Abstract
The safety of bowel-cleansing agents is an important issue in clinical practice, especially in patients with chronic diseases. Although the safety and efficacy of polyethylene glycol (PEG) has been investigated in many studies, few studies on PEG plus ascorbic acid exist. In this study, we compared the safety of 2 bowel-cleansing agents for patients with liver cirrhosis: 2-liter PEG (2 L PEG) plus ascorbic acid versus 4-liter PEG (4 L PEG). We performed a retrospective study on colonoscopy in patients with liver cirrhosis. Patients referred for colonoscopy were divided into 2 groups: 2 L PEG plus ascorbic acid (n = 105) and 4 L PEG (n = 61). Safety was assessed by comparing the clinical factors and laboratory findings as follows: blood biochemistry, electrolytes, weight change, and bowel-cleansing quality. Serum electrolytes, laboratory findings, and body weight showed no significant change between the 2 groups. There was no significant change in clinical factors before and after bowel preparation in the PEG group or the PEG plus ascorbic acid group. The acceptability and compliance of patients was better in the 2 L PEG plus ascorbic acid than the 4 L PEG group. In subgroup analysis, patients with compensated or decompensated cirrhosis showed no increased risk of electrolyte imbalances after bowel preparation. Child–Pugh scores did not influence the outcome after bowel cleansing. Successful cleansing was mostly achieved in both groups. Our analysis showed that of the use of 2 L PEG plus ascorbic acid could be a safe choice for colonoscopy in patients with liver cirrhosis.
Keywords: ascorbic acid, colonoscopy, liver cirrhosis, polyethylene glycol, safety
1. Introduction
Colonoscopy is an effective procedure for detecting colorectal cancer. The increasing demand for colonoscopy can be attributed to the widespread knowledge about cancer screening and surveillance.[1–4] For colonoscopy to be effective, bowel cleansing must be ensured, and it must be both safe and acceptable to ensure patients’ compliance.
Polyethylene glycol (PEG) solution has been used for bowel cleansing for a long time.[5] Since PEG is an isosmotic solution, it passes through the bowel without absorption or secretion.[6,7] Therefore, 4-liter PEG (4 L PEG) solutions have been widely used for bowel cleansing before colonoscopy. Previous studies have shown that PEG as a bowel-cleansing agent is safe and even helpful for hepatic encephalopathy in patients with liver cirrhosis (LC), with the added advantage of increased acceptability, compared with sodium phosphate.[8,9] However, PEG solutions are poorly tolerated by patients due to their poor flavor and the high volume of fluid that must be ingested. Furthermore, PEG induces nausea and vomiting in the majority of patients, even those in a healthy condition. Thus, laxative ingestion and colon cleansing are frequently perceived as the worst part of undergoing colonoscopy. This is a serious problem in patients with chronic diseases, such as those with LC, who have general weakness and loss of appetite. More than anything, in patients with LC and ascites, the high volume of 4 L PEG is a heavy burden that induces poor compliance.
A low-volume (2 L) PEG solution with ascorbic acid (PEG + Asc) has been used for bowel cleansing.[10–13] The safety of PEG + Asc has been proven in patients under special conditions.[14–17] Although PEG has been investigated in many studies, only few mention the efficacy, safety, and symptoms associated with PEG + Asc, in patients with chronic liver disease. Moreover, the influence of high-dose ascorbic acid in PEG + Asc solutions has not been studied in patients with LC. In this study, we compared the safety, effectiveness, and benefits of 2 bowel-cleansing agents: 2 L PEG + Asc versus 4 L PEG in patients with LC.
2. Methods
2.1. Study design
This was a single-center, retrospective study of patients referred to our hospital for colonoscopy between March 2012 and February 2016. We analyzed the demographic, laboratory, and endoscopic data of the patients. We reviewed the patients with LC who received either 4 L PEG or 2 L PEG + Asc for bowel preparation and underwent colonoscopy during the hospital stay. The 4 L PEG solution used was Colyte (TaeJoon Pharmaceuticals, Seoul, Korea) with the following composition: sodium chloride 1.46 g, potassium chloride 0.745 g, sodium sulfate 5.68 g, and PEG 60 g in a liter of the solution. The 2-L polyethylene glycol solutions with ascorbic acid (PEG-Asc) solution used was Coolprep (TaeJoon Pharmaceuticals) with the following composition: sodium chloride 2.691 g, potassium chloride 1.015 g, sodium sulfate 7.5 g, PEG 100 g, ascorbic acid 4.7 g, and sodium ascorbate 5.9 g in a liter of solution. This study was approved by the institutional review board of Korea University Anam Hospital (IRB number: AN17168-001).
2.2. Patient population
During the study period, we included patients with LC for whom colonoscopy was recommended. We collected data on patients who were over 18 years old and confirmed to have LC. LC was diagnosed either based on histology or on a combination of radiologic, laboratory, and clinical parameters. In the patients who performed liver biopsies, the results were used for confirming a diagnosis of cirrhosis. If liver biopsy was not performed, LC was diagnosed by using complete blood count, liver function tests, and imaging studies such as computed tomography, magnetic resonance imaging, and ultrasound. Patients with acute hepatitis or a vague diagnosis were excluded from this study. Patients were also excluded if they had a history of a severe comorbidity such as congestive heart failure or severe renal insufficiency.
2.3. Efficacy and safety endpoints
Tolerability and acceptability were assessed by patient questionnaire, which includes a standard medical history and assessment of health status. Patients were given a questionnaire before colonoscopy regarding bowel movements, nausea, abdominal pain, able to consume the entire preparation, taste of preparation agent, and total satisfaction. The data were retrospectively collected with confidentiality and anonymity. And we reviewed the endoscopic reports about bowel-cleansing quality according to the 4-point Boston Bowel Preparation Scale.[18] We analyzed the results of blood biochemistry and the patient's demographic characteristics before and after colonoscopy. The laboratory findings were defined as “before bowel preparation; within 24 hours before taking a bowel-cleansing agent” and “after bowel preparation; within 24 hours after taking a bowel-cleansing agent.” We excluded cases without timely data before or after colonoscopy. After that, we compared the differences between the PEG and PEG + Asc groups. We also performed a subgroup analysis according to the Child–Pugh classification of patients with LC (class A, B, and C) and whether they had compensated cirrhosis or not.
2.4. Statistical analysis
Data were presented as the mean value ± standard deviation or median ± interquartile range or as proportions. The efficacy and safety analyses were compared using χ2 statistics. Absolute values and percentage change in blood parameters were compared between the 2 groups using the Student's t test or the Mann–Whitney U test. Data were analyzed using the Statistical Package for the Social Sciences version 20.0 (IBM Corp., Armonk, NY). P values <.05 were defined as significant.
3. Results
3.1. Baseline characteristics of patients
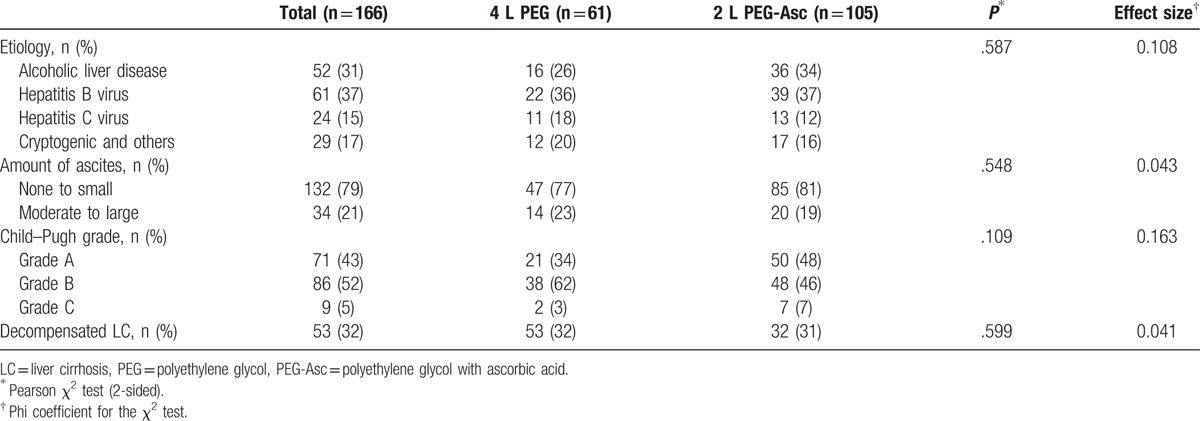
During the study period, 166 patients with LC underwent bowel preparation for colonoscopy. We selected 61 patients for the 4 L PEG solution group and 105 patients for the 2 L PEG + Asc group. Child–Pugh classes A/B/C included 71/86/9 patients, respectively. Demographics and other laboratory findings were similar in the 2 preparation groups at the time of inclusion. The baseline characteristics of these patients are shown in Tables 1 and 2.
Table 1.
Baseline characteristics of patients.
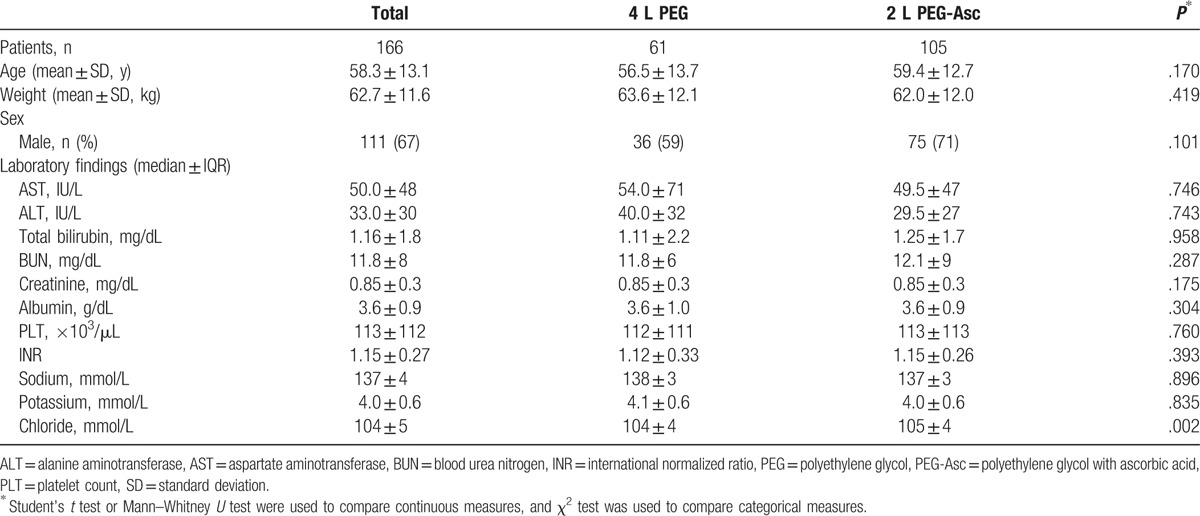
Table 2.
Etiology and degree of liver cirrhosis.
3.2. Quality of bowel preparation and patient acceptability
The outcomes of bowel cleansing are shown in Table 3. The mean score for abdominal discomfort was significantly lower in the 2 L PEG + Asc solution group (2.31 ± 0.81 score in the 4 L PEG group vs 1.72 ± 0.92 score in the 2 L PEG + Asc group, P = .039). Nausea was lower in the 2 L PEG + Asc group, but the difference was not statistically significant. Although patients’ reports regarding symptoms were equivocal, patient's acceptability for easy intake indicated a significant difference (easy or moderate degree, 35% in the 4 L PEG group vs 55% in 2 L PEG + Asc group, P = .010).
Table 3.
Outcome of bowel cleansing and patient's report.
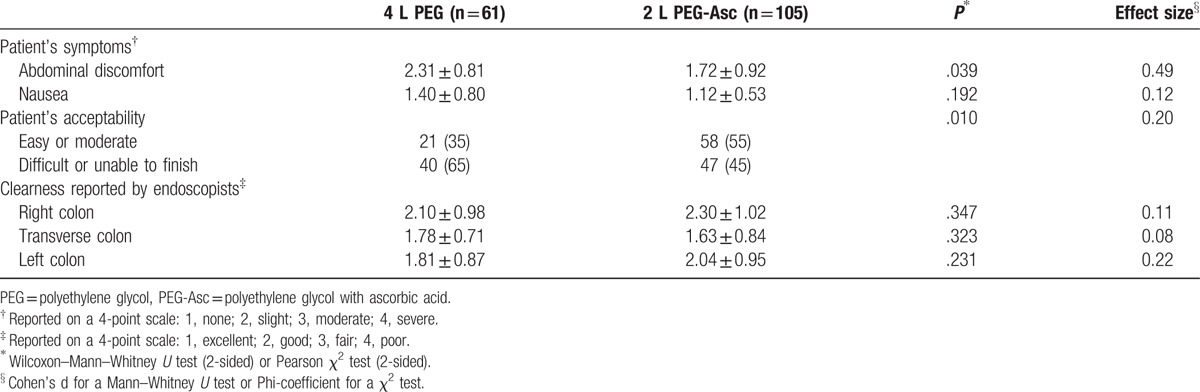
Based on the 4-point Boston Bowel Preparation Scale, there was no difference in preparation quality of each colon portion between the 2 groups.
3.3. Influence of bowel preparation in patients with LC
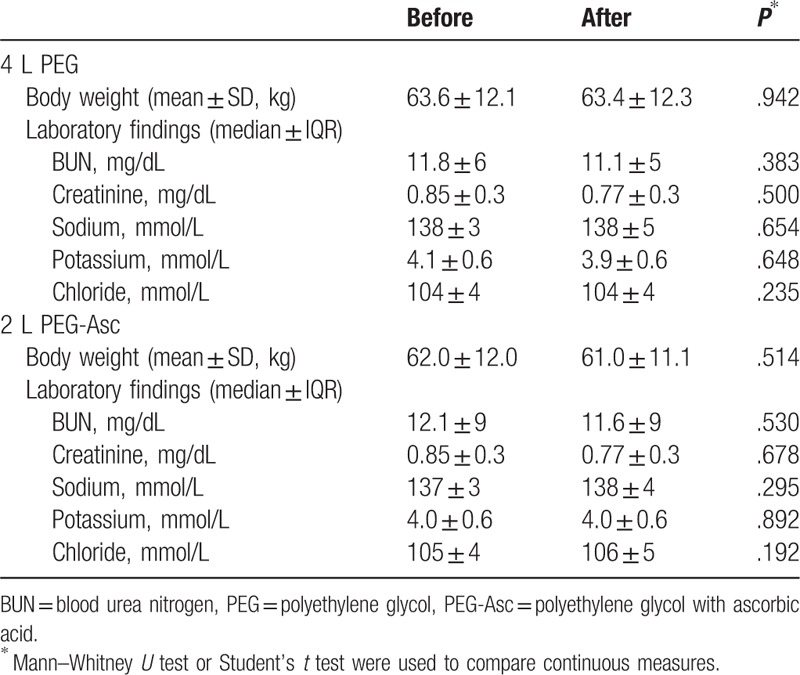
Table 4 shows the body weight and laboratory data with pairwise comparisons before and after ingestion of PEG or PEG + Asc. After bowel preparation, patient body weight was not changed in either group. No significant decrease in serum BUN, creatinine, sodium, potassium, or chloride was observed.
Table 4.
Comparison of laboratory and demographic values before and after bowel preparation.
3.4. Safety of PEG + Asc regarding the severity of LC
We also performed a subgroup analysis according to Child–Pugh classification of patients with LC (class A, B, and C) and existence of decompensation in the 2-L PEG + Asc group. Table 5 shows that there was no significant laboratory change in Child–Pugh grade B or even grade C after bowel cleansing. An increase of BUN and creatinine was observed in patients with Child–Pugh C, but it was not significant. And also, most laboratory findings were not shown significant difference between compensated LC and decompensated LC (Table 6). Serum sodium levels significantly decreased when 2 L PEG + Asc was used in the decompensated LC group, but only by as little as 0.2%. Albumin levels significantly increased by 4.9% after bowel cleansing in patients with decompensated LC.
Table 5.
Subgroup analysis according to Child–Pugh grade in the 2 L PEG + Asc group.
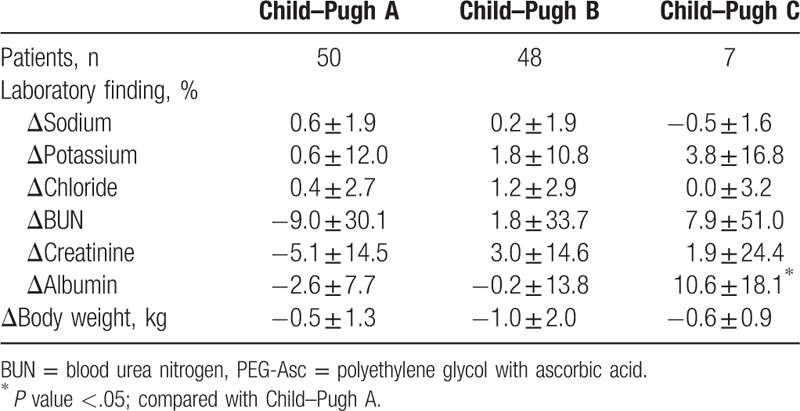
Table 6.
Subgroup analysis according to compensation in the 2 L PEG + Asc group.
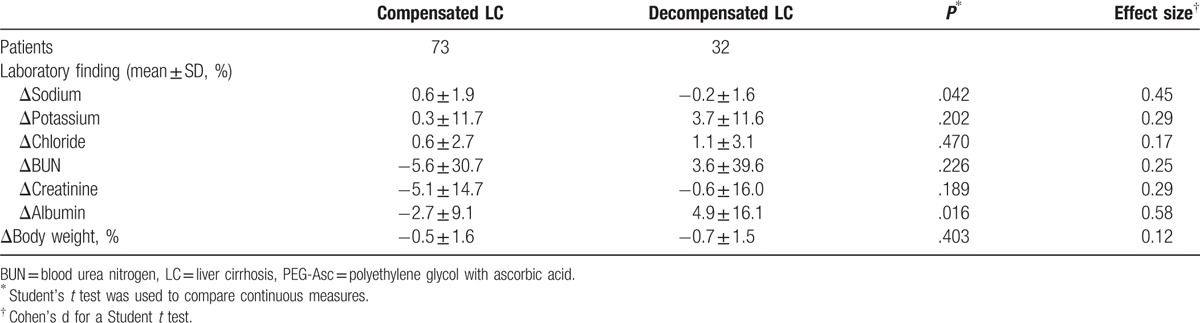
4. Discussion
Compared with those with healthy livers, patients with LC have a greater risk for electrolyte imbalances, hemodynamic changes, and ascites owing to the disease itself or the use of diuretics.[19–22] High-volume PEG is effective for bowel cleansing and is safe in patients with serum electrolyte imbalances, even with advanced hepatic dysfunction.[6,7,23] However, PEG ingestion is perceived as the worst part of the colonoscopy procedure by most patients because of the high volume that must be ingested. Thus, it may negatively affect patient compliance and reduce overall efficacy. Furthermore, its high volume is a severe burden in patients with ascites, as can be seen in decompensated LC.
Nowadays, low-volume PEG with additives is used for better acceptability of PEG solutions.[6,7,15,24] However, if the additives are not completely absorbed and remain in the colonic lumen, an osmotic effect may occur. Even though it is possible to reduce the PEG solution volume, this may cause problems during or after bowel preparation. Although most available PEG + Asc solutions contain high-dose ascorbic acid (10–20 g), the safety of high-dose ascorbic acid remains uncertain in patients with LC. Moreover, it is unclear whether PEG + Asc solution is related to body weight changes/electrolyte imbalances after colonoscopy preparation.
In this study, we demonstrated that the administration of low-volume PEG + Asc is efficient and safe in patients with LC, even those with ascites. The results showed that the percentage of patients with successful colon cleansing was not significantly different between 4 L PEG and 2 L PEG + Asc. Although a previous study had reported inferior results with the use of low-volume PEG solutions for bowel preparation,[25] 2 L PEG + Asc was not inferior to high-volume PEG in the efficacy of bowel cleansing in this study.
Despite the presence of LC, there was no significant electrolyte difference after bowel preparation in either the PEG group or the PEG + Asc group. Although the mean BUN and creatinine values increased after bowel cleansing, the change was not significant and was attributed to fasting during the colonoscopy procedure. In the PEG + Asc group in this study, electrolyte balance or weight was not influenced by the ingestion of high-dose ascorbic acid (10 g).
A subgroup analysis showed that 2 L PEG + Asc was a safe bowel-cleansing agent for patients with severe LC. There were no significant changes in blood biochemistry, including serum electrolytes and creatinine. Although there was an increase in the mean value of BUN after bowel preparation in the decompensated LC or Child–Pugh C group, it was not significant.
Above all, regarding tolerability and acceptability, excellent success rates were observed in the low-volume PEG + Asc group, especially in patients with LC and ascites. The fewer complaints of abdominal discomfort and abdominal bloating in the PEG + Asc group could be attributed to the reduced PEG volume. Patients’ questionnaire reports about the ease of bowel-cleansing solution intake indicated superior results with the use of the PEG + Asc solution compared with the use of PEG solution. This could be attributed not only to the low volume but also to the improved flavor provided by the ascorbic acid. In other questionnaire items, such as the willingness to take the solution again or judgment of the product and the procedure, PEG + Asc was also superior to 4 L PEG.
This study has several limitations. First, regarding the efficacy of bowel cleansing, we reviewed only the endoscopists’ judgment. Although the endoscopists described the score of bowel preparation using the same scale, lack of formal interrater reliability would be a limitation in this study. Second, other independent comorbidities in patients, like chronic kidney disease or congestive heart failure, could affect the results. Furthermore, collected data did not include information about chronic constipation or previous inadequate preparation in our study. Third, this study included a small number of patients and designed as a single-center study. Finally, above all, the main limitation of this study is its retrospective nature. Blood sampling time was not consistent before and after colonoscopy. Moreover, bowel cleansing using 4 L PEG had been performed in the previous years, whereas bowel cleansing using 2 L PEG + Asc was dominantly used in more recent years. These factors might affect the results, independent from the kind of bowel cleaning agents.
However, no study of proper bowel-cleansing agents for patients with LC or patients with ascites exists. To our knowledge, this was the first study to evaluate the efficacy and safety of bowel-cleansing agents in patients with LC after the ingestion of low-volume PEG + Asc.
In conclusion, this study suggests that 2 L PEG + Asc is effective, safe, and more acceptable than 4 L PEG, and therefore represents a more suitable option for patients with LC.
Footnotes
Abbreviations: LC = liver cirrhosis, PEG = polyethylene glycol, PEG-Asc = polyethylene glycol solutions with ascorbic acid.
JML and JHL contributed equally to this work.
This research was supported by a grant from Korea University and a 2014 Weolbong grant from the Korean Gastrointestinal Endoscopy Research Foundation, funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI14C3477).
The authors report no conflicts of interest.
References
- [1].Smith RA, Andrews K, Brooks D, et al. Cancer screening in the United States, 2016: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin 2016;66:96–114. [DOI] [PubMed] [Google Scholar]
- [2].Levin B, Lieberman DA, McFarland B, et al. Screening and surveillance for the early detection of colorectal cancer and adenomatous polyps, 2008: a joint guideline from the American Cancer Society, the US Multi-Society Task Force on Colorectal Cancer, and the American College of Radiology. CA Cancer J Clin 2008;58:130–60. [DOI] [PubMed] [Google Scholar]
- [3].Nelson RS, Thorson AG. Colorectal cancer screening. Curr Oncol Rep 2009;11:482–9. [DOI] [PubMed] [Google Scholar]
- [4].Kahi CJ, Rex DK. Screening and surveillance of colorectal cancer. Gastrointest Endosc Clin N Am 2005;15:533–47. ix. [DOI] [PubMed] [Google Scholar]
- [5].DiPalma JA, Brady CE., 3rd Colon cleansing for diagnostic and surgical procedures: polyethylene glycol-electrolyte lavage solution. Am J Gastroenterol 1989;84:1008–16. [PubMed] [Google Scholar]
- [6].Lichtenstein G. Bowel preparations for colonoscopy: a review. Am J Health Syst Pharm 2009;66:27–37. [DOI] [PubMed] [Google Scholar]
- [7].Belsey J, Epstein O, Heresbach D. Systematic review: oral bowel preparation for colonoscopy. Aliment Pharmacol Ther 2007;25:373–84. [DOI] [PubMed] [Google Scholar]
- [8].Frommer D. Cleansing ability and tolerance of three bowel preparations for colonoscopy. Dis Colon Rectum 1997;40:100–4. [DOI] [PubMed] [Google Scholar]
- [9].Shawki S, Wexner SD. Oral colorectal cleansing preparations in adults. Drugs 2008;68:417–37. [DOI] [PubMed] [Google Scholar]
- [10].Kim SH, Kim JW. Low volume polyethylene glycol (peg) plus ascorbic acid, a valid alternative to standard PEG. Gut Liver 2016;10:160–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Bitoun A, Ponchon T, Barthet M, et al. Norcol Group. Results of a prospective randomised multicentre controlled trial comparing a new 2-L ascorbic acid plus polyethylene glycol and electrolyte solution vs. sodium phosphate solution in patients undergoing elective colonoscopy. Aliment Pharmacol Ther 2006;24:1631–42. [DOI] [PubMed] [Google Scholar]
- [12].Ell C, Fischbach W, Bronisch HJ, et al. Randomized trial of low-volume PEG solution versus standard PEG + electrolytes for bowel cleansing before colonoscopy. Am J Gastroenterol 2008;103:883–93. [DOI] [PubMed] [Google Scholar]
- [13].Worthington J, Thyssen M, Chapman G, et al. A randomised controlled trial of a new 2 litre polyethylene glycol solution versus sodium picosulphate + magnesium citrate solution for bowel cleansing prior to colonoscopy. Curr Med Res Opin 2008;24:481–8. [DOI] [PubMed] [Google Scholar]
- [14].Tsunoda T, Sogo T, Iwasawa K, et al. Feasibility and safety of bowel cleansing using low-volume polyethylene glycol with ascorbic acid before pediatric colonoscopy: a pilot study. Dig Endosc 2016;29:160–7. [DOI] [PubMed] [Google Scholar]
- [15].Jung YS, Lee CK, Eun CS, et al. Low-volume polyethylene glycol with ascorbic acid for colonoscopy preparation in elderly patients: a randomized multicenter study. Digestion 2016;94:82–91. [DOI] [PubMed] [Google Scholar]
- [16].Lee JM, Keum B, Yoo IK, et al. Polyethylene glycol plus ascorbic acid for bowel preparation in chronic kidney disease. Medicine (Baltimore) 2016;95:e4755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Moon W. Optimal and safe bowel preparation for colonoscopy. Clin Endosc 2013;46:219–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Lai EJ, Calderwood AH, Doros G, et al. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc 2009;69(3 pt 2):620–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Papper S. Fluid and electrolyte disturbances in cirrhosis. Am J Med Sci 1976;272:53–6. [DOI] [PubMed] [Google Scholar]
- [20].Arroyo V, Jimenez W. Complications of cirrhosis. II. Renal and circulatory dysfunction. Lights and shadows in an important clinical problem. J Hepatol 2000;32(1 suppl):157–70. [DOI] [PubMed] [Google Scholar]
- [21].Knotek M, Rogachev B, Schrier RW. Update on peripheral arterial vasodilation, ascites and hepatorenal syndrome in cirrhosis. Can J Gastroenterol 2000;14(suppl D):112D–21D. [DOI] [PubMed] [Google Scholar]
- [22].Gentilini P, Vizzutti F, Gentilini A, et al. Update on ascites and hepatorenal syndrome. Dig Liver Dis 2002;34:592–605. [DOI] [PubMed] [Google Scholar]
- [23].Pontone S, Angelini R, Standoli M, et al. Low-volume plus ascorbic acid vs high-volume plus simethicone bowel preparation before colonoscopy. World J Gastroenterol 2011;17:4689–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kang MJ, Jung SA, Kim SE, et al. A prospective trial comparing 4 L polyethylene glycol with 2 L polyethylene glycol plus bisacodyl tablets for colon preparation. J Gastroenterol Hepatol 2008;23:A108–9. [Google Scholar]
- [25].Haapamaki MM, Lindstrom M, Sandzen B. Low-volume bowel preparation is inferior to standard 4 1 polyethylene glycol. Surg Endosc 2011;25:897–901. [DOI] [PubMed] [Google Scholar]


