Abstract
To enhance the production of phenolic compounds with high antioxidant activity and reduce the level of phototoxic fagopyrin, buckwheat leaves were extracted with subcritical water (SW) at 100~220°C for 10~50 min. The major phenolic compounds were quercetin, gallic acid, and protocatechuic acid. The cumulative amount of individual phenolic compounds increased with increasing extraction temperature from 100°C to 180°C and did not change significantly at 200°C and 220°C. The highest yield of individual phenolic compounds was 1,632.2 μg/g dry sample at 180°C, which was 4.7-fold higher than that (348.4 μg/g dry sample) at 100°C. Total phenolic content and total flavonoid content increased with increasing extraction temperature and decreased with increasing extraction time, and peaked at 41.1 mg gallic acid equivalents/g and 26.9 mg quercetin equivalents/g at 180°C/10 min, respectively. 2,2-Diphenyl-1-picrylhydrazyl free radical scavenging activity and ferric reducing ability of plasma reached 46.4 mg ascorbic acid equivalents/g and 72.3 mmol Fe2+/100 g at 180°C/10 min, respectively. The fagopyrin contents were reduced by 92.5~95.7%. Color values L* and b* decreased, and a* increased with increasing extraction temperature. SW extraction enhanced the yield of phenolic compounds with high antioxidant activity and reduced the fagopyrin content from buckwheat leaves.
Keywords: buckwheat leaves, subcritical water extraction, phenolic compounds, antioxidant activity, fagopyrin content
INTRODUCTION
Phenolic compounds are secondary metabolites in plants, and their structures vary from single aromatic phenolics to complex derived phenolics. They have various health benefits, such as antioxidant, antimicrobial, antiobesity, antidiabetic, antihypertensive, antimutagenic, and anticancer effects (1,2).
Buckwheat (Fagopyrum esculentum) belongs to the family Polygonaceae, which originates from East Asia, and it is commonly used in arid and cold regions worldwide (3). Phenolic compounds are present at higher levels in leaves than in seeds, and in decreasing order in flowers, leaves, stems, and roots (4–6). Phenolic compounds in buckwheat have various health benefits, such as antioxidant and antihyperlipidemic effects, prevention of neurological diseases, and enhancement of immune responses (4,7).
Phenolic compounds from buckwheat are extracted using organic solvents (mainly neat methanol) and hydrolyzed using an alkali solution (3,4,6). These solvents are not appropriate for the extraction and hydrolysis of phenolic compounds for use in food due to their toxicity, cost, and toxic residues (8). Phenolic compounds in plants exist in free form or bound to organic acids, carbohydrates, and lignins (9). The biological activity of some phenolic compounds can be increased following the decomposition of bound phenolics to an aglycone moiety (10).
Subcritical water (SW) is an alternative to organic solvents for extraction and hydrolysis of phenolic compounds from plant materials due to non-toxicity, and environmentally friendly nature. SW has a low dielectric constant and a high ion product compared to water at ambient temperature (11). Non-polar compounds, such as phenolic compounds, are soluble in SW. SW is an acidic medium favorable for hydrolysis reactions (12). These two properties make SW suitable for extraction and hydrolysis of phenolic compounds from plant materials without the use of acid, alkali, and/or toxic organic solvents.
Several studies have investigated SW extraction of phenolic compounds from plants. The yield of quercetin from onion skin at 165°C was about 3-fold higher than that at 100°C (13). The yields of narirutin and hesperidin from Citrus unshiu peel at 160°C were about 4- and 2-fold higher, respectively, than those at 110°C (14). The sum of the levels of chlorogenic, gallic, and procatechuic acids from potato peels at 180°C was about 4-fold higher than that at 100°C (15). The yields of procatechuic and vanillic acids from rice bran were maximal at 230 and 295°C, respectively (16). However, the yield of phenolic compounds from buckwheat leaves using a subcritical water extraction method has not been reported yet. Buckwheat leaves also contain the phototoxic compound fagopyrin, which causes inflammation of the skin upon exposure to sunlight (17). Thus, it is desirable to reduce the fagopyrin content in extracts of buckwheat leaves.
In this study, SW was used to extract phenolic compounds from buckwheat leaves and reduce the phototoxic fagopyrin content. The effects of SW extraction temperature and time were investigated in terms of the total phenolic content (TPC), total flavonoid content (TFC), levels of individual phenolic compounds, antioxidant activity, color, and fagopyrin content.
MATERIALS AND METHODS
Materials and chemicals
Buckwheat leaves were collected from a local farm on Jeju, Korea, washed, dried, ground (30 mesh), and stored at −20°C. 2,4,6-tripyridyl-S-triazine (TPTZ) and hypericin were supplied from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). Folin-Ciocalteau’s phenol reagent and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Ferrous sulfate heptahydrate and N,O-bis(trimethylsilyl)trifluoroacetamide (BSTFA) were supplied by Wako Pure Chemical Industries, Ltd. (Osaka, Japan) and Supelco Inc. (Bellefonte, PA, USA), respectively. p-Hydroxybenzoic acid, protocatechuic acid, gentisic acid, quercetin, and 3-(4-hydroxyphenyl)-1-propanol were purchased from Sigma Chemical Co.. Caffeic acid, catechin, p-coumaric acid, ferulic acid, and gallic acid were purchased from Fluka Chemical Corp. (Steinheim, Switzerland).
SW extraction (SWE)
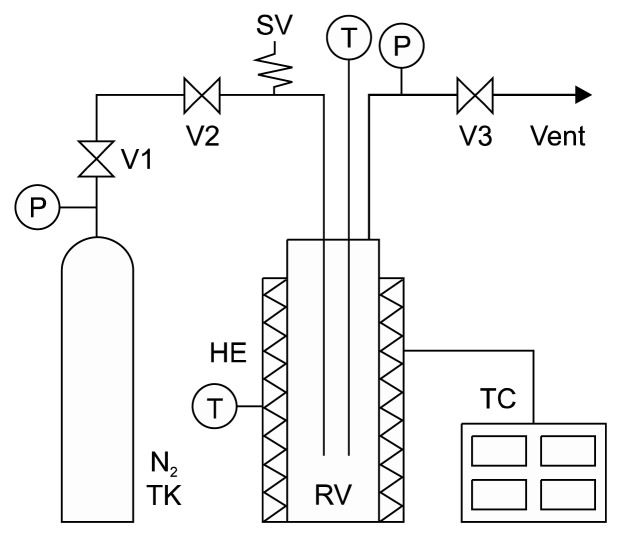
SWE was performed using our laboratory-built system (Fig. 1). This system consists of a reaction vessel, an electric band heater, a nitrogen gas tank, auxiliary valves, and piping systems. SWE of buckwheat leaves was carried out in a 300-mL extractor (Parker Autoclave Engineers, Erie, PA, USA). Powdered buckwheat leaves (2.0 g) and 100 mL of distilled water were loaded into the reactor. Nitrogen gas was used to force out oxygen dissolved in the sample solution to avoid oxidation. The reactor was heated electrically to the desired extraction temperature (100~220°C), which was maintained for the desired extraction time (10~50 min). Then, the reactor was rapidly cooled by soaking it in tap water. The extract was collected and filtered through filter paper (No. 5A, Advantec Toyo Kaisha, Ltd., Tokyo, Japan) (18).
Fig. 1.
Schematic diagram of the subcritical water extraction system. HE, heat exchanger; N2 TK, nitrogen tank; P, pressure indicator; RV, extractor; SV, safety valve; T, temperature indicator; TC, temperature controller; V, on/off valve.
Determination of levels of individual phenolic compounds by gas chromatography/mass spectrometry (GC/MS)
The extract (5 mL) was mixed with 15 mL of ethyl acetate in a conical tube and shaken for 1 min. After centrifugation for 10 min at 10,000 g, the upper layer was collected, and the residue was further extracted twice. 3-(4-Hydroxyphenyl)-1-propanol (0.25 mL), as an internal standard, was mixed with the combined extract, and the residual solvent was removed in a rotary vacuum evaporator at 40°C. A 0.25 mL of BSTFA was added to the residue and derivatized for 40 min at 75°C.
Quantitative analysis of each phenolic compound was performed using an Agilent GC 6890N with an HP 5973 MS detector (electron ionization, 70 eV) (Agilent Technologies, Santa Clara, CA, USA). An HP-5 MS capillary column (30 m×0.25 mm, 0.25 μm film thickness) was used at a split ratio of 1:20. Helium was the carrier gas at a flow rate of 0.6 mL/min. The injector and transfer line temperatures were 280 and 300°C, respectively. The oven temperature was held at 120°C for 1 min, increased to 220°C at 5°C/min, then to 300°C at 10°C/min, and held for 10 min. A selective ion monitoring GC/MS method was applied for the detection of target phenolic compounds.
Chromatographic peaks were identified by comparing the retention times and three fragment ions of each phenolic compound with those of reference compounds. The quantification was performed using 3-(4-hydroxy-phenyl)-1-propanol as the internal standard at target ion m/z 206 and qualifiers 191 and 179. Target and qualifier ions for nine phenolic compounds were set as follows: caffeic acid, 396, 381, 219; catechin, 368, 355, 346; p-coumaric acid, 293, 308, 219; ferulic acid, 338, 323, 308; gentisic acid, 355, 356, 357; gallic acid, 281, 443, 458; p-hydroxybenzoic acid, 267, 223, 193; protocatechuic acid, 193, 355, 370; and quercetin, 647, 559, 575. The quantification of each phenolic compound was performed using standard curves for each phenolic compound (19).
Determination of TPC and TFC
TPC was measured using the Folin-Denis method (20). A diluted extract (0.2 mL) was added to 0.1 mL of 2 M Folin-Ciocalteau’s phenol reagent and 0.9 mL of distilled water. Then, 0.3 mL of Na2CO3 (20% w/v) was added after 5 min, and the volume was adjusted to 2 mL. This solution was reacted for 1 h, and the absorbance was determined at 760 nm. TPC was expressed as mg gallic acid equivalents (GAE)/g dry sample.
TFC was determined by the aluminum chloride colorimetric method (20). A diluted extract (0.2 mL) was mixed with 60 μL of NaNO2 (5% w/v) and 0.8 mL of ethanol. After 5 min, 60 μL of AlCl3 (10% w/v) was added and reacted for 5 min. Then, 0.4 mL of 1 M NaOH was added, and the mixture was reacted for 1 min. The absorbance was determined at 415 nm. TFC was expressed as mg quercetin equivalents (QE)/g dry sample.
Determination of antioxidant activity
DPPH free radical scavenging activity was measured as follows (21). A diluted extract (0.1 mL) was mixed with 0.1 mM DPPH (2.0 mL), and the solution was reacted for 30 min. The absorbance was measured at 517 nm. The activity was expressed as mg ascorbic acid equivalents (AAE)/g dry sample.
Ferric reducing ability of plasma (FRAP) was determined as follows (22). The FRAP solution was prepared by mixing 20 mM FeCl3·6H2O solution, 300 mM acetate buffer, and 10 mM TPTZ solution (in 40 mM HCl) at a ratio of 1:1:10 (v/v). A diluted extract (0.1 mL) was mixed with the FRAP solution (3 mL) and distilled water (0.3 mL), and reacted for 30 min at 37°C. The absorbance was determined at 595 nm. FRAP was expressed as mmol Fe2+/100 g dry sample.
Determination of fagopyrin content
Untreated powdered buckwheat leaves (2.0 g) and 100 mL of methanol were loaded into a round-bottom flask and extracted at 65°C for 2 h. The extract was collected and filtered through filter paper. The absorbances of the methanol extract and SW extracts were measured at 590 nm after passing through a 0.45-μm syringe filter.
Unfortunately, no fagopyrin reference standard is currently available. Therefore, the concentration of fagopyrin was calculated using hypericin as a secondary standard, which is similar to fagopyrin in structure and UV spectrum. It is expressed as hypericin equivalents because the absorbance depends on complex structures and thus cannot be directly compared between fagopyrin and hypericin (17,23). The calibration curve was constructed between the absorbance at 590 nm and the concentration of hypericin, which ranged from 1.0 μg/mL to 5.0 μg/mL, where the correlation coefficient was 0.999.
Color measurement
Color values (L*, a*, and b*) were measured using a color spectrophotometer (HunterLab UltraScan VIS, Hunter Associates Laboratory, Inc., Reston, VA, USA).
Statistical analysis
The statistical significance of the results was measured by the SPSS software (version 18.0, SPSS Inc., Chicago, IL, USA). Significant differences (P<0.05) among the treatment means were determined by Duncan’s multiple range test.
RESULTS AND DISCUSSION
Yields of individual phenolic compounds
SW extraction was performed at 100~220°C for 20 min and at 180°C for 10~50 min (Table 1). Nine phenolic compounds were identified: caffeic acid, p-coumaric acid, catechin, ferulic acid, gallic acid, p-hydroxybenzoic acid, gentisic acid, protocatechuic acid, and quercetin. The major phenolic compounds were quercetin, gallic acid, and protocatechuic acid. The cumulative amount of individual phenolic compounds increased with increasing extraction temperature from 100°C to 180°C and did not change significantly at 200 and 220°C. The highest yield of total individual phenolic compounds was 1,632.2 μg/g dry sample at 180°C, which was 4.7-fold higher than that (348.4 μg/g dry sample) at 100°C.
Table 1.
Profile of individual phenolic compounds in subcritical water extracts from buckwheat leaves at different temperatures for 20 min and for different times at 180°C
| Extraction condition | Yields (μg/g dry sample) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Caffeic acid | Catechin | p-Coumaric acid | Ferulic acid | Gentisic acid | Gallic acid | p-Hydroxybenzoic acid | Protocatechuic acid | Quercetin | Total | |
| Temperature (°C) for 20 min | ||||||||||
| 100 | ND1) | 57.3±3.3b | ND | ND | ND | ND | ND | ND | 291.1±19.2d | 348.4±17.0d |
| 120 | ND | 142.1±11.0a | ND | ND | ND | ND | ND | 25.0±4.9c | 301.5±11.1d | 460.3±8.8d |
| 140 | 74.0±33.3ab | 147.8±33.3a | 60.9±7.2d | 26.1±10.9b | ND | ND | 10.9±1.6e | 54.3±37.6c | 442.3±71.0c | 812.7±83.8c |
| 160 | 91.1±19.0a | 60.1±19.4b | 107.4±4.4a | 48.7±7.8a | 10.4±0.2d | 132.2±17.8b | 42.5±3.9d | 195.1±13.7a | 471.5±3.5c | 1,155.6±21.8b |
| 180 | 64.2±2.5ab | 34.0±3.1b | 93.1±3.8b | 39.2±2.9a | 15.8±0.6c | 212.9±8.8a | 79.6±2.5c | 182.3±7.3a | 910.9±67.7b | 1,632.2±91.5a |
| 200 | 45.2±0.6bc | 27.5±0.4b | 74.3±1.8c | 22.2±0.3b | 31.8±1.2a | 104.4±5.8c | 107.7±7.1b | 110.9±10.1b | 1,088.1±116.6a | 1,603.0±138.0a |
| 220 | 27.0±0.8c | ND | 56.1±4.5d | ND | 22.0±2.5b | 34.4±1.2d | 132.3±17.3a | 47.4±9.5c | 1,218.4±131.5a | 1,537.7±157.4a |
| Time (min) at 180°C | ||||||||||
| 10 | 72.0±1.2A | 119.6±8.5A | 102.5±3.2A | 44.5±2.2A | 11.9±0.5E | 190.3±17.5B | 75.7±4.4B | 189.1±4.6A | 1,004.3±116.5AB | 1,810.0±129.0NS2) |
| 20 | 64.2±2.5B | 34.0±3.0C | 93.1±3.8B | 39.2±2.9B | 15.8±0.6D | 212.9±8.7A | 79.6±2.5B | 182.4±7.3A | 910.9±67.7B | 1,632.2±91.5 |
| 30 | 57.2±0.9C | 55.8±12.4B | 85.3±1.8C | 32.8±1.3C | 18.6±0.1C | 192.0±5.2B | 90.9±2.4A | 164.0±6.1B | 1,012.1±40.6AB | 1,708.8±56.1 |
| 40 | 52.0±1.3D | 44.8±11.5BC | 78.7±2.7D | 28.5±1.9D | 21.4±1.3B | 171.9±7.2BC | 92.6±6.3A | 145.8±7.4C | 1,028.8±107.6AB | 1,664.5±146.2 |
| 50 | 49.7±0.6D | 28.3±0.2C | 74.1±0.6D | 23.1±0.8E | 24.2±0.1A | 152.7±3.5C | 81.1±1.6A | 136.7±6.8C | 1,172.7±91.2A | 1,742.4±84.0 |
Data are given as mean±standard deviation (n=3).
The means in each column followed by the same letter (a–e, A–E) are not significantly different by Duncan’s multiple range test at P<0.05.
Not detected.
Not significant.
The yield of hydroxybenzoic acid derivatives (gallic acid, gentisic acid, p-hydroxybenzoic acid, and protocatechuic acid) was higher at higher temperatures (180~ 220°C), while that of hydroxycinnamic acid derivatives (caffeic acid, p-coumaric acid, and ferulic acid) was higher at lower temperatures (160 and 180°C). Therefore, the thermal stability of hydroxycinnamic acid derivatives is lower than that of hydroxybenzoic acid derivatives, likely due to disruption of the C=C bonds in hydroxycinnamic acid derivatives at higher temperatures (24,25).
The yield of catechin was higher at lower temperatures (120 and 140°C), while that of quercetin was higher at higher temperatures (200 and 220°C) (Table 1). Elhamirad and Zamanipoor (26) reported that quercetin had high thermal stability, and its Q10 value (1.65) was lower than those of caffeic (2.22) and gallic (2.22) acids.
The effect of extraction time on the yield of phenolic compounds is also shown in Table 1. The sum of individual phenolic compounds did not change significantly with increasing extraction time. However, the yields of individual phenolic compounds–for example, caffeic acid, catechin, p-coumaric acid, ferulic acid, gallic acid, and protocatechuic acid–decreased with increasing extraction time, possibly due to thermal degradation (15).
TPC and TFC
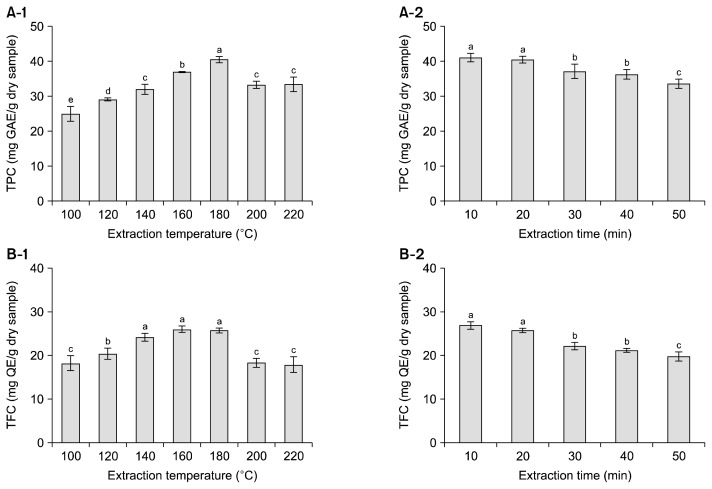
TPC in SW extracts increased with increasing extraction temperature from 100°C to 180°C and then decreased up to 220°C (Fig. 2A-1). The maximum TPC was 40.5 mg GAE/g dry sample at 180°C. TPC in SW extracts decreased with increasing extraction time from 10 to 50 min, and the maximum TPC was 41.1 mg GAE/g dry sample at 10 min (Fig. 2A-2). TFC showed a similar pattern to TPC (Fig. 2B). TFC increased with increasing extraction temperature from 100°C to 180°C, and the maximum TFC was 26.0 mg QE/g dry sample at 160°C (Fig. 2B-1). TFC decreased with increasing extraction time from 10 to 50 min, and the maximum TFC was 26.9 mg QE/g dry sample at 10 min (Fig. 2B-2). Tunchaiyaphum et al. (8) extracted phenolic compounds from mango peel using SW, and they reported that TPC peaked at 180°C (30.6 mg GAE/g DW) and then decreased up to 220°C (20.1 mg GAE/g DW), possibly due to the thermal degradation of phenolic compounds.
Fig. 2.
Total phenolic content (TPC) (A) and total flavonoid content (TFC) (B) in subcritical water extracts from buckwheat leaves at different temperatures for 20 min and for different times at 180°C. Each bar represents the mean±standard deviation (n=3). The means with different letters (a–e) are significantly different by Duncan’s multiple range test at P<0.05. GAE, gallic acid equivalents; QE, quercetin equivalents.
Antioxidant activity
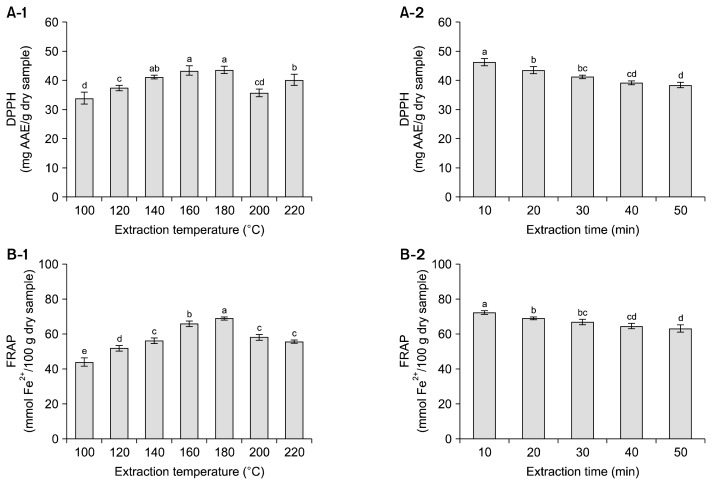
Phenolic compounds have strong antioxidant activity due to the presence of hydroxyl substituents in aromatic rings, which enable them to scavenge free radicals (1). Fig. 3 shows the effect of extraction conditions on the antioxidant activities of SW extracts from buckwheat leaves. DPPH radical scavenging activity increased with increasing extraction temperature from 100°C to 180°C, and then decreased up to 220°C (Fig. 3A-1). The maximum DPPH was 43.6 mg AAE/g dry sample at 180°C. DPPH radical scavenging activity decreased with increasing extraction time, and it was highest (46.4 mg AAE/g dry sample) at 10 min (Fig. 3A-2). FRAP values showed a similar pattern to DPPH radical scavenging activity. The maximum FRAP values were 69.0 mmol Fe2+/100 g dry sample at 180°C (Fig. 3B-1) and 72.3 Fe2+/100 g dry sample at 10 min (Fig. 3B-2). The effects of extraction temperature and time on antioxidant activity were similar to those on TPC and TFC. Pourali et al. (16) reported that DPPH of SW extracts from rice bran showed a similar trend to TPC. Jo et al. (27) also reported strong linear correlations (r=0.98, 0.97, and 0.99) between TPC and antioxidant activities (DPPH, ABTS, and FRAP) of SW extracts from golden oyster mushrooms.
Fig. 3.
2,2-Diphenyl-1-picrylhydrazyl (DPPH) free radical scavenging activity (A) and ferric reduction antioxidant power (FRAP) (B) in subcritical water extracts from buckwheat leaves at different temperatures for 20 min and for different times at 180°C. Each bar represents the mean±standard deviation (n=3). The means with different letters (a–e) are significantly different by Duncan’s multiple range test at P<0.05. AAE, ascorbic acid equivalents.
Fagopyrin content
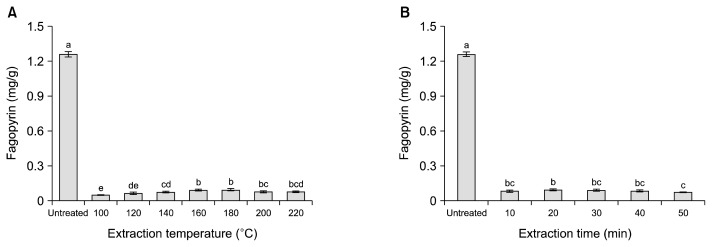
The fagopyrin contents of SW extracts are shown in Fig. 4. The fagopyrin content of untreated buckwheat leaves was 1.261 mg/g dry sample, and those of SW extracts were 92.5~95.7% lower due to decomposition at >100°C. Mannila and Wai (28) reported that the hypericin level in pressurized water extracts from St. John’s wort decreased as extraction temperature increased from 25°C to 100°C. Therefore, it was concluded that fagopyrin was heat-labile and thus was destroyed by SW treatment at higher temperatures.
Fig. 4.
Fagopyrin content (hypericin equivalents) in subcritical water extracts from buckwheat leaves at different temperatures for 20 min (A) and for different times at 180°C (B). Each bar represents the mean±standard deviation (n=3). The means with different letters (a–e) are significantly different by Duncan’s multiple range test at P<0.05.
Color values
The color values of SW extracts from buckwheat leaves according to extraction temperature and time are shown in Table 2. As the extraction temperature increased from 100°C to 160°C, the L* and b* values of the SW extract decreased gradually. With a further increase in extraction temperature, the L* value plateaued, and the b* value increased slightly. The a* value remained constant from 100°C to 160°C, and increased at higher temperatures. All color values increased with increasing extraction time from 10 to 30 min, and they remained constant from 30 to 50 min. Song et al. (29) reported that the L* values of hydrothermal extracts of Saururus chinensis leaves decreased, and the a* values increased with increasing temperature and time due to the Maillard reaction.
Table 2.
Color difference values in subcritical water extracts from buckwheat leaves at different extraction temperatures for 20 min and for different extraction times at 180°C
| Extraction condition | L* | a* | b* |
|---|---|---|---|
| Temperature (°C) for 20 min | |||
| 100 | 39.28±0.74a | 2.29±0.20b | 17.92±0.88a |
| 120 | 36.41±0.42b | 2.59±0.37b | 14.45±0.75b |
| 140 | 32.38±0.75c | 3.21±0.68b | 9.81±1.89c |
| 160 | 30.33±0.14d | 2.94±0.96b | 7.31±1.28d |
| 180 | 30.37±0.76d | 5.12±0.32a | 9.80±0.86c |
| 200 | 32.31±0.67c | 5.69±0.53a | 12.35±1.36b |
| 220 | 30.71±1.21d | 4.79±0.81a | 9.54±2.14cd |
| Time (min) at 180°C | |||
| 10 | 29.79±0.35C | 4.80±0.74B | 8.96±0.51C |
| 20 | 30.37±0.76C | 5.12±0.32B | 9.80±0.86C |
| 30 | 34.08±0.19A | 7.12±0.40A | 16.05±0.40A |
| 40 | 33.29±0.20B | 6.94±0.10A | 14.71±0.46B |
| 50 | 34.15±0.17A | 7.21±0.29A | 15.74±0.28A |
Data are given as mean±standard deviation (n=3).
The means in each column followed by the same letter are not significantly different by Duncan’s multiple range test at P< 0.05.
In conclusion, the cumulative amount of individual phenolic compounds at 180°C was 4.7-fold higher than that at 100°C. Antioxidant activities, expressed as DPPH radical scavenging activity and FRAP, were 1.5-fold higher at 180°C than those at 100°C. The fagopyrin contents of buckwheat leaves were reduced by more than 92% by SW treatment. Therefore SW can be used as an environmentally friendly extraction solvent to enhance the yield of phenolic compounds with high antioxidant activity and reduce the phototoxic fagopyrin level from buckwheat leaves.
ACKNOWLEDGEMENTS
This research was supported by the 2016 scientific promotion program funded by Jeju National University.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Tsao R. Chemistry and biochemistry of dietary polyphenols. Nutrients. 2010;2:1231–1246. doi: 10.3390/nu2121231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kunyanga CN, Imungi JK, Okoth MW, Biesalski HK, Vadivel V. Total phenolic content, antioxidant and antidiabetic properties of methanolic extract of raw and traditionally processed Kenyan indigenous food ingredients. LWT-Food Sci Technol. 2012;45:269–276. doi: 10.1016/j.lwt.2011.08.006. [DOI] [Google Scholar]
- 3.Seo JM, Lee DB, Arasu MV, Wu Q, Suzuki T, Yoon YH, Lee SW, Park SU, Kim SJ. Quantitative differentiation of phenolic compounds in different varieties of buckwheat cultivars from China, Japan and Korea. J Agric Chem Environ. 2013;2:109–116. [Google Scholar]
- 4.Inglett GE, Rose DJ, Chen D, Stevenson DG, Biswas A. Phenolic content and antioxidant activity of extracts from whole buckwheat (Fagopyrum esculentum Möench) with or without microwave irradiation. Food Chem. 2010;119:1216–1219. doi: 10.1016/j.foodchem.2009.07.041. [DOI] [Google Scholar]
- 5.Verardo V, Gomez-Caravaca AM, Segura-Carretero A, Caboni MF, Fernández-Gutiérrez A. Development of a CE-ESI-microTOF-MS method for a rapid identification of phenolic compounds in buckwheat. Electrophoresis. 2011;32:669–673. doi: 10.1002/elps.201000609. [DOI] [PubMed] [Google Scholar]
- 6.Uddin MR, Li X, Park WT, Kim YB, Kim SJ, Kim YS, Lee MY, Park CH, Park SU. Phenolic compound content in different organs of Korean common buckwheat cultivars. Asian J Chem. 2013;25:424–426. [Google Scholar]
- 7.Kreft M. Buckwheat phenolic metabolites in health and disease. Nutr Res Rev. 2016;29:30–39. doi: 10.1017/S0954422415000190. [DOI] [PubMed] [Google Scholar]
- 8.Tunchaiyaphum S, Eshtiaghi MN, Yoswathana N. Extraction of bioactive compounds from mango peels using green technology. Int J Chem Eng Appl. 2013;4:194–198. [Google Scholar]
- 9.Liu RH. Whole grain phytochemicals and health. J Cereal Sci. 2007;46:207–219. doi: 10.1016/j.jcs.2007.06.010. [DOI] [Google Scholar]
- 10.Das AK, Singh V. Antioxidative free and bound phenolic constituents in botanical fractions of Indian specialty maize (Zea mays L.) genotypes. Food Chem. 2016;201:298–306. doi: 10.1016/j.foodchem.2016.01.099. [DOI] [PubMed] [Google Scholar]
- 11.Plaza M, Turner C. Pressurized hot water extraction of bioactives. Trends Anal Chem. 2015;71:39–54. doi: 10.1016/j.trac.2015.02.022. [DOI] [Google Scholar]
- 12.Shitu A, Izhar A, Tahir TM. Sub-critical water as a green solvent for production of valuable materials from agricultural waste biomass: a review of recent work. Global J Environ Sci Manage. 2015;1:255–264. [Google Scholar]
- 13.Ko MJ, Cheigh CI, Cho SW, Chung MS. Subcritical water extraction of flavonol quercetin from onion skin. J Food Eng. 2011;102:327–333. doi: 10.1016/j.jfoodeng.2010.09.008. [DOI] [Google Scholar]
- 14.Cheigh CI, Chung EY, Chung MS. Enhanced extraction of flavanones hesperidin and narirutin from Citrus unshiu peel using subcritical water. J Food Eng. 2012;110:472–477. doi: 10.1016/j.jfoodeng.2011.12.019. [DOI] [Google Scholar]
- 15.Singh PP, Saldaña MDA. Subcritical water extraction of phenolic compounds from potato peel. Food Res Int. 2011;44:2452–2458. doi: 10.1016/j.foodres.2011.02.006. [DOI] [Google Scholar]
- 16.Pourali O, Asghari FS, Yoshida H. Production of phenolic compounds from rice bran biomass under subcritical water conditions. Chem Eng J. 2010;160:259–266. doi: 10.1016/j.cej.2010.02.057. [DOI] [Google Scholar]
- 17.Benković ET, Kreft S. Fagopyrins and protofagopyrins: detection, analysis, and potential phototoxicity in buckwheat. J Agric Food Chem. 2015;63:5715–5724. doi: 10.1021/acs.jafc.5b01163. [DOI] [PubMed] [Google Scholar]
- 18.Ko JY, Ko MO, Kim DS, Lim SB. Enhanced production of phenolic compounds from pumpkin leaves by subcritical water hydrolysis. Prev Nutr Food Sci. 2016;21:132–137. doi: 10.3746/pnf.2016.21.2.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim MB, Park JS, Lim SB. Antioxidant activity and cell toxicity of pressurised liquid extracts from 20 selected plant species in Jeju, Korea. Food Chem. 2010;122:546–552. doi: 10.1016/j.foodchem.2010.03.007. [DOI] [Google Scholar]
- 20.Yi MR, Hwang JH, Oh YS, Oh HJ, Lim SB. Quality characteristics and antioxidant activity of immature Citrus unshiu vinegar. J Korean Soc Food Sci Nutr. 2014;43:250–257. doi: 10.3746/jkfn.2014.43.2.250. [DOI] [Google Scholar]
- 21.Kamiloglu S, Pasli AA, Ozcelik B, Camp JV, Capanoglu E. Colour retention, anthocyanin stability and antioxidant capacity in black carrot (Daucus carota) jams and marmalades: effect of processing, storage conditions and in vitro gastrointestinal digestion. J Funct Foods. 2015;13:1–10. doi: 10.1016/j.jff.2014.12.021. [DOI] [Google Scholar]
- 22.Thaipong K, Boonprakob U, Crosby K, Cisneros-Zevallos L, Byrne DH. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J Food Compost Anal. 2006;19:669–675. doi: 10.1016/j.jfca.2006.01.003. [DOI] [Google Scholar]
- 23.Hinneburg I, Reinhard H, Neubert H. Influence of extraction parameters on the phytochemical characteristics of extracts from buckwheat (Fagopyrum esculentum) herb. J Agric Food Chem. 2005;53:3–7. doi: 10.1021/jf049118f. [DOI] [PubMed] [Google Scholar]
- 24.Cheng Y, Xu Q, Liu J, Zhao C, Xue F, Zhao Y. Decomposition of five phenolic compounds in high temperature water. J Braz Chem Soc. 2014;25:2102–2107. [Google Scholar]
- 25.Khuwijitjaru P, Suaylam B, Adachi S. Degradation of caffeic acid in subcritical water and online HPLC-DPPH assay of degradation products. J Agric Food Chem. 2014;62:1945–1949. doi: 10.1021/jf404850a. [DOI] [PubMed] [Google Scholar]
- 26.Elhamirad AH, Zamanipoor MH. Thermal stability of some flavonoids and phenolic acids in sheep tallow olein. Eur J Lipid Sci Technol. 2012;114:602–606. doi: 10.1002/ejlt.201100240. [DOI] [Google Scholar]
- 27.Jo EK, Heo DJ, Kim JH, Lee YH, Ju YC, Lee SC. The effects of subcritical water treatment on antioxidant activity of golden oyster mushroom. Food Bioprocess Technol. 2013;6:2555–2561. doi: 10.1007/s11947-012-0793-x. [DOI] [Google Scholar]
- 28.Mannila M, Wai CM. Pressurized water extraction of naphtodianthrones in St. John’s wort (Hypericum perforatum L.) Green Chem. 2003;5:387–391. doi: 10.1039/b212351g. [DOI] [Google Scholar]
- 29.Song AS, Lim SW, Kim SJ, Lee SC. Effect of hydrothermal treatment on the antioxidant activity of Sambaekcho (Saururus chinensis) leaves. Food Sci Biotechnol. 2013;22:825–829. doi: 10.1007/s10068-013-0151-4. [DOI] [Google Scholar]