Abstract
Kaempferol has been shown to inhibit vascular formation in endothelial cells. However, the underlying mechanisms are not fully understood. In the present study, we evaluated whether kaempferol exerts antiangiogenic effects by targeting extracellular signal-regulated kinase (ERK)/p38 mitogen-activated protein kinase (MAPK) and phosphoinositide 3-kinase (PI3K)/Akt/mechanistic target of rapamycin (mTOR) signaling pathways in endothelial cells. Endothelial cells were treated with various concentrations of kaempferol for 24 h. Cell viability was determined by the 3- (4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide assay; vascular formation was analyzed by tube formation, wound healing, and mouse aortic ring assays. Activation of hypoxia-inducible factor-1α (HIF-1α), vascular endothelial growth factor receptor 2 (VEGFR2), ERK/p38 MAPK, and PI3K/Akt/mTOR was analyzed by Western blotting. Kaempferol significantly inhibited cell migration and tube formation in endothelial cells, and suppressed microvessel sprouting in the mouse aortic ring assay. Moreover, kaempferol suppressed the activation of HIF-1α, VEGFR2, and other markers of ERK/p38 MAPK and PI3K/Akt/mTOR signaling pathways in endothelial cells. These results suggest that kaempferol inhibits angiogenesis by suppressing HIF-1α and VEGFR2 activation via ERK/p38 MAPK and PI3K/Akt/mTOR signaling in endothelial cells.
Keywords: kaempferol, angiogenesis, HUVECS
INTRODUCTION
Angiogenesis is the development of new blood vessels, and is required for the growth of new tissues. This is a normal physiological phenomenon associated with routine processes, including wound healing and embryogenesis. However, it also occurs in response to the expansion of solid tumors and their subsequent growth away from the existing blood supply (1). Therefore, the study of angiogenic processes and the identification of novel antiangiogenic agents are important for cancer therapy. Angiogenesis depends on cell proliferation, migration, and invasion of endothelial cells (2). It requires the activation of several signaling molecules, such as extracellular signal-regulated kinase (ERK) (3,4), p38 mitogen-activated protein kinase (MAPK) (5), and Akt (6). Therefore, regulating these angiogenic factors could inhibit angiogenesis (7). Vascular endothelial growth factor receptor 2 (VEGFR2) is a predominant inducer of both normal and pathophysiological angiogenesis (8). It is activated by the transcription factor, hypoxia-inducible factor-1α (HIF-1α), which binds to the hypoxia response element within the vascular endothelial growth factor (VEGF) gene promoter (9). Mechanistic target of rapamycin (mTOR), a protein kinase of the phosphoinositide 3-kinase (PI3K)/Akt pathway, regulates several fundamental cellular functions; its deregulation may be associated with tumorigenesis (10). PI3K/Akt/mTOR signaling has been reported to play a key role in the proliferation and angiogenesis of endothelial cells (11).
Flavonoids are natural polyphenols present in a wide variety of fruits and vegetables (12). They are reported to reduce the risk of cardiovascular diseases in humans and modulate various signaling pathways to regulate cell proliferation, angiogenesis, and metastasis (13–15). Kaempferol, 3,5,7-trihydroxy-2-(4-hydroxyphenyl)-4H-1-benzopyran-4-one, is a bioflavonoid structurally similar to quercetin (Fig. 1A); it is one of the most common dietary flavonoids that possesses antioxidant and antitumor properties. It is reported to exert antiproliferative effects on human endothelial and lung cancer cells by activating MAPK signaling (16–18). However, the mechanisms underlying its antiangiogenic activity are not fully understood.
Fig. 1.
Chemical structure of kaempferol (A) and the effects of kaempferol on endothelial cell viability (B). Endothelial cell viability was evaluated by the MTT assay after exposure to kaempferol (50, 100, 200, 400, and 800 μM) for 24 h; cell viability is expressed as the percentage of viable cells cultured in the absence of kaempferol. Values represent the mean±SD. *P<0.05 and **P<0.01 compared with control (0 μM).
Therefore, we evaluated whether kaempferol inhibits angiogenesis by targeting ERK/p38 MAPK and PI3K/Akt/mTOR signaling pathways in endothelial cells.
MATERIALS AND METHODS
Reagents
Kaempferol (Fig. 1A) was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA) and dissolved in 100% dimethyl sulfoxide (DMSO). A stock solution of 100 mmol/L kaempferol was prepared and stored as small aliquots at −20°C until use. We purchased 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), DMSO, gelatin, and horseradish peroxidase (HRP)-conjugated anti-mouse and anti-rabbit antibodies from Sigma-Aldrich Co.. Growth factor-reduced Matrigel was purchased from BD Biosciences (San Jose, CA, USA). The phospho-specific antibodies (anti-p38, anti-PI3K, anti-AKT, anti-mTOR, anti-p70S6K, anti-VEGFR2, and anti-HIF-1α) and the MAPK inhibitors (SB203580 and PD 98059) were purchased from Cell Signaling Technology (Danvers, MA, USA). The HRP-conjugated β-actin and phospho-ERK antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Endothelial cell culture
Human umbilical vein endothelial cells (HUVECs) were obtained from ATCC (Rockville, MD, USA) and cultured in endothelial basal medium-2 (EBM-2) growth medium (Lonza, Walkersville, MD, USA), containing hydrocortisone, epidermal growth factor, basic fibroblast growth factor, insulin-like growth factor-1, VEGF, ascorbic acid, heparin, and 10% fetal bovine serum (FBS) at 37°C in a humidified atmosphere of 5% CO2. The cells were seeded in culture flasks or on plates coated with 1% gelatin and allowed to grow to confluence before experimental treatment. The HUVECs used in the experiment were between passages 3 and 5. The commercially available vascular endothelial cell-specific supplement EGM®-2 MV Bullet Kit (Lonza) was used (19).
Cell viability assay
Endothelial cell viability was assessed by the MTT assay. The effects of kaempferol on cell viability were calculated as a percentage relative to solvent-treated control. The cells (5×103 cells/well) were seeded into a 96-well plate with EGM-2 medium supplemented with 10% FBS. After the cells were allowed to adhere, the culture medium was removed, the cells were rinsed twice with phosphate-buffered saline (PBS), and then incubated with serum-free medium for 12 h. After serum starvation, the cells were cultured in fresh medium supplemented with 2% FBS and various concentrations of kaempferol at 37 °C for 24 h. Following incubation, the MTT solution was added, and the plate was incubated for an additional 4 h. The resulting formazan deposit was solubilized in DMSO, and the absorbance at 570 nm was measured by using a VersaMax ELISA microplate reader (Molecular Devices, Sunnyvale, CA, USA).
Scratch-wound migration assay
Endothelial cells were allowed to grow to confluence in 6-well plates pre-coated with 0.1% gelatin and then incubated with 10 mg/mL mitomycin C (Sigma-Aldrich Co.) at 37°C under a 5% CO2 atmosphere for 2 h to inactivate the cells. The monolayers of endothelial cells were wounded by scratching with a 0.2-mL pipette tip, and fresh medium containing various concentrations of kaempferol was added. Images were captured with an inverted phase contrast light microscope (Olympus Optical Co. Ltd., Tokyo, Japan) after incubation for 24 h. The migrated cells from three randomly selected fields were quantified by manual counting (DMC advanced program) under an optical microscope at 200× magnification, and the inhibition was calculated as a percentage relative to the control.
Tube formation assay with endothelial cells on Matrigel
Matrigel (70 μL/well) was added to a 96-well plate and polymerized for 30 min at 37°C. The endothelial cells (3×104 cells) were seeded onto each well of the Matrigel-coated 96-well plate and then incubated in 2% FBSEBM-2 with various concentrations of kaempferol. After incubation for 8 h, the formation of the endothelial cell tubular structures was visualized under an inverted microscope and photographed at 40× magnification. Furthermore, the tube formation was quantified from the calculation of the tube length, normalized, and expressed as a percentage relative to untreated control cells.
Western blotting analysis
The cells were treated with kaempferol for 24 h, harvested, and lysed in protein extraction solution (Intron Biotechnology, Inc., Gyeonggi, Korea) containing protease inhibitors and phosphatase inhibitors for 10 min at 4°C. The total protein concentration in the supernatant was measured by using the Bradford assay. After heating at 95°C for 5 min, the samples of total protein (40 μg) were subjected to 6~15% sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The proteins were transferred onto polyvinylidene difluoride membranes (Millipore, Bedford, MA, USA) by the application of 100 V for 60~100 min. The membranes were incubated with 5% bovine serum albumin (BSA) in Tris-buffered saline (TBS) with 0.05% Tween 20 (TBST) for 30 min at room temperature and then with primary antibodies diluted 1: 200~1:1,000 in 5% BSA in TBST overnight at 4°C. The membranes were washed three times with TBST and incubated with the appropriate secondary antibodies. The protein bands were detected by using an enhanced chemiluminescence detection kit (Intron Biotechnology, Inc.) and an LAS-1000 Imager (Fuji Film Corp., Tokyo, Japan).
Mouse aortic ring assay
The mouse aortic ring assay was performed as previously described (20). First, 48-well plates were covered with 150 μL Matrigel and incubated at 37°C in 5% CO2 for 30 min. The aortas isolated from mice (Central Laboratory Animal Inc., Seoul, Korea) were cleaned of periadventitial fat and connective tissues and cut into 1~1.5 mm long rings. The aortas were rinsed with PBS, placed in the Matrigel-covered wells, and covered with an additional 200 μL Matrigel. After the artery rings were cultured in 1 mL serum-free EGM-2 medium for 24 h, the medium was replaced with 1 mL EGM-2 medium supplemented with vehicle or kaempferol (0 or 40 μM). The medium was replaced every 2 days with medium described above. After 7 days, the microvessel growth was measured through photographs captured with an OLYMPUS inverted microscope (40× objective, Olympus Optical Co. Ltd.,). The length of the capillary was estimated by using a phase-contrast microscope to measure the distance from the cut end of the aortic segment to the approximate center point of the capillary. The length of the capillary was measured by using Adobe Photoshop software (DMC advanced program). Each value represents an average of 3~4 culture samples.
Statistical analysis
The results are expressed as the mean±standard deviation (SD). Statistical significance was determined by using one-way analysis of variance (ANOVA) and Student’s t-test for paired data. A P-value of <0.05 indicated statistical significance. All statistical calculations were computed using SPSS for Windows Version 10.0 (SPSS, Chicago, IL, USA).
RESULTS
Effect of kaempferol treatment on endothelial cell viability
To determine the non-cytotoxic concentration of kaempferol in endothelial cells, we evaluated their viability by the MTT assay. The cells were treated with different concentrations of kaempferol (50, 100, 200, 400, and 800 μM) for 24 h. It was found that concentrations higher than 100 μM significantly decreased endothelial cell viability (Fig. 1B). Therefore, subsequent studies on the biological activity of kaempferol were conducted at lower concentrations (10, 20, and 40 μM).
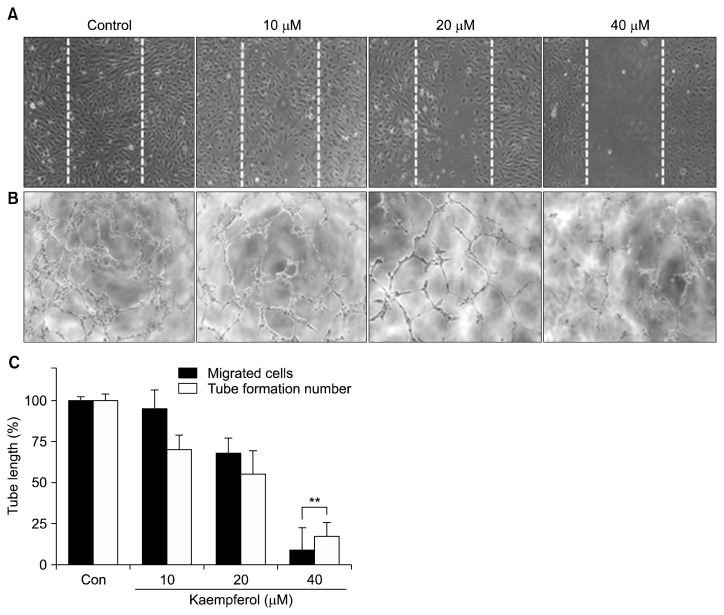
Inhibition of endothelial cell migration and tube formation by kaempferol
Endothelial cell migration and tube formation are essential steps in angiogenesis. Therefore, we determined the effects of kaempferol on endothelial cell migration by a wound healing assay. Wound healing by endothelial cell migration was almost complete after 24 h of incubation; however, it was inhibited by kaempferol in a concentration-dependent manner (Fig. 2A and 2C); it was significantly suppressed by treatment with 40 μM kaempferol (P<0.01). We also found that kaempferol significantly inhibits tube formation in cultured endothelial cells in a concentration-dependent manner (P<0.01) (Fig. 2B and 2C).
Fig. 2.
Effects of kaempferol treatment on migration and tube formation in endothelial cells. (A) Cells were grown to confluence in 6-well plates, scratch-wounded, and treated with the indicated concentrations of kaempferol. (B) Cells were seeded in 96-well plates coated with Matrigel and incubated in the absence or presence of kaempferol for 4~8 h. The tubular structures were photographed, and the migrated cells were manually counted. (C) The numbers of migrated cells and tube formations in HUVECs after kaempferol treatment are shown. Values represent the mean±SD. **P<0.01 compared with control.
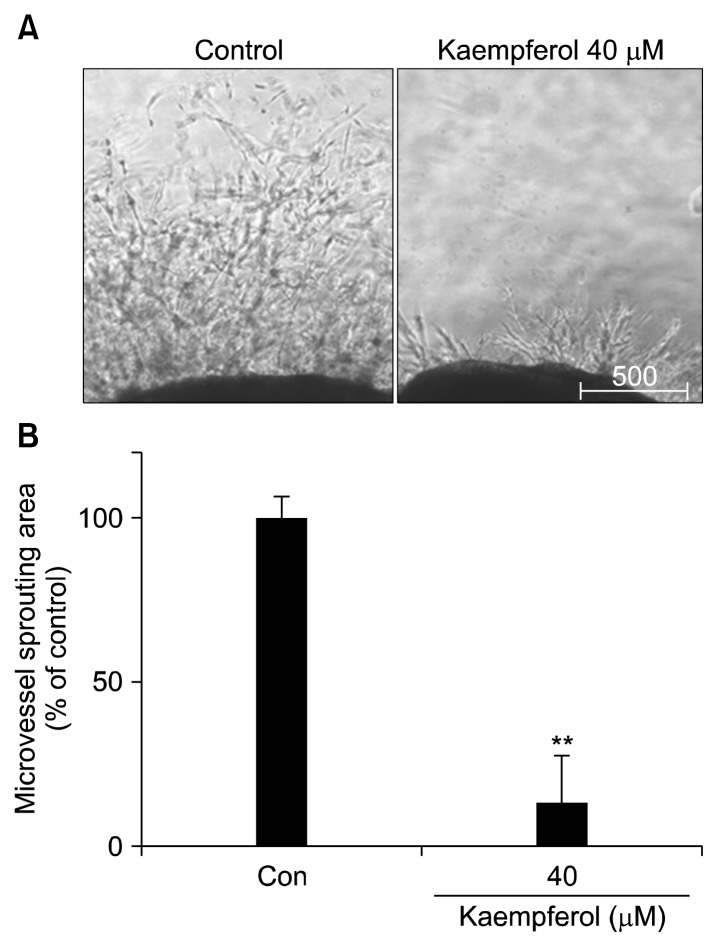
Suppression of capillary sprouting by kaempferol in a mouse aortic ring assay
A mouse aortic ring assay was used to investigate the effect of kaempferol on capillary sprouting/vascular formation. Treatment with 40 μM kaempferol significantly suppressed microvessel outgrowth from aortic rings (P< 0.01) (Fig. 3).
Fig. 3.
Effect of kaempferol treatment on microvessel outgrowth from mouse aortic rings. Aortic rings isolated from mice were embedded in Matrigel-coated 48-well plates and cultured in media containing various concentrations of kaempferol for seven days followed by the measurement of microvessel length. Representative photographs of three independent experiments are shown. Values represent the mean±SD. **P<0.01 compared with control.
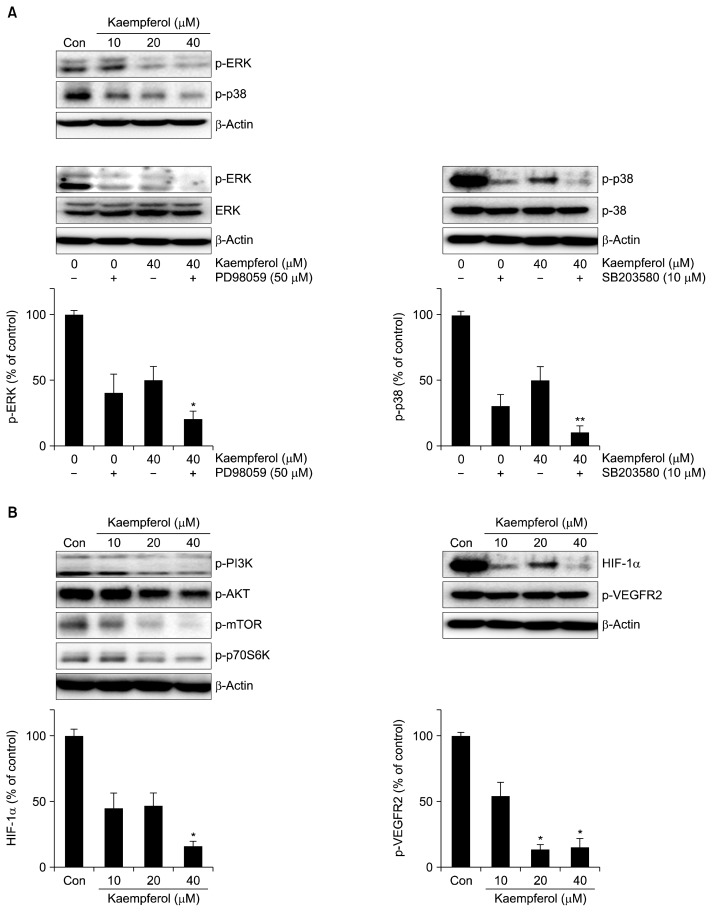
Suppression of ERK/p38 MAPK and PI3K/Akt/mTOR signaling pathways by kaempferol in endothelial cells
To determine whether kaempferol exerts antiangiogenic effects by targeting ERK/p38 MAPK and PI3K/Akt/mTOR signaling pathways, we investigated the phosphorylation of ERK, p38, and JNK by western blotting. Kaempferol treatment (10, 20, and 40 μM) reduced the phosphorylation of ERK and p38 in endothelial cells (Fig. 4A). To further confirm its effects on the MAPK signaling pathway, we co-treated the cells with kaempferol along with an ERK inhibitor (PD98059) or a p38 inhibitor (SB203580). A synergistic suppression of ERK and p38 phosphorylation was observed in these co-treated cells compared to that in cells treated with either inhibitor alone. We found that kaempferol suppresses the activation of Akt, mTOR, and a downstream effector, p70S6K (Fig. 4B). It also reduced the activation of HIF-1α and VEGFR2 in endothelial cells. Our results suggest that kaempferol inhibits the phosphorylation of ERK, p38, Akt, and mTOR to reduce HIF-1α and VEGFR2 phosphorylation.
Fig. 4.
Effect of kaempferol treatment on (A) MAPK and (B) PI3K/Akt/mTOR signaling pathways. Endothelial cells were treated with the indicated concentrations of kaempferol for 24 h. Protein samples (40 μg) were subjected to 10% SDS-PAGE, and phosphorylated ERK, p38, PI3K, Akt, mTOR, p70S6K, VEGFR2, and HIF-1α were detected by western blotting. The cells were treated with kaempferol in combination with MAPK inhibitors (ERK inhibitor, PD98059; p38 inhibitor, SB203580), and the activation of ERK and p38 was detected by western blotting; β-actin was used as an internal control. Values represent the mean±SD of three independent experiments. *P<0.05 and **P<0.01 compared with control.
DISCUSSION
Flavonoids are one of the most abundant constituents of fruits and vegetables; they exert anticancer effects through their antioxidant, antiestrogenic, antiproliferative, antiangiogenic, and anti-inflammatory properties (21). Plant-based antiangiogenic drugs are gaining importance in cancer treatment (22–24). We demonstrated that kaempferol inhibits vascular formation both in vitro and ex vivo by suppressing HIF-1α and VEGFR2 activation via ERK/p38 MAPK and PI3K/Akt/mTOR signaling in endothelial cells. Kaempferol exhibits notable anti-proliferative activity in cancer cells (16,17). However, we demonstrated that it exerts antiangiogenic effects at non-cytotoxic concentrations in endothelial cells. These cells spontaneously form capillary-like networks in Matrigel in vitro (25). Tube formation, which depends on the maturation of migrated endothelial cells, is also involved in the initial steps of angiogenesis. Further, the mouse aortic ring assay is an ex vivo assay system widely used for the evaluation of antiangiogenic activities of test compounds that effectively simulates the interaction among endothelial cells, fibroblasts, pericytes, and smooth muscle cells (26). At a concentration of 40 μM, kaempferol significantly inhibited migration and tube formation in endothelial cells, and suppressed microvessel outgrowth from aortic rings. Angiogenesis is critical for tumor growth, invasion, and metastasis (27). ERK/p38 MAPK and PI3K/Akt/mTOR signaling pathways regulate the growth, survival, and migration of vascular endothelial cells in angiogenesis (21,28). PI3K is an important intracellular signal-transducing enzyme (29), and Akt stimulates angiogenesis by increasing cell migration and invasion (30). The deregulated expression of phosphatase and tensin homolog leads to the aberrant activation of Akt as well as its downstream effectors, including mTOR, in prostate cancer (25). Further, the increased activation of ERK/MAPK signaling is observed in many prostate tumors (17). We observed that kaempferol reduces the activation of ERK/p38 MAPK and PI3K/Akt/mTOR signaling pathways to suppress the phosphorylation of VEGFR2 and HIF-1α in endothelial cells.
In conclusion, we demonstrated that kaempferol significantly inhibits migration and tube formation in vitro, as well as microvessel outgrowth in an ex vivo aortic ring model by suppressing HIF-1α and VEGFR2 activation via ERK/p38 MAPK and PI3K/Akt/mTOR signaling in endothelial cells. These findings provide an understanding of the mechanisms involved in the antiangiogenic actions of kaempferol and highlight its anticancer potential.
ACKNOWLEDGEMENTS
This study was supported by the Kyungnam University Foundation Grant, No 20160109.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The author declares no conflict of interest.
REFERENCES
- 1.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358:2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cristi E, Perrone G, Toscano G, Verzì A, Nori S, Santini D, Tonini G, Vetrani A, Fabiano A, Rabitti C. Tumour proliferation, angiogenesis, and ploidy status in human colon cancer. J Clin Pathol. 2005;58:1170–1174. doi: 10.1136/jcp.2004.025536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanno S, Oda N, Abe M, Terai Y, Ito M, Shitara K, Tabayashi K, Shibuya M, Sato Y. Roles of two VEGF receptors, Flt-1 and KDR, in the signal transduction of VEGF effects in human vascular endothelial cells. Oncogene. 2000;19:2138–2146. doi: 10.1038/sj.onc.1203533. [DOI] [PubMed] [Google Scholar]
- 4.Meadows KN, Bryant P, Vincent PA, Pumiglia KM. Activated Ras induces a proangiogenic phenotype in primary endothelial cells. Oncogene. 2004;23:192–200. doi: 10.1038/sj.onc.1206921. [DOI] [PubMed] [Google Scholar]
- 5.Wu G, Luo J, Rana JS, Laham R, Sellke FW, Li J. Involvement of COX-2 in VEGF-induced angiogenesis via P38 and JNK pathways in vascular endothelial cells. Cardiovasc Res. 2006;69:512–519. doi: 10.1016/j.cardiores.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 6.Bai X, Cerimele F, Ushio-Fukai M, Waqas M, Campbell PM, Govindarajan B, Der CJ, Battle T, Frank DA, Ye K, Murad E, Dubiel W, Soff G, Arbiser JL. Honokiol, a small molecular weight natural product, inhibits angiogenesis in vitro and tumor growth in vivo. J Biol Chem. 2003;278:35501–35507. doi: 10.1074/jbc.M302967200. [DOI] [PubMed] [Google Scholar]
- 7.Sharma R, Sharma R, Khaket TP, Dutta C, Chakraborty B, Mukherjee TK. Breast cancer metastasis: putative therapeutic role of vascular cell adhesion molecule-1. Cell Oncol. 2017;40:199–208. doi: 10.1007/s13402-017-0324-x. [DOI] [PubMed] [Google Scholar]
- 8.Hoeben A, Landuyt B, Highley MS, Wildiers H, Van Oosterom AT, De Bruijn EA. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56:549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- 9.Forsythe JA, Jiang BH, Iyer NV, Agani F, Leung SW, Koos RD, Semenza GL. Activation of vascular endothelial growth factor gene transcription by hypoxia-inducible factor 1. Mol Cell Biol. 1996;16:4604–4613. doi: 10.1128/MCB.16.9.4604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fasolo A, Sessa C. mTOR inhibitors in the treatment of cancer. Expert Opin Investig Drugs. 2008;17:1717–1734. doi: 10.1517/13543784.17.11.1717. [DOI] [PubMed] [Google Scholar]
- 11.Humar R, Kiefer FN, Berns H, Resink TJ, Battegay EJ. Hypoxia enhances vascular cell proliferation and angiogenesis in vitro via rapamycin (mTOR)-dependent signaling. FASEB J. 2002;16:771–780. doi: 10.1096/fj.01-0658com. [DOI] [PubMed] [Google Scholar]
- 12.Bosetti C, Rossi M, McLaughlin JK, Negri E, Talamini R, Lagiou P, Montella M, Ramazzotti V, Franceschi S, LaVecchia C. Flavonoids and the risk of renal cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2007;16:98–101. doi: 10.1158/1055-9965.EPI-06-0769. [DOI] [PubMed] [Google Scholar]
- 13.Moon YJ, Wang X, Morris ME. Dietary flavonoids: effects on xenobiotic and carcinogen metabolism. Toxicol In Vitro. 2006;20:187–210. doi: 10.1016/j.tiv.2005.06.048. [DOI] [PubMed] [Google Scholar]
- 14.Mink PJ, Scrafford CG, Barraj LM, Harnack L, Hong CP, Nettleton JA, Jacobs DR., Jr Flavonoid intake and cardiovascular disease mortality: a prospective study in postmenopausal women. Am J Clin Nutr. 2007;85:895–909. doi: 10.1093/ajcn/85.3.895. [DOI] [PubMed] [Google Scholar]
- 15.Luo H, Jiang BH, King SM, Chen YC. Inhibition of cell growth and VEGF expression in ovarian cancer cells by flavonoids. Nutr Cancer. 2008;60:800–809. doi: 10.1080/01635580802100851. [DOI] [PubMed] [Google Scholar]
- 16.Hung H. Inhibition of estrogen receptor alpha expression and function in MCF-7 cells by kaempferol. J Cell Physiol. 2004;198:197–208. doi: 10.1002/jcp.10398. [DOI] [PubMed] [Google Scholar]
- 17.Leung HWC, Lin CJ, Hour MJ, Yang WH, Wang MY, Lee HZ. Kaempferol induces apoptosis in human lung non-small carcinoma cells accompanied by an induction of antioxidant enzymes. Food Chem Toxicol. 2007;45:2005–2013. doi: 10.1016/j.fct.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 18.Kim JD, Liu L, Guo W, Meydani M. Chemical structure of flavonols in relation to modulation of angiogenesis and immune-endothelial cell adhesion. J Nutr Biochem. 2006;17:165–176. doi: 10.1016/j.jnutbio.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 19.Kim GD, Kim GJ, Seok JH, Chung HM, Chee KM, Rhee GS. Differentiation of endothelial cells derived from mouse embryoid bodies: a possible in vitro vasculogenesis model. Toxicol Lett. 2008;180:166–173. doi: 10.1016/j.toxlet.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 20.Baker M, Robinson SD, Lechertier T, Barber PR, Tavora B, D’Amico G, Jones DT, Vojnovic B, Hodivala-Dilke K. Use of the mouse aortic ring assay to study angiogenesis. Nat Protoc. 2012;7:89–104. doi: 10.1038/nprot.2011.435. [DOI] [PubMed] [Google Scholar]
- 21.Gates MA, Tworoger SS, Hecht JL, De Vivo I, Rosner B, Hankinson SE. A prospective study of dietary flavonoid intake and incidence of epithelial ovarian cancer. Int J Cancer. 2007;121:2225–2232. doi: 10.1002/ijc.22790. [DOI] [PubMed] [Google Scholar]
- 22.Cao Y, Cao R, Bråkenhielm E. Antiangiogenic mechanisms of diet-derived polyphenols. J Nutr Biochem. 2002;13:380–390. doi: 10.1016/S0955-2863(02)00204-8. [DOI] [PubMed] [Google Scholar]
- 23.Tosetti F, Ferrari N, De Flora S, Albini A. ‘Angioprevention’: angiogenesis is a common and key target for cancer chemopreventive agents. FASEB J. 2002;16:2–14. doi: 10.1096/fj.01-0300rev. [DOI] [PubMed] [Google Scholar]
- 24.Dorai T, Aggarwal BB. Role of chemopreventive agents in cancer therapy. Cancer Lett. 2004;215:129–140. doi: 10.1016/j.canlet.2004.07.013. [DOI] [PubMed] [Google Scholar]
- 25.Tozer GM, Kanthou C, Baguley BC. Disrupting tumour blood vessels. Nat Rev Cancer. 2005;5:423–435. doi: 10.1038/nrc1628. [DOI] [PubMed] [Google Scholar]
- 26.Kruger EA, Duray PH, Tsokos MG, Venzon DJ, Libutti SK, Dixon SC, Rudek MA, Pluda J, Allegra C, Figg WD. Endostatin inhibits microvessel formation in the ex vivo rat aortic ring angiogenesis assay. Biochem Biophys Res Commun. 2000;268:183–191. doi: 10.1006/bbrc.1999.2018. [DOI] [PubMed] [Google Scholar]
- 27.Risau W. Mechanisms of angiogenesis. Nature. 1997;386:671–674. doi: 10.1038/386671a0. [DOI] [PubMed] [Google Scholar]
- 28.Karar J, Maity A. PI3K/AKT/mTOR pathway in angiogenesis. Front Mol Neurosci. 2011;4:51. doi: 10.3389/fnmol.2011.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou Q, Lui VWY, Yeo W. Targeting the PI3K/Akt/mTOR pathway in hepatocellular carcinoma. Future Oncol. 2011;7:1149–1167. doi: 10.2217/fon.11.95. [DOI] [PubMed] [Google Scholar]
- 30.Aksamitiene E, Kiyatkin A, Kholodenko BN. Cross-talk between mitogenic Ras/MAPK and survival PI3K/Akt pathways: a fine balance. Biochem Soc Trans. 2012;40:139–146. doi: 10.1042/BST20110609. [DOI] [PubMed] [Google Scholar]