroxidase medium. Zentralbl Bakteriol Mikrobiol Hyg A. 1985;259:151–154. doi: 10.1016/s0176-6724(85)80045-6. [DOI] [PubMed] [Google Scholar]
- 18.Yeung SY, Lan WH, Huang CS, Lin CP, Chan CP, Chang MC, Jeng JH. Scavenging property of three cresol isomers against H2O2, hypochlorite, superoxide and hydroxyl radicals. Food Chem Toxicol. 2002;40:1403–1413. doi: 10.1016/S0278-6915(02)00102-3. [DOI] [PubMed] [Google Scholar]
- 19.Lee DS, Cho YS, Je JY. Antioxidant and antibacterial activities of chitosan-phloroglucinol conjugate. Fish Aquat Sci. 2013;16:229–235. [Google Scholar]
- 20.Lahaye M. Marine algae as sources of fibres: Determination of soluble and insoluble dietary fibre contents in some ‘sea vegetables’. J Sci Food Agric. 1991;54:587–594. doi: 10.1002/jsfa.2740540410. [DOI] [Google Scholar]
- 21.Wong KH, Cheung PCK. Nutritional evaluation of some subtropical red and green seaweeds. Part I–proximate composition, amino acid profiles and some physico-chemical properties. Food Chem. 2000;71:475–482. doi: 10.1016/S0308-8146(00)00175-8. [DOI] [Google Scholar]
- 22.Je JY, Park PJ, Kim EK, Park JS, Yoon HD, Kim KR, Ahn CB. Antioxidant activity of enzymatic extracts from the brown seaweed Undaria pinnatifida by electron spin resonance spectroscopy. LWT-Food Sci Technol. 2009;42:874–878. doi: 10.1016/j.lwt.2008.10.012. [DOI] [Google Scholar]
- 23.Kang MC, Kim KN, Kang SM, Yang X, Kim EA, Song CB, Nah JW, Jang MK, Lee JS, Jung WK, Jeon YJ. Protective effect of dieckol isolated from Ecklonia cava against ethanol caused damage in vitro and in zebrafish model. Environ Toxicol Pharmacol. 2013;36:1217–1226. doi: 10.1016/j.etap.2013.09.018. [DOI] [PubMed] [Google Scholar]
- 24.Lee SH, Ko CI, Jee Y, Jeong Y, Kim M, Kim JS, Jeon YJ. Anti-inflammatory effect of fucoidan extracted from Ecklonia cava in zebrafish model. Carbohydr Polym. 2013;92:84–89. doi: 10.1016/j.carbpol.2012.09.066. [DOI] [PubMed] [Google Scholar]
- 25.Wijesinghe WAJP, Athukorala Y, Jeon YJ. Effect of anticoagulative sulfated polysaccharide purified from enzymeassistant extract of a brown seaweed Ecklonia cava on Wistar rats. Carbohydr Polym. 2011;86:917–921. doi: 10.1016/j.carbpol.2011.05.047. [DOI] [Google Scholar]
- 26.Lee SH, Jeon YJ. Anti-diabetic effects of brown algae derived phlorotannins, marine polyphenols through diverse mechanisms. Fitoterapia. 2013;86:129–136. doi: 10.1016/j.fitote.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 27.Ou B, Hampsch-Woodill M, Prior RL. Development and validation of an improved oxygen radical absorbance capacity assay using fluorescein as the fluorescent probe. J Agric Food Chem. 2001;49:4619–4626. doi: 10.1021/jf010586o. [DOI] [PubMed] [Google Scholar]
- 28.Nkhili E, Brat P. Reexamination of the ORAC assay: effect of metal ions. Anal Bioanal Chem. 2011;400:1451–1458. doi: 10.1007/s00216-011-4884-8. [DOI] [PubMed] [Google Scholar]
- 29.Prior RL, Wu X, Schaich K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J Agric Food Chem. 2005;53:4290–4302. doi: 10.1021/jf0502698. [DOI] [PubMed] [Google Scholar]
- 30.Lin S, Yang B, Chen F, Jiang G, Li Q, Duan X, Jiang Y. Enhanced DPPH radical scavenging activity and DNA protection effect of litchi pericarp extract by Aspergillus awamori bioconversion. Chem Cent J. 2012;6:108. doi: 10.1186/1752-153X-6-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- 32.Li S, Tan HY, Wang N, Zhang ZJ, Lao L, Wong CW, Feng Y. The role of oxidative stress and antioxidants in liver diseases. Int J Mol Sci. 2015;16:26087–26124. doi: 10.3390/ijms161125942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li AN, Li S, Zhang YJ, Xu XR, Chen YM, Li HB. Resources and biological activities of natural polyphenols. Nutrients. 2014;6:6020–6047. doi: 10.3390/nu6126020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Medina J, Moreno-Otero R. Pathophysiological basis for antioxidant therapy in chronic liver disease. Drugs. 2015;65:2445–2461. doi: 10.2165/00003495-200565170-00003. [DOI] [PubMed] [Google Scholar]
- 35.Kim KC, Kang KA, Zhang R, Piao MJ, Kim GY, Kang MY, Lee SJ, Lee NH, Surh YJ, Hyun JW. Up-regulation of Nrf2-mediated heme oxygenase-1 expression by eckol, a phlorotannin compound, through activation of Erk and PI3K/Akt. Int J Biochem Cell Biol. 2010;42:297–305. doi: 10.1016/j.biocel.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 36.Lee HG, Li MH, Joung EJ, Na HK, Cha YN, Surh YJ. Nrf2-Mediated heme oxygenase-1 upregulation as adaptive survival response to glucose deprivation-induced apoptosis in HepG2 cells. Antioxid Redox Signal. 2010;13:1639–1648. doi: 10.1089/ars.2010.3226. [DOI] [PubMed] [Google Scholar]
- 37.Li L, Dong H, Song E, Xu X, Liu L, Song Y. Nrf2/ARE pathway activation, HO-1 and NQO1 induction by polychlorinated biphenyl quinone is associated with reactive oxygen species and PI3K/AKT signaling. Chem Biol Interact. 2014;209:56–67. doi: 10.1016/j.cbi.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 38.Je JY, Lee DB. Nelumbo nucifera leaves protect hydrogen peroxide-induced hepatic damage via antioxidant enzymes and HO-1/Nrf2 activation. Food Funct. 2015;6:1911–1918. doi: 10.1039/C5FO00201J. [DOI] [PubMed] [Google Scholar]
- 39.Park SY, Kim YS, Ahn CB, Je JY. Partial purification and identification of three antioxidant peptides with hepatoprotective effects from blue mussel (Mytilus edulis) hydrolysate by peptic hydrolysis. J Funct Foods. 2016;20:88–95. doi: 10.1016/j.jff.2015.10.023. [DOI] [Google Scholar]
- 40.Kumar KJ, Yang HL, Tsai YC, Hung PC, Chang SH, Lo HW, Shen PC, Chen SC, Wang HM, Wang SY, Chou CW, Hseu YC. Lucidone protects human skin keratinocytes against free radical-induced oxidative damage and inflammation through the up-regulation of HO-1/Nrf2 antioxidant genes and down-regulation of NF-κB signaling pathway. Food Chem Toxicol. 2013;59:55–66. doi: 10.1016/j.fct.2013.04.055. [DOI] [PubMed] [Google Scholar]
- 41.Luo Z, Dong X, Ke Q, Duan Q, Shen L. Chitooligosaccharides inhibit ethanol-induced oxidative stress via activation of Nrf2 and reduction of MAPK phosphorylation. Oncol Rep. 2014;32:2215–2222. doi: 10.3892/or.2014.3463. [DOI] [PubMed] [Google Scholar]
- 42.Ji LL, Sheng YC, Zheng ZY, Shi L, Wang ZT. The involvement of p62-Keap1-Nrf2 antioxidative signaling pathway and JNK in the protection of natural flavonoid quercetin against hepatotoxicity. Free Radic Biol Med. 2015;85:12–23. doi: 10.1016/j.freeradbiomed.2015.03.035. [DOI] [PubMed] [Google Scholar]
- 43.Lee DS, Li B, Kim KS, Jeong GS, Kim EC, Kim YC. Butein protects human dental pulp cells from hydrogen peroxide-induced oxidative toxicity via Nrf2 pathway-dependent heme oxygenase-1 expressions. Toxicol In Vitro. 2013;27:874–881. doi: 10.1016/j.tiv.2013.01.003. [DOI] [PubMed] [Google Scholar]
- 44.Sarmadi BH, Ismail A. Antioxidative peptides from food proteins: a review. Peptides. 2010;31:1949–1956. doi: 10.1016/j.peptides.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 45.Udenigwe CC, Aluko RE. Chemometric analysis of the amino acid requirements of antioxidant food protein hydrolysates. Int J Mol Sci. 2011;12:3148–3161. doi: 10.3390/ijms12053148. [DOI] [PMC free article] [PubMed] [Google Scholar]