Abstract
Currently, alternatives to prebiotics for skin treatment are receiving much interest. However, little is known about the efficacy of topically applied prebiotics in skin anti-aging. This study was conducted to observe the anti-aging effects of DuOligo, which is composed of lactulose and galactooligosaccharides (GOS). We investigated wrinkle-related parameters by quantitative and qualitative skin evaluation in healthy women who consumed DuOligo for 8 weeks. The double-blind, randomized, and placebo-controlled study included subjects who were divided into two groups (Placebo: dextrin 4.5 g/d, n=14, 51.50 y vs. DuOligo: DuOligo 4.5 g/d, n=14, 52.65 y). The DuOligo group showed a reduction in mean wrinkle length and depth measured via quantitative skin evaluation after 8 weeks, whereas the Placebo group showed slight increases in these parameters (P<0.001). The wrinkle severity rating scale in the DuOligo group was decreased after 8 weeks, but it increased in the Placebo group (Placebo group: 0.14 vs. DuOligo group: −0.86, P<0.001). The global aesthetic improvement scale for the DuOligo group was significantly higher than that for the Placebo group at week 8 (P<0.001). In conclusion, our findings suggest that oral consumption of DuOligo is beneficial to the skin, and present the possibility of new nutritional strategies for wrinkle care.
Keywords: lactulose, oligosaccharides, prebiotics, aging, skin
INTRODUCTION
Prebiotics are non-digestible, selectively fermented dietary fibers. Prebiotic research has largely focused on two major carbohydrates, lactulose and galactooligosaccharides (GOS), which have been successfully used as food ingredients to stimulate the growth of health-promoting bacteria in the gastrointestinal tract (1,2). Prebiotics promote the growth of one or more bacterial genera in the gastrointestinal tract and thus provide health benefits to the host (2). It has been found that, similarly to the gut microflora, the skin’s microbiota plays a beneficial role (3). New applications for prebiotics in anti-aging could be to inhibit the formation of fragmented elastin fibers, scavenge free radicals, and activate dermal microcirculation (4).
Currently, alternatives to prebiotics for skin treatment are receiving much interest. However, there are not many studies regarding the use of prebiotics for skin care, and in this context, their clinical use is controversial. Especially, little is known about the efficacy of topically applied prebiotics in skin anti-aging. In this study, we investigated whether the consumption of DuOligo, composed of lactulose and GOS, has anti-aging effects on the skin and whether it could represent a novel approach for skin care. Lactulose [β-(1→4)-galactosyl-fructose] is a synthetic disaccharide, produced by isomerization of lactose in basic media or enzyme-catalysed synthesis, with a significant impact on human digestion (5,6). Lactulose is among the most studied prebiotics and the first one successfully used as a food ingredient to stimulate the growth of health-promoting bacteria in the gastrointestinal tract (1). Galactooligosaccharides (GOS), considered non-digestible carbohydrates which are mainly constituted by galactose units, also belong to the group of prebiotics (7). Currently, there is a great interest in obtaining new prebiotic carbohydrates with improved properties. To this end, we investigated the wrinkle-related parameters by quantitative and qualitative skin evaluation in healthy women who consumed DuOligo for 8 weeks.
SUBJECTS AND METHODS
Preparation of DuOligo
The DuOligo used in this study was provided by Neo Cremar Co., Ltd. (Seoul, Korea). Lactose 400~600 g was added to 500 mL of distilled water, and dissolved to a final sugar content of 45°brix with constant stirring. The 1% (w/w) sodium carbonate was added to the amount of lactose added, followed by stirring for 60 min at 75 to 80°C. After completion of the reaction, the reaction mixture was cooled to 60°C, and the pH was adjusted to 6.5 for the enzyme reaction. β-galactosidase (0.1%) (w/w) was treated for 24 h. Ion exchange treatment was carried out for the removal of byproducts. The reaction mixture was finally filtered through a 0.22 μm filter and concentrated to 75°brix. The DuOligo was composed of lactulose (51.67%), GOS (15.80%), lactose (14.22%), a mixture of glucose and galactose (12.32%), and other ingredients (12.32%).
Subjects
Subjects were recruited through advertisements in a local newspaper. Thirty healthy Korean women, aged 40~ 60 years, with fine wrinkles at the outer corner of their eyes, called lateral canthal lines, were chosen for this study. The exclusion criteria were Fitzpatrick skin types I or IV, allergies, photosensitivity, tanning sunburns, infections, pregnancy, and breastfeeding. Also excluded were subjects who had undergone wrinkle removal or peeling procedures within the previous 6 months.
Study protocol
The study was approved by the Jeonju University, and all subjects gave written informed consent (jjIRB-160415-HR-2016-0608). The double-blind, randomized, and placebo-controlled study included subjects who were divided into two groups (Placebo and DuOligo groups) by stratified block randomization. For 8 weeks, the DuOligo group was asked to take a DuOligo 4.5 g tablet once a day. This dosage was selected based on the findings of a preliminary study (8). The Placebo group consumed only the vehicle (100% dextrin), which was the same size and color tablet as DuOligo. The subjects were asked to not change their diet or lifestyle during the study.
Skin evaluation
Wrinkle length and depth were measured using a replica method at baseline and week 8. The crow’s foot area was wiped with ethanol and was measured in a room at 20~ 25°C and relative humidity of 30~40%. After an adhesive paper (diameter, 11 mm) was attached, translucent silicon was mixed in a cup containing two components, a basic substance and a catalyst (Courage+Khazaka electronic GmbH, Köln, Germany). A layer of the silicone mixture was spread over the restricted area of the adhesive paper and left to dry for 5 min. Then the skin replica was analyzed using a Skin-Visiometer SV 600 (Courage+ Khazaka electronic GmbH) (9).
Clinical efficacy was assessed by the wrinkle severity rating scale (WSRS) and global aesthetic improvement scale (GAIS) at baseline and week 8 (10). The WSRS is a photograph-based outcome instrument that is designed specifically for quantifying facial wrinkles. Wrinkle severity scoring is based on a visual assessment of wrinkle length and depth (Table 1). The GAIS is a relative, rather than absolute, scale: the investigator grades the over-all improvement in wrinkles by comparing the subject’s appearance at follow-up against a photograph taken before treatment (Table 1). After week 8, adverse effects including erythema, edema, bruising, and altered pigmentation were assessed by questioning subjects and observing skin responses.
Table 1.
Scales for qualitative skin evaluation on wrinkles
| Score | Rating | Description |
|---|---|---|
| WSRS (wrinkle severity rating scale) | ||
| 5 | Extreme | Extremely deep and long folds, detrimental to facial appearance; 2- to 4-mm visible V-shaped fold when stretched; unlikely to have satisfactory correction with injectable implant alone |
| 4 | Severe | Very long and deep folds; prominent facial feature; less than 2-mm visible fold when stretched; significant improvement is expected from injectable implant |
| 3 | Moderate | Moderately deep folds; clear facial feature visible at normal appearance but not when stretched; excellent correction is expected from injectable implant |
| 2 | Mild | Shallow but visible fold with a slight indentation; minor facial feature; implant is expected to produce a slight improvement in appearance |
| 1 | Absent | No visible fold; continuous skin line |
| GAIS (global aesthetic improvement scale) | ||
| 4 | Very much improved | Optimal cosmetic result for the implant in this patient |
| 3 | Much improved | Marked improvement in appearance from the initial condition, but not completely optimal for this patient. A touch-up would slightly improve the result |
| 2 | Improved | Obvious improvement in appearance from the initial condition, but a touch-up or retreatment is indicated |
| 1 | No change | The appearance is essentially the same as the original condition |
| 0 | Worse | The appearance is worse than the original condition |
Statistical analysis
All statistical analyses were performed using the Statistical Package for Social Sciences version 12.0 (SPSS Inc., Chicago, IL, USA). Differences between the two groups were statistically evaluated by t-tests. Paired t-tests were used to assess the differences in the changes from baseline within groups. All data were two-sided at the 5% significance level and were reported as mean±standard error of the mean (SEM).
RESULTS
Subjects
Thirty women were selected for participation in this study. Of them, two were withdrawn from the trial as follows: one failed to complete the study and one was noncompliant. As a result, 28 participants met the study requirements. None of the participants withdrew from the study protocol because of DuOligo treatment-related adverse effects. No adverse effects were experienced by the subjects, and none of the withdrawals were considered to be due to the study products (data not shown). The Placebo (mean age: 51.50 y) and DuOligo groups (mean age: 52.65 y) ultimately consisted of 14 women each. The Placebo and DuOligo groups had similar results regarding the wrinkle-related parameters. The initial values of all the variables did not significantly differ between the two groups (data not shown).
Skin evaluation
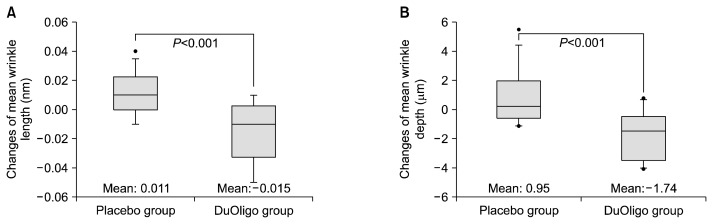
Fig. 1 presents the changes in wrinkle length and depth measured by a replica method at baseline and week 8. The DuOligo group showed a reduced mean wrinkle length and depth after 8 weeks, whereas the Placebo group showed slight increases in these parameters. The differences in the changes of mean wrinkle length and depth between two groups were significant (P<0.001). As shown in Table 2, the results of the clinical efficacy assessments for qualitative skin evaluation also revealed that participants’ wrinkles were improved by the oral treatment of DuOligo. The WSRS in the DuOligo group was decreased after 8 weeks, whereas it was increased in the Placebo group (Placebo group: 0.14 vs. DuOligo group: −0.86, P<0.001). The photographs of subjects who consumed DuOligo for 8 weeks showed that wrinkles in the crow’s feet region were markedly improved compared with the baseline (DuOligo group WSRS, baseline: 4.01 vs. Week 8: 3.14, P<0.01). The GAIS for DuOl-igo-treated subjects (DuOligo group) was significantly higher than that for DuOligo-non-treated subjects (Placebo group) at week 8 (P<0.001).
Fig. 1.
Effects of DuOligo on wrinkle-related parameters by quantitative skin evaluation (A, wrinkle mean length; B, wrinkle mean depth). P-value indicates a significant difference between changes (from baseline to week 8) in the two groups as determined by t-test. Each box plot is composed of five horizontal lines that display the 10th, 25th, 50th, 75th, and 90th percentiles, respectively. All values above the 90th and below the 10th percentile are plotted separately.
Table 2.
Effects of DuOligo on wrinkle-related parameters by qualitative skin evaluation
| Placebo group | DuOligo group | P-value | |||||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Baseline | Week 8 | Change | Baseline | Week 8 | Change | ||
| WSRS | 3.86±0.29 | 4.02±0.23 | 0.14±0.18 | 4.01±0.31 | 3.14±0.29 | −0.86±0.21** | <0.001 |
| GAIS | 1.14±0.14 | 2.57±0.23 | <0.001 | ||||
WSRS, wrinkle severity rating scale; GAIS, global aesthetic improvement scale.
P-value indicates a significant difference between changes (from baseline to week 8) in the two groups as determined by t-test.
Significant difference in the changes from baseline within groups determined by paired t-test at P<0.01.
All data were two-sided at the 5% significance level and were reported as means±SEM.
DISCUSSION
The role of prebiotics in the function of epidermal tight junctions and lipid lamellae between corneocytes may represent a new application for skin health (4). Prebiotics can be applied to the skin microbiota directly to selectively increase the activity and growth of beneficial normal skin microbiota (11). Normalization of intestinal microflora with the use of prebiotics has a positive effect on several skin conditions (12). Recent studies demonstrate the successful development of a prebiotic cosmetic approach to balance the composition of the cutaneous microflora. It is generally feasible to improve the composition of the skin microflora, i.e., to limit or reduce the growth of pathogenic species and simultaneously preserve or even stimulate the growth of beneficial bacteria (13). As such, the relationship between the skin and its microflora provide the basis for developing prebiotic strategies for skin care (12). In a preliminary study (14), researchers found that in hairless mice exposed to ultraviolet B irradiation, GOS administration suppressed increases in transepidermal water loss (TEWL) and concomitant decreases in skin hydration, which reflect barrier function perturbation. GOS administration also increased gene expression of CD44, which was associated with maintenance of hyaluronic acid homeostasis (15). Additionally, the TEWL and wrinkle area percentage of healthy adults who consumed DuOligo, composed of lactulose and GOS, decreased significantly compared with these values in adults given placebo (8). These results suggested that DuOligo treatment improves skin parameters and has potential as a functional cosmetic material. Our results indicate that DuOligo improved wrinkle-related parameters in healthy women compared with women given placebo. In conclusion, our findings support that the oral treatment of DuOligo is beneficial not only to the intestines but also to the skin and present the possibility of new nutritional strategies for skin care. However, further research is necessary to fully understand the effects of DuOligo on skin health and to understand in detail how ingested prebiotics might influence the skin through intestine-mediated changes. In order to determine the effect of wrinkles, the experimental period of 8 weeks was rather short. The effects of wrinkles on long term follow-up studies should be investigated, and the mechanism of wrinkle effects should be studied.
ACKNOWLEDGEMENTS
This research was supported by High Value-added Food Technology Development Program, Ministry of Agriculture, Food and Rural Affairs.
Footnotes
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
REFERENCES
- 1.Olano A, Corzo N. Lactulose as a food ingredient. J Sci Food Agric. 2009;89:1987–1990. doi: 10.1002/jsfa.3694. [DOI] [Google Scholar]
- 2.Wilson B, Whelan K. Prebiotic inulin-type fructans and galacto-oligosaccharides: definition, specificity, function, and application in gastrointestinal disorders. J Gastroenterol Hepatol. 2017;32:64–68. doi: 10.1111/jgh.13700. [DOI] [PubMed] [Google Scholar]
- 3.Schagen SK, Zampeli VA, Makrantonaki E, Zouboulis CC. Discovering the link between nutrition and skin aging. Dermatoendocrinol. 2012;4:298–307. doi: 10.4161/derm.22876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Foisy M, Boyle RJ, Chalmers JR, Simpson EL, Williams HC. The prevention of eczema in infants and children: an overview of Cochrane and non-Cochrane reviews. Evid Based Child Health. 2011;6:1322–1339. doi: 10.1002/ebch.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sitanggang AB, Drews A, Kraume M. Recent advances on prebiotic lactulose production. World J Microbiol Biotechnol. 2016;32:154. doi: 10.1007/s11274-016-2103-7. [DOI] [PubMed] [Google Scholar]
- 6.Panesar PS, Kumari S. Lactulose: production, purification and potential applications. Biotechnol Adv. 2011;29:940–948. doi: 10.1016/j.biotechadv.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Cardelle-Cobas A, Corzo N, Olano A, Peláez C, Requena T, Ávila M. Galactooligosaccharides derived from lactose and lactulose: influence of structure on Lactobacillus, Streptococcus and Bifidobacterium growth. Int J Food Microbiol. 2011;149:81–87. doi: 10.1016/j.ijfoodmicro.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 8.Hong YH, Jung EY, Suh HJ, Han SH. Skin health effect of DuOligo intake containing lactulose. J Soc Cosmet Sci Korea. 2016;42:367–375. [Google Scholar]
- 9.Lee HK, Seo YK, Baek JH, Koh JS. Comparison between ultrasonography (Dermascan C version 3) and transparency profilometry (Skin Visiometer SV600) Skin Res Technol. 2008;14:8–12. doi: 10.1111/j.1600-0846.2007.00257.x. [DOI] [PubMed] [Google Scholar]
- 10.Beer K. A randomized, evaluator-blinded comparison of efficacy of hyaluronic acid gel and avian-sourced hylan B plus gel for correction of nasolabial folds. Dermatol Surg. 2007;33:928–936. doi: 10.1111/j.1524-4725.2007.33194.x. [DOI] [PubMed] [Google Scholar]
- 11.Al-Ghazzewi FH, Tester RF. Impact of prebiotics and probiotics on skin health. Benef Microbes. 2014;5:99–107. doi: 10.3920/BM2013.0040. [DOI] [PubMed] [Google Scholar]
- 12.Baquerizo Nole KL, Yim E, Keri JE. Probiotics and prebiotics in dermatology. J Am Acad Dermatol. 2014;71:814–821. doi: 10.1016/j.jaad.2014.04.050. [DOI] [PubMed] [Google Scholar]
- 13.de Jongh GJ, Zeeuwen PL, Kucharekova M, Pfundt R, van der Valk PG, Blokx W, Dogan A, Hiemstra PS, van de Kerkhof PC, Schalkwijk J. High expression levels of keratinocyte antimicrobial proteins in psoriasis compared with atopic dermatitis. J Invest Dermatol. 2005;125:1163–1173. doi: 10.1111/j.0022-202X.2005.23935.x. [DOI] [PubMed] [Google Scholar]
- 14.Hong KB, Jeong MG, Kim JH, Park Y, Suh HJ. Photoprotective effects of reinforced galactooligosaccharides supplementation against skin damage in hairless mice (LB357) FASEB J. 2014;28:LB357. [Google Scholar]
- 15.Kukkonen K, Savilahti E, Haahtela T, Juntunen-Backman K, Korpela R, Poussa T, Tuure T, Kuitunen M. Probiotics and prebiotic galacto-oligosaccharides in the prevention of allergic diseases: a randomized, double-blind, placebo-controlled trial. J Allergy Clin Immunol. 2007;119:192–198. doi: 10.1016/j.jaci.2006.09.009. [DOI] [PubMed] [Google Scholar]