Abstract
To develop functionally and nutritionally improved tofu, the effects of partial (0.2~0.8%) replacement with licorice powder (LP) on the quality characteristics of tofu were investigated. The pH and turbidity values decreased upon addition of increasing amounts of LP (P<0.05). The yield of LP-supplemented tofu was higher than that of the control tofu, and it increased as the concentration of LP increased (P<0.05). Substituting 0.6% and above of LP significantly hardened the texture of tofu (P<0.05) while control and 0.2~0.4% samples were not significantly different among them (P> 0.05). Lightness significantly decreased with higher LP content in the formulation (P<0.05), as indicated by visual observation that the color of tofu became darker. Redness and yellowness significantly increased (P<0.05). 2,2-Diphenyl-1-picrylhydrazyl and 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid radical scavenging activities significantly increased (P<0.05) with higher substitution of LP, and they were well correlated. Tofu incorporated with LP (0.2~0.8%) had a better shelf life which was approximately 4.32~26.64 h longer than the control tofu at the elevated temperature of 15°C. Finally, consumer acceptance test revealed that supplementation of LP more than 0.4% had an adverse effect on general consumer acceptance. On the basis of the overall observations, tofu samples supplemented with 0.2% (w/w) LP were found to benefit from the functional properties of LP, without compromising consumer acceptance.
Keywords: tofu, licorice powder, physicochemical properties, consumer acceptances, storage stability
INTRODUCTION
Soybeans (Glycine max L.) contain high amounts of protein (35~40% on a dry wet basis) and special biological active compounds, which have been regarded as cholesterol-free sources of high quality nutrients (1,2). In addition to their nutritional benefits, isoflavones (genistein, daidzein, and glycitein) in tofu have several health-promoting functions like anti-carcinogenicity, lowering blood cholesterol and sugar, and reducing the risk of cardiovascular disease (3–8). Soybeans have been utilized in various forms of foods, with tofu being the most widely accepted. Tofu, a traditional soybean food, has been a prime component of Asian food culture (9). Tofu is gaining increasing consumption and popularity throughout the world as a healthy vegetable protein source as compared to meat, fish, and cheese (10,11).
Licorice comes from the roots and stolons of some Glycyrrhiza species (G. glabra, G. uralensis, G. inflata, G. eurycarpa, G. aspera, and G. korshinskyi) and has been used in traditional herbal medicine for at least 4,000 years (12) due to its emollient, anti-inflammatory, antiviral, anti-allergic, antioxidant, and anti-cancerous properties (13). Licorice also reduced cyclosporine bioavailability by activating P-glycoprotein and cytochrome P450 3A4 (14), and compounds containing plant extracts from red yeast rice, bitter gourd, chlorella, soy protein, and licorice reduced total cholesterol, low-density lipoprotein cholesterol, ratio of total-to-high-density lipoprotein cholesterol, and triglyceride levels in subjects with metabolic syndrome (15). Due to its sweet taste, licorice has been extensively used in food, confectionery, pharmaceutical, and tobacco products worldwide (16), and it is considered as a “generally recognized as safe” by the US Food and Drug Administration (17). Considering its physiological activities and therapeutic effects, it would be beneficial to develop a novel functional food based on licorice. A literature review indicates that licorice powder (LP) or extract has been successfully applied to the processing of cheonggukjang (18), muffin (19), bread (20), kimchi (21), takju (22,23), doenjang (24), kochujang (25), and pound cake (26).
Rapidly growing concerns about healthy diets and increased demand for the use of novel food ingredients, especially soybean products, have led to investigations into tofu with value-added ingredients such as LP. To the best of our knowledge, little to no information is available on the effects of LP on the physicochemical and sensory properties as well as storage stability of tofu. LP contains antioxidants and other nutritional components, and if added to foods as a supplement, it can provide beneficial effects on nutrition and health. In this study, LP was supplemented with soybean milk in order to improve its functional and nutritional value. The aim of this research was to determine the effects of LP addition (0.2~ 0.8%) on the physicochemical and sensory characteristics of tofu. The antioxidant properties and storage stability of LP-supplemented tofu were also determined.
MATERIALS AND METHODS
Materials
LP was purchased from Garunara (Seoul, Korea), and soybeans were purchased from Orga Whole Foods (Seoul, Korea). Salt and brown rice vinegar (Ottogi, Seoul, Korea) were purchased from the local market in Gyeongsan, Korea.
Preparation of tofu
Washed soybeans (500 g) were socked in tap water at 25°C for 24 h. The hydrated beans were placed in a basket to remove excess water and ground with distilled water (3,600 mL) in a blender (DA700-G, Daesung Artlon, Seoul, Korea) for 2 min at high speed, followed by straining through a muslin cloth and pressing to obtain soymilk. Soymilk (2,800 mL) was heated to a boil for 6 min while removing the foam, and stirred for 2 min with a natural coagulant (mixture of 10 g of salt and 8 g of vinegar) and held for 5 min to coagulate and used as the control. The other samples were made by substituting 0.2~ 0.8% of LP based on the total weight of the soybean milk. The curd was gently transferred to a specially designed, perforated mold (10×11×10 cm depth) lined with cheese cloth and pressed for 20 min using bricks weighing 5 kg.
Physicochemical analyses
A sample (10 g) of tofu was mixed with 90 mL of distilled water and homogenized for 1 min. The mixture was held at ambient temperature for 1 h to separate the solid and liquid phases. The pH of the supernatant was measured using a pH meter (MP230, Mettler Toledo, Zürich, Switzerland). The yield was expressed as the weight of tofu obtained from 100 g of soybeans. Turbidity of filtered soybean whey using a Whatman No. 2 filter (GE Healthcare UK Ltd., Little Chalfont, UK) was measured at 600 nm using a spectrophotometer (Optizen 2120UV Plus, Mecasys Co., Ltd., Daejeon, Korea). All measurements were done in triplicate and mean values were compared.
Color characteristics (CIE L*, a*, and b*) were measured using a Minolta Spectrophotometer (CM-600d, Minolta Co., Osaka, Japan). Texture profile analysis (TPA) of tofu samples (3×3×3 cm) was performed using a computer-controlled Advanced Universal Testing System (LRXPlus, Lloyd Instrument Ltd., Fareham, UK) at room temperature. Each sample was compressed twice to 30% of its original height using a disc-shaped probe (5 cm in diameter) at 1 mm/s speed, and the trigger was set at 0.1 gf. Hardness was expressed in Newtons as the peak height of the force on the first compression. Color measurements were repeated 5 times while TPA was conducted 9 times.
Determination of free radical scavenging activities
2,2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activities of the samples were measured in terms of their hydrogen-donating or radical scavenging activity using the stable DPPH radical. The assay was performed as previously described by Blois (27) with slight modifications. Briefly, 0.2 mM solution of DPPH radical in ethanol was prepared, after which 5 mL of this solution was added to 1 mL of sample solution in ethanol at different concentrations and then shaken and left to stand for 10 min. Decolorization of DPPH-donated protons was determined by measuring the absorbance at 517 nm using a spectrophotometer (Optizen 2020 UV Plus, Mecasys Co., Ltd., Daejeon, Korea). The scavenging activity of the DPPH radical was calculated using the following equation:
The spectrophotometric analysis of 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid cation radical (ABTS+·) scavenging activity was determined according to the method used by Re et al. (28) with slight modifications. The ABTS+· was produced by a reaction between 7.4 mM ABTS+· in H2O and 2.6 mM potassium persulfate during storage in the dark at room temperature for 12~ 16 h. Before use, the ABTS+· solution was diluted with methanol to obtain an absorbance of 1.1±0.02 at 734 nm. Subsequently, 3 mL of ABTS+· solution was added to 0.1 mL of sample. After 10 min, the percent inhibition at 734 nm was calculated for each concentration relative to the blank absorbance.
Storage test of tofu
The tofu was placed in a polypropylene container with 1,200 mL of sterilized distilled water as an immersion solution and stored at 15°C for 4 d, sterilized at 85°C for 15 min, and cooled down for 20 min using running water. The tofu and immersion solution were homogenized, and the supernatant was diluted with 0.1% peptone water. Plate count agar was used for the determination of total viable counts. All plates were triplicated, incubated at 38°C for 48 h, and viable cell numbers were determined as colony forming units (log CFU) per g.
Sensory evaluation
Tofu samples with and without supplementation of LP were subjected to sensory evaluation with forty untrained volunteer consumers (18 males and 22 females, aged from 20 to 58). Panelists received a tray containing five samples presented in random order at room temperature (randomly coded using a three-digit number), a glass of water, and an evaluation sheet. Participants were instructed on how to evaluate the samples and were not required to expectorate or consume the entire volume served. Panelists were asked to evaluate the samples for consumer acceptance of color, flavor, softness, taste, and overall acceptance. The ratings were carried on a 9-point Hedonic scale ranging from 9 (like extremely) to 1 (dislike extremely). The orders of serving were completely randomized.
Overall acceptance was evaluated first, and another session was held to evaluate the rest of the attributes. There was an inter-stimulus interval of 30 s imposed between samples, to allow time to recover from adaptation. Participants were advised to rinse their palates between samples. Enough space was given to handle the samples and questionnaire, and evaluation time was not constrained. No specific compensation was given to the participants.
This study was approved by the Daegu University Institutional Review Board (IRB # 1040621-201703-HR-012-02).
Statistical analysis
The experimental data were subjected to analysis of variance (ANOVA) using the general linear models procedure to identify significant differences among the samples. Mean values were compared using Duncan’s multiple range test. The significance was defined at the 5% level.
RESULTS AND DISCUSSION
Physicochemical characteristics
Table 1 presents the physicochemical characterization of tofu supplemented with different levels of LP. The pH values assessed in the samples ranged from 5.93 to 6.18, and decreased upon addition of increasing amounts of LP. Nevertheless, only minor changes were observed. Thus, it seems that LP supplementation resulted in tofu of slightly lower pH. A similar change of pH was observed for breads (20) and pound cakes (26) with incorporated LP, probably due to the weak acidic nature of the supplemented powder. The pH of LP was 5.68 in this study.
Table 1.
Physicochemical properties of tofu incorporated with different levels of LP
| Property | LP level (%) | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| 0 | 0.2 | 0.4 | 0.6 | 0.8 | ||
| pH | 6.18±0.13a | 6.10±0.07ab | 6.05±0.05bc | 5.95±0.14c | 5.93±0.06c | |
| Yield (g) | 125.21±2.71d | 131.31±1.01c | 131.65±3.34c | 139.16±0.75b | 148.26±1.69a | |
| Turbidity | 3.03±0.03a | 1.52±0.20b | 1.39±0.07b | 1.32±0.01b | 0.31±0.01c | |
| Texture (N) | 6.73±0.74c | 8.09±1.60c | 8.16±1.36c | 10.19±2.51b | 15.18±2.00a | |
| Color | L* | 87.89±1.02a | 83.13±0.68b | 79.64±0.61c | 74.68±1.33d | 72.81±0.40e |
| a* | 0.37±0.12d | 0.56±0.11c | 0.68±0.11c | 1.19±0.16b | 1.39±0.09a | |
| b* | 14.63±0.15d | 14.99±0.43d | 16.15±0.70c | 17.42±0.55b | 18.16±0.26a | |
Means with different letters (a–e) in the same row are significantly different according to Duncan’s multiple range test (P<0.05).
The yield of tofu with LP was higher than that of the control tofu and increased as the concentration of LP increased (P<0.05). The yield of tofu was directly influenced by the water-soluble protein and lipid contents of soybeans (29). When the LP was incorporated, soymilk proteins were better coagulated; thus, more water was entrapped in the gel network, and the yield of tofu increased (30). Similar results were reported for tofu containing chungkukjang powder (31), shrimp powder (32), loquat leaf powder (33), and Lagocephalus lunaris powder (34).
Turbidity of soybean whey tended to decrease with increased levels of LP incorporation where the values of 0.2%, 0.4%, and 0.6% samples were significantly different from those of the control and 0.8% sample (P<0.05). Turbidity values may indicate the degree of the aggregation of protein molecules or dispersion of color pigments due to the ingredients incorporated in the tofu formulation (35). The decrease in the turbidity values in this experiment is probably due to a strong protein aggregation between whey and LP. This resulted in a less amount of dispersed or suspended solids to block light, resulting in decreased turbidity. Similar decreases in the turbidity values were observed for tofu prepared with spinach juice (36).
The middle portion of tofu, which has a homogeneous texture, was analyzed for textural characteristics imparted by LP tofu. Substituting 0.6% and higher amounts of LP significantly hardened the texture of tofu (P<0.05) while the control and 0.2~0.4% samples were not significantly different among them with respect to hardness (P>0.05). This suggests that incorporation of LP up to 0.4% in tofu formulation would not change hardness, which is one of the important attributes influencing consumer acceptability. Increased hardness in tofu was also observed with Rhynchosia volubilis (35), Enreromorpha intenstinalis (37), and pine needle (38). The hardening of tofu can be affected by the whey protein interacts with coagulants and other constituents (e.g. phytic acid) in soy milk and anions to form the microstructure into gel. During the process of gelation, the intermolecular interaction of soy protein is somewhat slowed by LP and resulted in a more homogeneous and regular network, and the final result is a stronger tofu structure (39,40).
Tristimulus colorimetry of LP tofu was used to assess the extent of color change with different levels of LP incorporation. The L*-value (lightness) of the control is comparable to that of tofu with pine needle powder (38) and water dropwort juice (41). Tofu samples made with LP were significantly different from the control (P<0.05). A significant decrease (P<0.05) in lightness with increasing levels of LP incorporation was observed, and these are probably due to the dark color pigment of LP. On the other hand, the a*- and b*-values increased significantly upon addition of an increased amount of LP (P<0.05). The a*- and b*-values showed similar trends but no significant differences were found in the 0.2~0.4% samples for the a*-values, and the control and 0.2% samples for b*-values. Our results are in good agreement with the findings of Park and Lee (26) who reported the color characteristics of pound cakes incorporated with 0~0.8 % LP.
Free radical scavenging activities
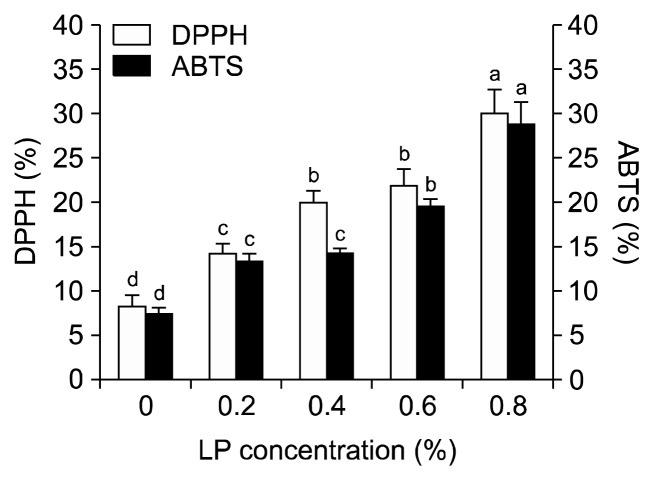
The antioxidant activities were examined based on the DPPH radical-scavenging activity and ABTS radical cation assay. The usage of LP in the tofu formulation enhanced the levels of antioxidant activity (Fig. 1). This can be attributed to the inherent rich antioxidant capacities of LP as compared to soybean powder. There were significant increases in both activity values of bound phenolic extracts in the samples with LP as compared to those of the control sample (P<0.05). They increased significantly with increasing addition of LP (P<0.05), whereas no significant differences were found in DPPH values between the 0.4% and 0.6% samples and ABTS values between the 0.2% and 0.4% samples (P>0.05), respectively. Samples enriched with 0.8% LP showed the highest antioxidant potential. The data also showed a positive correlation between the antioxidant capacities, DPPH increased as ABTS increased. A similar trend was reported by Park and Lee (26) for pound cakes supplemented with LP. The antioxidant capacities of the extracts are mainly due to the total phenolic content and total phenolic acids (42).
Fig. 1.
2,2-Diphenyl-1-picrylhydrazyl (DPPH) and 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS) radical scavenging activities of tofu incorporated with different levels of licorice powder (LP). Means within the same activity without a common letter (a–d) are significantly different (P<0.05).
Storage stability
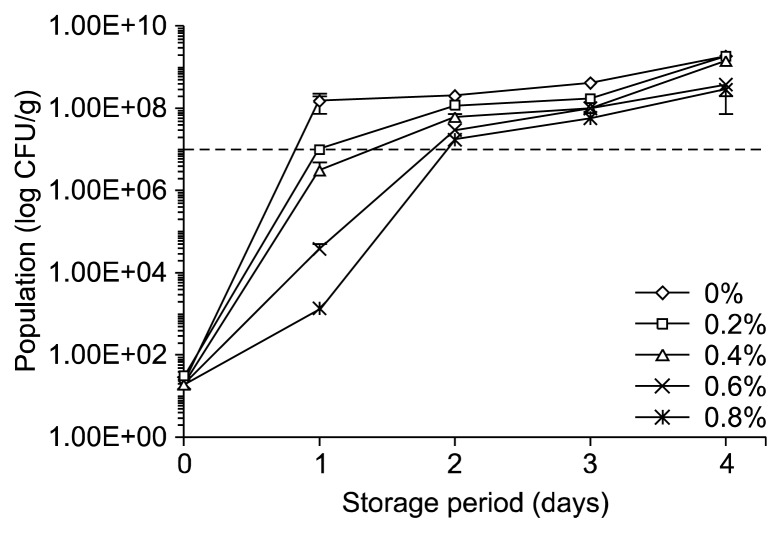
The changes of the viable microbial counts of tofu incorporated with LP and a control tofu during storage at 15°C for 4 days are shown in Fig. 2. The initial total plate count of the fresh control and LP-supplemented tofu samples at the beginning of the experiments was approximately 30 CFU/g. These values are less than the initial concentrations reported for tofu prepared with Rhynchosia volubilis (35). Total viable counts of all the tofu samples increased exponentially during storage. However, the results indicated that LP-supplementation had a significant effect on the inhibition of microbial growth. It can be seen that the viable microbial counts of the control tofu increased more rapidly than those of tofu prepared with LP during longer storage periods. With the postulation that tofu spoilage would start when viable counts were above 107 CFU/g, which is the maximum acceptable microbial limit (43–45), incorporation of LP in the range of the tested concentrations can extend the shelf life of tofu. Tofu incorporated with LP (0.2~0.8%) had a better shelf life, which was approximately 4.32~ 26.64 h longer than the control tofu at the elevated temperature of 15°C. This was probably due to the antimicrobial activities of phenolic compounds contained in LP (46). Such extension effect on shelf life of Japanese sea bass fillets incorporated with licorice extract was also reported by Qiu et al. (47).
Fig. 2.
Total microbial count of tofu incorporated with different levels of licorice powder (LP) during storage at 15°C.
Sensory findings
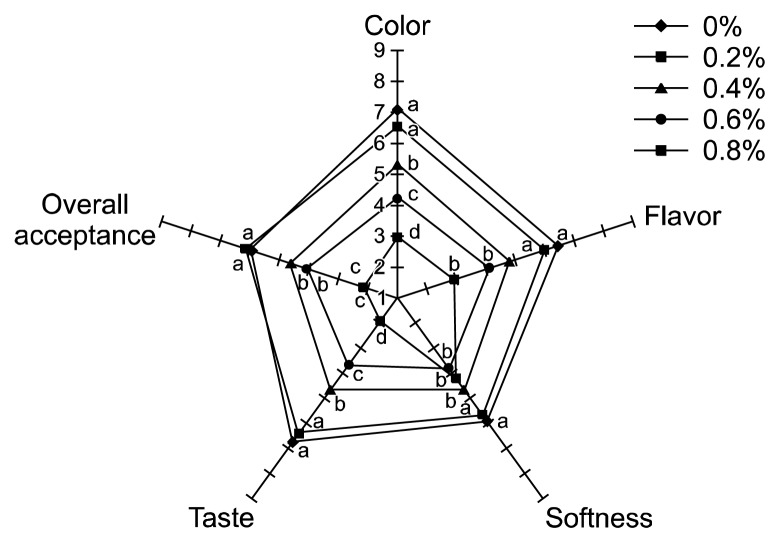
Color, flavor, taste, softness, and overall acceptance of control tofu and LP-supplemented tofu were evaluated, and the results are presented as a radar plot (Fig. 3). The highest color, flavor, taste, and softness scores were obtained for the control (7.13, 6.45, 6.75, and 5.93, respectively), whereas the 0.2% sample received the highest score (6.13) with respect to overall acceptance (Table 2). However, no significant differences were found between the control and the 0.2% sample in all attributes (P>0.05). This is probably due to the fact that most of the consumers were not familiar with LP-supplemented tofu and perhaps they tested them for the first time. When the supplementation is beyond 0.4%, all the values decreased with increasing addition of LP (P<0.05). Excessive addition of LP seemed to have a deteriorative effect on all the attributes evaluated.
Fig. 3.
Radar plot of sensory results of tofu supplemented with different levels of licorice powder (LP). Means within the same attribute without a common letter (a–d) are significantly different (P<0.05).
Table 2.
Consumer preference of tofu incorporated with different levels of LP
| Attribute | LP level (%) | ||||
|---|---|---|---|---|---|
|
| |||||
| 0 | 0.2 | 0.4 | 0.6 | 0.8 | |
| Color | 7.13±1.59a | 6.55±1.48a | 5.33±1.69b | 4.25±1.92c | 2.98±2.26d |
| Flavor | 6.45±1.50a | 6.00±1.87a | 4.80±1.71b | 4.13±1.67b | 2.93±1.94c |
| Taste | 6.75±1.58a | 6.40±1.66a | 4.68±1.62b | 3.68±1.61c | 1.93±1.44d |
| Softness | 5.93±2.10a | 5.68±1.99a | 4.68±1.80b | 3.83±1.87b | 4.23±2.68b |
| Overall acceptance | 6.00±2.01a | 6.13±1.73a | 4.63±2.05b | 4.08±2.00b | 2.15±1.67c |
Means with different letters (a–d) in the same row are significantly different according to Duncan’s multiple range test (P<0.05).
Overall acceptance scores of LP tofu were the best with the 0.2% sample. The results of the sensory analysis showed that supplementation with 0.2% LP improved most of the sensory attributes evaluated without significant differences. However, further increasing the substitution levels above 0.4% seemed to have some undesirable sensorial effects.
In conclusion, the present study established the feasibility of using LP as a supplement for enhancing the nutritional quality of tofu. Overall, our results suggest that nutritious tofu can be prepared by supplementing 0.2% LP, with increased physicochemical and antioxidant characteristics without sacrificing the sensorial qualities.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Jenkins DJ, Mirrahimi A, Srichaikul K, Berryman CE, Wang L, Carleton A, Abdulnour S, Sievenpiper JL, Kendall CW, Kris-Etherton PM. Soy protein reduces serum cholesterol by both intrinsic and food displacement mechanisms. J Nutr. 2010;140:2302S–2311S. doi: 10.3945/jn.110.124958. [DOI] [PubMed] [Google Scholar]
- 2.Lee CY, Kuo MI. Effect of γ-polyglutamate on the rheological properties and microstructure of tofu. Food Hydrocolloids. 2011;25:1034–1040. doi: 10.1016/j.foodhyd.2010.10.001. [DOI] [Google Scholar]
- 3.Mousavi Y, Adlercreutz H. Genistein is an effective stimulator of sex hormone-binding globulin production in hepatocarcinoma human liver cancer cells and suppresses proliferation of these cells in culture. Steroids. 1993;58:301–304. doi: 10.1016/0039-128X(93)90088-5. [DOI] [PubMed] [Google Scholar]
- 4.Messina MJ, Persky V, Setchell KD, Barnes S. Soy intake and cancer risk: a review of the in vitro and in vivo data. Nutr Cancer. 1994;21:113–131. doi: 10.1080/01635589409514310. [DOI] [PubMed] [Google Scholar]
- 5.Anderson JW, Johnstone BM, Cook-Newell ME. Metaanalysis of the effects of soy protein intake on serum lipids. N Engl J Med. 1995;332:276–282. doi: 10.1056/NEJM199508033330502. [DOI] [PubMed] [Google Scholar]
- 6.Mei J, Yeung SSC, Kung AWC. High dietary phytoestrogen intake is associated with higher bone mineral density in postmenopausal but not premenopausal women. J Clin Endocrinol Metab. 2001;86:5217–5221. doi: 10.1210/jcem.86.11.8040. [DOI] [PubMed] [Google Scholar]
- 7.Somekawa Y, Chiguchi M, Ishibashi T, Aso T. Soy in-take related to menopausal symptoms, serum lipids, and bone mineral density in postmenopausal Japanese women. Obstet Gynecol. 2001;97:109–115. doi: 10.1016/s0029-7844(00)01080-2. [DOI] [PubMed] [Google Scholar]
- 8.Morabito N, Crisafulli A, Vergara C, Gaudio A, Lasco A, Frisina N, D’Anna R, Corrado F, Pizzoleo MA, Cincotta M, Altavilla D, Ientile R, Squadrito F. Effects of genistein and hormone-replacement therapy on bone loss in early postmenopausal women: a randomized double-blind placebo-controlled study. J Bone Miner Res. 2002;17:1904–1912. doi: 10.1359/jbmr.2002.17.10.1904. [DOI] [PubMed] [Google Scholar]
- 9.Cui H, Zhao C, Lin L. The specific antibacterial activity of liposome-encapsulated Clove oil and its application in tofu. Food Control. 2015;56:128–134. doi: 10.1016/j.foodcont.2015.03.026. [DOI] [Google Scholar]
- 10.Lee JH. Physicochemical and sensory evaluation of whole soybean curd supplemented with pine needle powder. Prev Nutr Food Sci. 2015;20:148–152. doi: 10.3746/pnf.2015.20.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li M, Chen F, Yang B, Lai S, Yang H, Liu K, Bu G, Fu C, Deng Y. Preparation of organic tofu using organic compatible magnesium chloride incorporated with polysaccharide coagulants. Food Chem. 2015;167:168–174. doi: 10.1016/j.foodchem.2014.06.102. [DOI] [PubMed] [Google Scholar]
- 12.Fukai T, Marumo A, Kaitou K, Kanda T, Terada S, Nomura T. Anti-Helicobacter pylori flavonoids from licorice extract. Life Sci. 2002;71:1449–1463. doi: 10.1016/S0024-3205(02)01864-7. [DOI] [PubMed] [Google Scholar]
- 13.Shabkhiz MA, Eikani MH, Bashiri Sadr Z, Golmohammad F. Superheated water extraction of glycyrrhizic acid from licorice root. Food Chem. 2016;210:396–401. doi: 10.1016/j.foodchem.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 14.Hou YC, Lin SP, Lee Chao PD. Liquorice reduced cyclosporine bioavailability by activating P-glycoprotein and CYP 3A. Food Chem. 2012;135:2307–2312. doi: 10.1016/j.foodchem.2012.07.061. [DOI] [PubMed] [Google Scholar]
- 15.Lee IT, Lee WJ, Tsai CM, Su IJ, Yen HT, Sheu WHH. Combined extractives of red yeast rice, bitter gourd, chlorella, soy protein, and licorice improve total cholesterol, low-density lipoprotein cholesterol, and triglyceride in subjects with metabolic syndrome. Nutr Res. 2012;32:85–92. doi: 10.1016/j.nutres.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 16.Fu Y, Chen J, Li YJ, Zheng YF, Li P. Antioxidant and anti-inflammatory activities of six flavonoids separated from licorice. Food Chem. 2013;141:1063–1071. doi: 10.1016/j.foodchem.2013.03.089. [DOI] [PubMed] [Google Scholar]
- 17.Zhou S, Cao J, Qiu F, Kong W, Yang S, Yang M. Simultaneous determination of five bioactive components in Radix Glycyrrhizae by pressurised liquid extraction combined with UPLC-PDA and UPLC/ESI-QTOF-MS confirmation. Phytochem Anal. 2013;24:527–533. doi: 10.1002/pca.2427. [DOI] [PubMed] [Google Scholar]
- 18.Hwang SH, Chung HS, Kim SD, Youn KS. Effect of Glycyrrhizia uralensis extract addition on the quality of Cheonggukjang. J East Asian Soc Diet Life. 2004;14:571–575. [Google Scholar]
- 19.Kim YS, Choi HS, Woo IA, Song TH. The effect on the sensory and mechanical characteristics of functional muffin using Glycyrrhizae radix extract. Korean J Food Cook Sci. 2004;20:95–99. [Google Scholar]
- 20.Lee SY, Choi JS, Choi MO, Cho SH, Kim KBWR, Lee WH, Park SM, Ahn DH. Effect of extract from Glycyorrhiza uralensis and Curcula longa on shelf-life and quality of bread. J Korean Soc Food Sci Nutr. 2006;35:912–918. doi: 10.3746/jkfn.2006.35.7.912. [DOI] [Google Scholar]
- 21.Ko YT, Lee JY. Quality of licorice (Glycyrrhiza uralensis) powder added kimchi. Korean J Food Sci Technol. 2006;38:143–146. [Google Scholar]
- 22.Kim JH, Lee SY, Kim KBWR, Song EJ, Kim AR, Kim MJ, Ji KW, Ahn IS, Ahn DH. Effects of Glycyrrhiza uralensis, Menthae herba, Schizandra chinensis and chitosan on the shelflife and quality of Takju. J Korean Soc Food Sci Nutr. 2007;36:1436–1443. doi: 10.3746/jkfn.2007.36.11.1436. [DOI] [Google Scholar]
- 23.Kim AR, Lee SY, Kim KBWR, Song EJ, Kim JH, Kim MJ, Ji KW, Ahn IS, Ahn DH. Effect of Glycyrrhiza uralensis on shelf-life and quality of Takju. Korean J Food Sci Technol. 2008;40:194–200. [Google Scholar]
- 24.Lim SI, Song SM. Fermentation properties of low-salted doenjang supplemented with licorice, mustard, and chitosan. Korean J Food Sci Technol. 2010;42:323–328. [Google Scholar]
- 25.Lim SI, Song SM. Changes in characteristics of low-salted kochujang with licorice (Glycyrrhiza glabra), mustard (Brassica juncea), and chitosan during fermentation. J Korean Soc Food Sci Nutr. 2010;39:560–566. doi: 10.3746/jkfn.2010.39.4.560. [DOI] [Google Scholar]
- 26.Park GH, Lee JH. The quality and antioxidant properties of pound cakes containing licorice powder. Korean J Food Sci Technol. 2014;46:56–60. doi: 10.9721/KJFST.2014.46.1.56. [DOI] [Google Scholar]
- 27.Blois MS. Antioxidant determinations by the use of a stable free radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- 28.Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic Biol Med. 1999;26:1231–1237. doi: 10.1016/S0891-5849(98)00315-3. [DOI] [PubMed] [Google Scholar]
- 29.Smith AK, Watanabe T, Nash AM. Tofu from Japanese and United States soybeans. Food Technol. 1960;14:332–336. [Google Scholar]
- 30.Zuo F, Chen Z, Shi X, Wang R, Guo S. Yield and textural properties of tofu as affected by soymilk coagulation prepared by a high-temperature pressure cooking process. Food Chem. 2016;213:561–566. doi: 10.1016/j.foodchem.2016.07.008. [DOI] [PubMed] [Google Scholar]
- 31.An SH, Lee SH, Park GS. Quality characteristics of tofu prepared with various concentrations of commercial chung-kukjang powder. Korean J Food Cook Sci. 2008;24:258–265. [Google Scholar]
- 32.Cho HS, Kim KH. Quality characteristics of tofu added with shrimp powder. J East Asian Soc Diet Life. 2009;19:743–749. [Google Scholar]
- 33.Park ID. Quality characteristics of tofu added with loquat (Eriobotrya japonica Lindl.) leaf powder. Korean J Food Culture. 2012;27:521–527. doi: 10.7318/KJFC/2012.27.5.521. [DOI] [Google Scholar]
- 34.Yoo JY, Cho HS, Park BH. Quality characteristics of tofu prepared with Lagocephalus lunaris powder. Korean J Food Preserv. 2013;20:495–501. doi: 10.11002/kjfp.2013.20.4.495. [DOI] [Google Scholar]
- 35.Lee JH, Ahn MJ. Texture and storage stability of tofu incorporated with Rhynchosia volubilis. J Food Sci Nutr. 2009;14:71–75. [Google Scholar]
- 36.Choi YO, Chung HS, Youn KS. Effects of various concentrations of natural materials on the manufacturing of soybean curd. Korean J Postharvest Sci Technol. 2000;7:256–261. [Google Scholar]
- 37.Chung DO. Characteristics of tofu (soybean curd) quality mixed with Enteromorpha intenstinalis powder. J Korean Soc Food Sci Nutr. 2010;39:745–749. doi: 10.3746/jkfn.2010.39.5.745. [DOI] [Google Scholar]
- 38.Son BG, Kim HE, Lee JH. Physicochemical and consumer preference characteristics of tofu incorporated with pine needle powder. J Korean Soc Food Sci Nutr. 2015;44:296–301. doi: 10.3746/jkfn.2015.44.2.296. [DOI] [Google Scholar]
- 39.Bernal VM, Smajda CH, Smith JL, Stanley DW. Interactions in protein/polysaccharide/calcium gels. J Food Sci. 1987;52:1121–1125. doi: 10.1111/j.1365-2621.1987.tb14023.x. [DOI] [Google Scholar]
- 40.Lim BT, DeMan JM, DeMan L, Buzzel RI. Yield and quality of tofu as affected by soybeans and soymilk characteristics. Calcium sulphate coagulant. J Food Sci. 1990;55:1088–1092. doi: 10.1111/j.1365-2621.1990.tb01605.x. [DOI] [Google Scholar]
- 41.Seog EJ, Kim HR, Lee JH. Physical characteristics and consumer acceptance on tofu as influenced by water dropwort. J Food Sci Nutr. 2008;13:117–121. [Google Scholar]
- 42.Fang Z, Hu Y, Liu D, Chen J, Ye X. Changes of phenolic acids and antioxidant activities during potherb mustard (Brassica juncea, Coss.) pickling. Food Chem. 2008;108:811–817. doi: 10.1016/j.foodchem.2007.11.033. [DOI] [PubMed] [Google Scholar]
- 43.Kim DH, Lee KS. Effects of coagulants on storage of packed tofu. Korean J Food Sci Technol. 1992;24:92–96. [Google Scholar]
- 44.Kim J, Jeon JR. Quality characteristics of tofu added with black soybean hull powder. Korean J Food Culture. 2005;20:633–637. [Google Scholar]
- 45.Li C, Dong M, Chen X, Jiang M, Lü X, Nuerguli R, Hashim MM. Construction of microbial growth kinetics prediction model of kurthia in tofu and shelf life prediction. Trans Chin Soc Agric Eng. 2009;25:82–86. [Google Scholar]
- 46.Wang L, Yang R, Yuan B, Liu Y, Liu C. The antiviral and antimicrobial activities of licorice, a widely-used Chinese herb. Acta Pharm Sin B. 2015;5:310–315. doi: 10.1016/j.apsb.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qiu X, Chen S, Liu G, Yang Q. Quality enhancement in the Japanese sea bass (Lateolabrax japonicas) fillets stored at 4°C by chitosan coating incorporated with citric acid or licorice extract. Food Chem. 2014;162:156–160. doi: 10.1016/j.foodchem.2014.04.037. [DOI] [PubMed] [Google Scholar]