Abstract
Early neurological deterioration (END) is associated with increased risk of functional disability and mortality. However, data are limited regarding the long-term risk of poor functional outcomes. Thus we explored the association between END and long-term outcomes in patients with acute ischemic stroke.
A total of 1064 patients were enrolled with acute ischemic stroke who were consecutively admitted to the 3 stroke units of Huai-He Hospital, Kaifeng, China. END was defined as an increment change of at least one point in motor power or total National Institute of Health Stroke Scale (NIHSS) score deterioration ≥2 points within the first week after admission. We retrospectively assessed the risk factors of END and prospectively explored the relationship between END and the long-term outcomes by multivariable regression models after adjusting the potential confounding factors. Outcomes were evaluated at 18 months based on modified Rankin scale (MRS) scores.
Approximately 32% of first-ever ischemic stroke patients experienced END during the acute phase. END was associated with diabetes (odds ratio [OR], 2.218; 95% confidence interval [CI] 1.619–3.037), NIHSS score at admission (OR, 1.052; 95% CI 1.023–1.082), C-reactive protein (CRP) levels (OR, 1.224; 95% CI 1.066–1.406]), and homocysteine (HCY) levels (OR, 1.203; 95% CI 1.061–1.365) after adjusting related factors, such as hypertension, diabetes, NIHSS at admission, and some blood laboratory values, including direct bilirubin, total cholesterol, low-density lipoprotein, glucose, CRP, HCY, and D-dimer levels. During the follow-up period, 52 (4.9%) patients died, 160 (15.0%) recrudesced, and 317 (29.8%) suffered poor outcomes. Multivariate logistic regression analyses revealed that poor outcome was associated with END (OR, 3.366; 95% CI 2.495–4.542), age (OR, 1.028; 95% CI 1.015–1.041), body mass index (OR, 1.096; 95% CI 1.051–1.144), coronary heart disease (OR, 1.637; 95% CI 1.108–2.416), and CRP (OR, 2.474; 95% CI 1.840–3.326).
The risk factors of END are multifaceted. Diabetes, NIHSS score at admission, CRP, and HCY are independent predictors of END. In addition, the results of this study indicate that END is an important predictor of poor functional outcome.
Keywords: acute ischemic stroke, early neurological deterioration, follow-up, outcome
1. Introduction
Worldwide, Asia, Russia, and Eastern Europe experience the highest rates of mortality and disability-adjusted life years lost, as a result of stroke.[1] Furthermore, it reported that, the incidence rate of ischemic stroke increased by an average of 8.7% annually between 1984 and 2004 in China.[2] The consequences of stroke-related disability and death are significant for both society and individuals. Therefore, all measures capable of decreasing disability are therefore extremely important.
Early neurological deterioration (END), also known as “stroke progression,’, “early stroke progression,” “stroke-in-progression,” and “stroke-in-evolution,” has been observed in approximately 5% to 40% of patients with acute ischemic stroke,[3–5] and is frequently associated with increased risk of functional disability and mortality.[6–11]
The majority of studies have focused on the early risk of END[5,7,12] and short-term assessments (up to 6 months),[8,11,13,14,15] but data regarding the long-term risk of poor functional outcomes are limited. Despite Millikan and Siekert[16] first proposed the concept of progressive stroke 50 years ago, the effects of prognosis are still not clear. Our current study aimed to explore the risk factors of END in patients with acute ischemic stroke, as well as stroke recurrence, stroke disability, and all-cause death after END during a follow-up period of approximately 18 months.
2. Subjects and methods
We studied 3015 subjects who were consecutively admitted to 3 Stroke Units (2 of them were from the Department of Neurology and the other was from the Department of Rehabilitation) of Huai-He Hospital, Kaifeng, China between January 1, 2014 and November 30, 2015. All of the patients with first-ever ischemic stroke during the acute phase (symptoms occurring within 7 days[17]) were diagnosed by a neurologist according to the fourth Chinese National Conference's recommendations for the diagnosis of cerebrovascular diseases.[18] All potentially eligible patients were screened. The inclusion criteria were as follows: (1) 18 years of age or older, (2) ischemic stroke diagnosed via computed tomography and/or magnetic resonance imaging, (3) and first-ever ischemic stroke occurring within 7 days before admission. The exclusion criteria were as follows: (1) diagnosis of transient ischemic attack, intracerebral hemorrhage, subarachnoid hemorrhage, brain tumors or unspecified stroke; (2) history of more serious medical disease other than ischemic stroke, such as cancer, renal failure, and Parkinson's disease; (3) the interference of the C- reaction protein (CRP) level by any disorder, including asthma, arthritis, liver disease, bronchitis, sinus infection, aspiration pneumonia or urinary tract infection on acute stroke phase; (4) death during the hospital admission; (5) incomplete clinical data; and (6) lack of follow-up data, including no information and refusal to participate. Finally, 1064 patients met the inclusion criteria and provided informed consent for their participation. A flowchart illustrating the selection of study subjects is presented in Figure 1.
Figure 1.
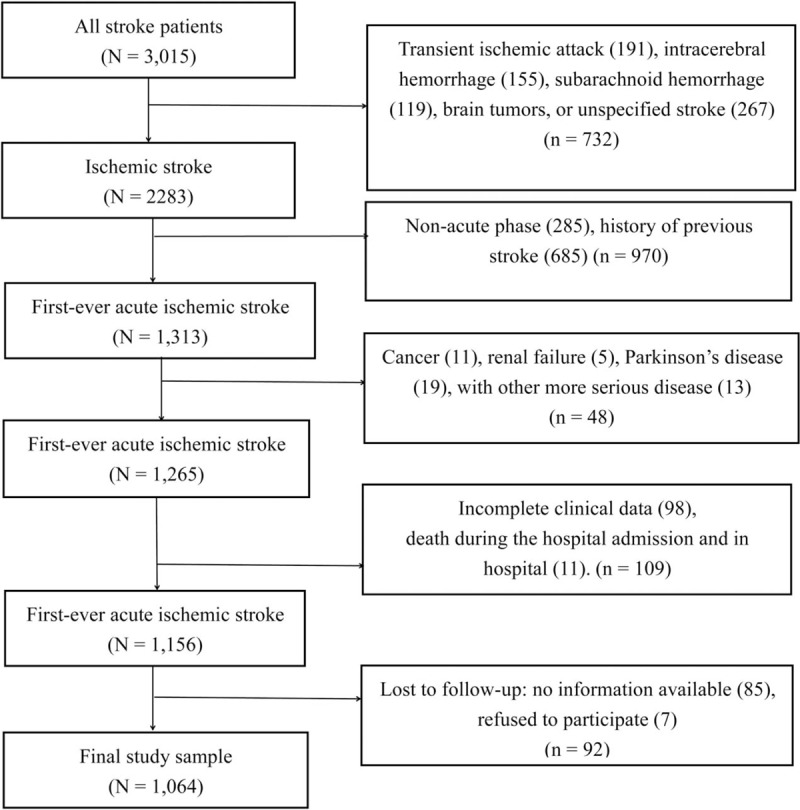
Flow diagram representing participant selection process.
2.1. Study protocol
Data were collected including patients’ demography, medical history, stroke risk factors, and blood samples. Risk factors were defined according to protocol. Body mass index (BMI) was calculated by dividing measured weight in kilograms by the square of measured height in meters. Hypertension was defined as treatment with antihypertensive drugs before stroke onset or introduction of antihypertensive drugs before discharge. Diabetes was defined as treatment with glucose-lowering medications or diet prior to stroke onset, or a fasting serum glucose level > 7.7 mmol/L and / or 2-hour postprandial blood glucose > 11.1 mol/L according to the WHO and the International Diabetes Federation standard during the hospitalization. Coronary heart disease was documented by using previous history of myocardial infarction >6 months but <5 years before enrollment in the study, or angina pectoris confirmed by positive coronary angiography, nuclear scintigraphy, or exercise test.[19] Smoking was defined as the use of at least 1 cigarette per day prior to stroke onset. Alcohol drinkers were defined as those who drank alcohol on average more than once per week over the past year. Blood samples were obtained from all patients within 24 hours of admission, after an overnight fast of 12 to 14 hours. Blood samples, that is, the peripheral venous blood, were drawn from the antecubital vein of the patients, and then placed into plasma separator tubes with ethylene diamine tetraacetic acid and serum separator tubes for centrifugation. Levels of direct bilirubin, total bilirubin, albumin, total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL), glucose, C-reactive protein (CRP), homocysteine (HCY), fibrinogen, and D-dimer were measured within 2 hours of collection.
2.2. Neurological assessment and definition of END
Stroke severity was assessed at admission by a certified neurologist using the National Institutes of Health Stroke Scale (NIHSS).[20] In our study, END was defined as an increment of at least one point in motor power or total NIHSS score deterioration ≥2 points within the first week after admission.[10,17,21–24] Trained neurologists assessed the neurological status of the patients on a daily basis.
2.3. Follow-up and outcome assessment
Follow-up was performed by telephone interview. Patients who were unable to answer orientation questions had a proxy interview. Trained research personnel making the phone calls were blinded to patients’ baseline clinical status. Patients were asked standardized follow-up questions approximately 18 months after stroke onset. The collected outcome data included stroke recurrence, stroke disability, and all-cause death. Recurrent cerebrovascular events included ischemic stroke, intracranial hemorrhage, and subarachnoid hemorrhage. Cases of recurrent stroke were cross-checked with the treating hospitals to ensure the accuracy of diagnosis. In case of suspected stroke recurrence without hospitalization, the case was adjudicated by the trial executive committee. Stroke disability was defined as modified Rankin scale (MRS) of 3 to 6 at approximately 18 months after stroke onset. All-cause mortality was defined as death from any cause confirmed by either a death certificate from the local citizen registry or records from the treating hospital. When no official documentation was available, case fatality was determined if death was reported on 2 consecutive follow-up periods by different proxies.
2.4. Ethics Statement
This study was conducted in accordance with the principles of the Declaration of Helsinki, and the protocol was approved by the Ethics Committee of Henan University, China. Oral consent for use of clinical records was obtained from patients during follow-up. Written consent was not required due to the retrospective nature of the study. All patient data were anonymized prior to analysis.
2.5. Statistical analyses
Categorical variables were reported as proportions, while continuous variables were reported as median values or means ± standard deviations (SD). We examined the differences in continuous variables between patients with good and poor outcomes using Student's t- and nonparametric tests for normally and non-normally distributed data, respectively. Categorical variables were compared using Pearson's chi-square tests. Multivariate logistic regression models were used to investigate the risk factors of functional outcome with a forward, stepwise selection strategy. A parsimonious final logistic regression model was fitted by retaining all predictor variables with P < .05. Odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. Two-tailed P values <.05 were considered statistically significant. All statistical analyses were performed using SPSS software package, version 17.0 (SPSS, Inc., Chicago, IL).
3. Results
A total of 3015 patients were recruited between January 1, 2014 and November 30, 2015. Of these, 732 with other types of stroke or brain tumors, 685 with history of previous stroke, and 285 in the nonacute phase at admission were excluded. In addition, 48 patients with more serious disease and 109 patients with incomplete clinical data and death before discharge were excluded, and 85 patients without available content information and 7 patients who refused to participate were also excluded. Finally, 1064 patients participated in the follow-up study, and 92.0% completed the follow-up at 18 months after stroke onset (Fig. 1).
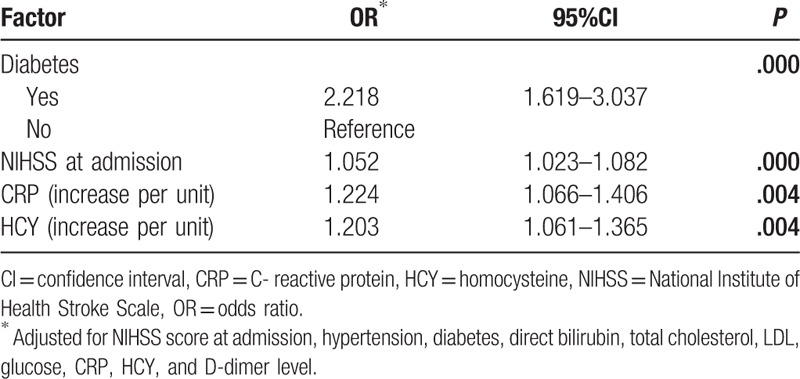
To examine the effect of early neurological deterioration, the patients were classified into 2 groups: those with (1) early neurological deterioration (END), and (2) nonearly neurological deterioration (non-END). Table 1 shows the baseline characteristics of patients in 2 groups. The END group contained 340 (32.0%) patients. The patients’ ages ranged from 18 to 96 (63.82 ± 12.59), and their BMI ranged from 17.7 to 36.3 kg/m2 (25.15 ± 3.57). Univariate analyses revealed that END was significantly associated with the increasing hypertension, diabetes, NIHSS at admission, and some blood laboratory values such as direct bilirubin, total cholesterol, LDL, glucose, CRP, HCY, and D-dimer level (Table 1). During the follow-up time of 18 months, 52 (4.9%) patients died, 160 (15.0%) patients recrudesced, and 317 (29.8%) patients had poor outcomes (Table 1). In addition, Table 1 showed that END was significantly associated with death and poor outcomes, but was not associated with recurrence.
Table 1.
Univariate comparison of demographic and clinical characteristics between first-ever ischemic stroke patients who showed early neurological deterioration and patients who did not.
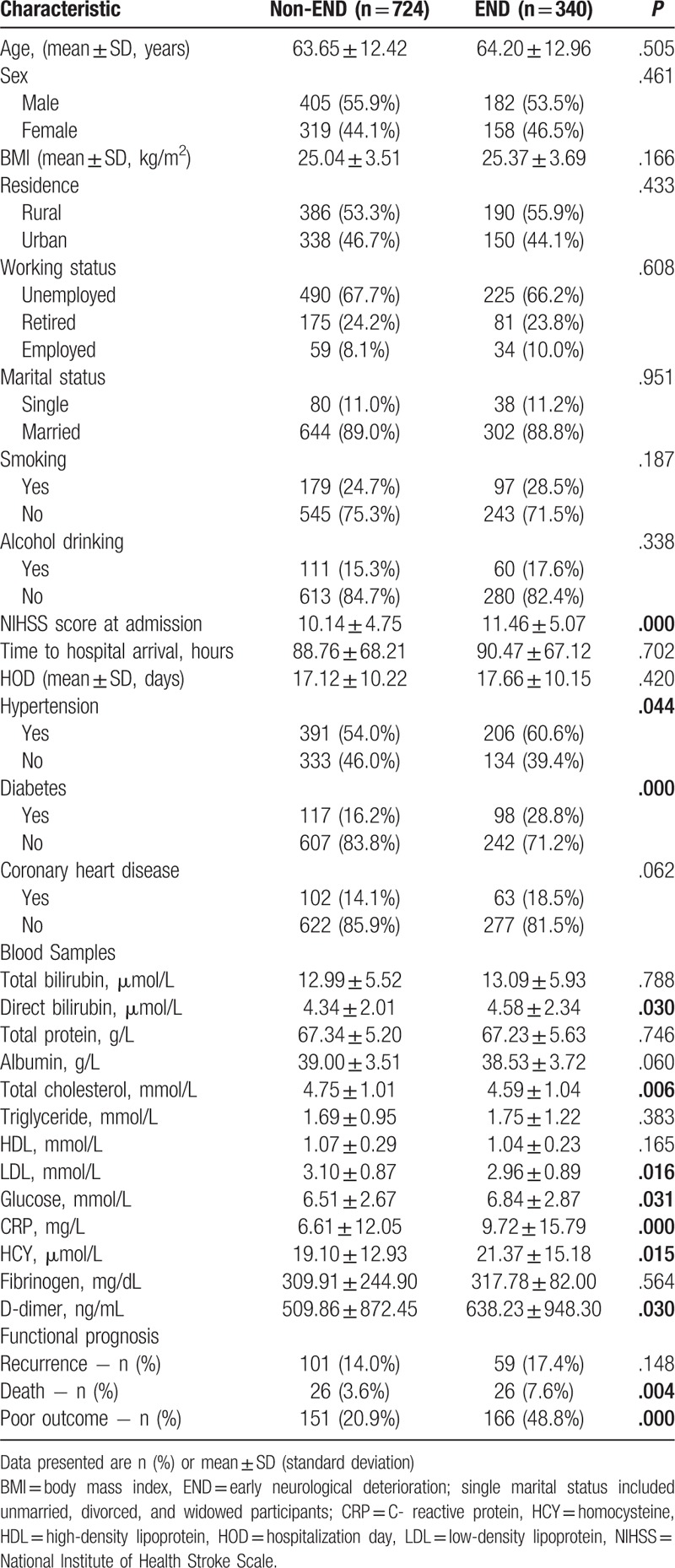
Table 2 showed the results of the multivariate logistic regression analysis for determination of the predictors of END. Diabetes (OR, 2.218; 95% CI 1.619–3.037), NIHSS score at admission (OR, 1.052; 95% CI 1.023–1.082), CRP (OR, 1.224; 95% CI 1.066–1.406]), and HCY (OR, 1.203; 95% CI 1.061–1.365) were significantly independent predictors of END.
Table 2.
Multivariate logistic regression analysis of significant risk factors for early neurological deterioration in acute ischemic stroke patients.
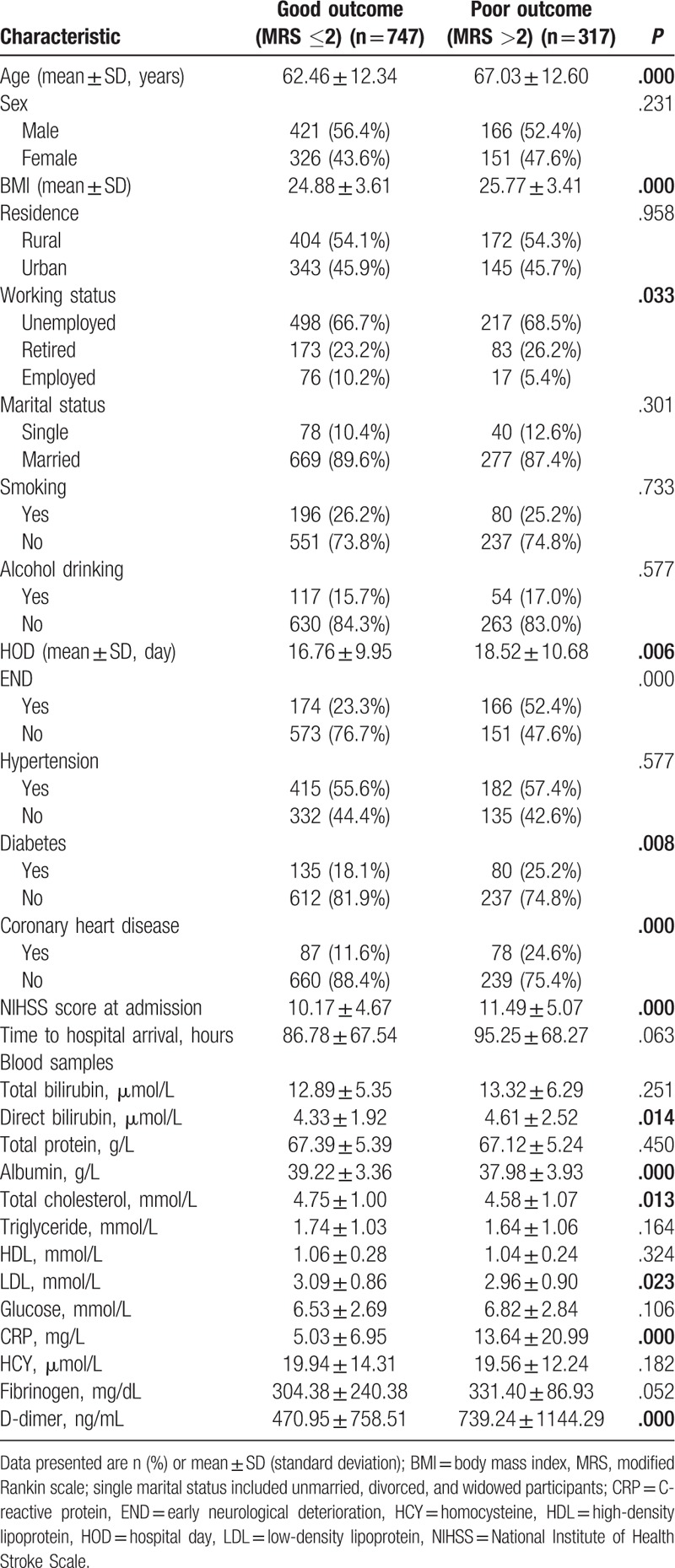
As shown in Table 3, poor outcome by the 18-month follow-up was not associated with sex, residence, marital status, smoking, alcohol drinking, hypertension, or time from onset of stroke to admission, but was significantly related to working status (P = .033), hospitalization day (HOD; P = .006), END (P< .001), diabetes (P = .008), coronary heart disease (P< .001), and NIHSS score at admission (P = .001). Compared to patients with good outcomes, those who with poor outcomes were elderly (67.03 ± 12.60 vs. 62.46 ± 12.34 years, P< .001), with higher BMI (25.77 ± 3.41 vs. 24.88 ± 3.61 kg/m2, P< .001), higher direct bilirubin level (4.61 ± 2.52 vs. 4.33 ± 1.92 umol/L, P = .014), lower albumin level (37.98 ± 3.93 vs. 39.22 ± 3.36 g/L, P< .001), lower total cholesterol level (4.58 ± 1.07 vs. 4.75 ± 1.00 mmol/L, P = .013), lower LDL level (2.96 ± 0.90 vs. 3.09 ± 0.86 mmol/L, P = .023), higher CRP level (13.64 ± 20.99 vs. 5.03 ± 6.95 mg/L, P< .001), and higher D-dimer level (739.24 ± 1144.29 vs. 470.95 ± 758.51 ng/mL, P< .001). The other blood values did not differ significantly between the 2 groups.
Table 3.
Comparison of baseline demographic and clinical characteristics between patients with poor and good outcomes.
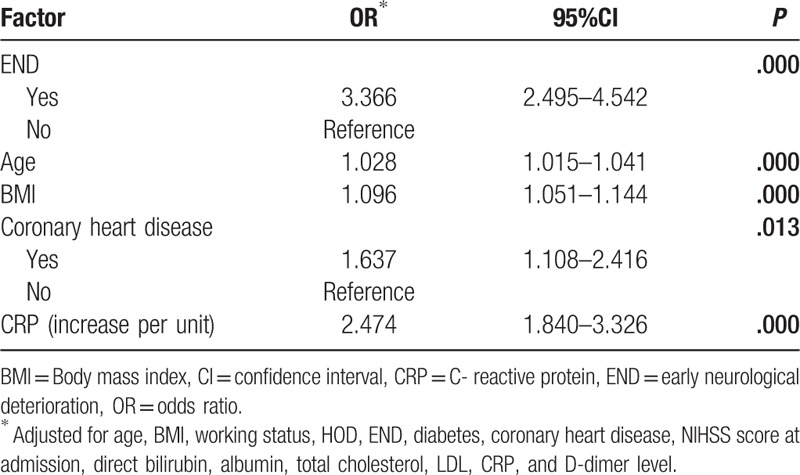
A number of independent predictors of poor outcome after acute ischemic stroke were revealed in multivariate logistic regression analyses (Table 4). It showed that poor outcome was associated with END (OR, 3.366; 95% CI 2.495–4.542), age (OR, 1.028; 95% CI 1.015–1.041), BMI (OR, 1.096; 95% CI 1.051–1.144), coronary heart disease (OR, 1.637; 95% CI 1.108–2.416), and CRP (OR, 2.474; 95% CI 1.840–3.326). The associations with working status, HOD, diabetes, NIHSS score at admission, direct bilirubin, albumin, total cholesterol, LDL, and D-dimer levels were not statistically significant, and were therefore not included in the final parsimonious regression model.
Table 4.
Factors related to poor outcome in acute ischemic stroke patients, based on multivariate logistic regression models.
4. Discussion
In our work, the most important finding was that END was a strong predictor of long-term unfavorable outcome during the acute phase in the first-ever ischemic stroke patients. In addition, older age, higher BMI and CRP level, and concomitant coronary heart disease were all associated with the long-term clinical unfavorable outcomes. It suggests our findings might provide new etiological factors as well as practical implications for stroke patients.
It also revealed that 32% of the first-ever ischemic stroke patients have experienced END during the acute phase. The proportion of patients with END was ranging from 5% to 40% which was consistent with the previous studies.[3–5] The incidence of END after acute ischemic stroke may vary widely in previous studies, depending on the definition used (i.e., different stroke scales and time frames used to assess deterioration). In fact, because some patients may be going trough their “natural” worsening in the early stage, the time of evaluation after first acute ischemic stroke is an important influencing factor on the incidence of END. Dávalos et al[7] reported a 20.3% incidence rate of patients with relatively late neurological deterioration after 3 to 7 days. In addition, Siegler et al[25] reported that NIHSS scores ≥2 points is a sensitive indicator of poor outcome compared to NIHSS scores ≥4 points. However, regardless of the definition used, END is consistently associated with poor three-month clinical outcomes.[26]
In recent years, many investigators have reported diabetes and NIHSS score at admission could be independent predictors of END.[7,13,27,28,29] Similarly, we also found these factors were related to END in the acute phase, even after adjusting for other significant covariates. A recently published cohort study also reported NIHSS score at admission to be independently associated with an increased risk of END in the acute phase.[30] Nevertheless, Zhang et al[21] found that the NIHSS score at admission was not associated with END. The probable causes may be not only their sample size was 208, relatively small, but also the object of their study included mild neurological disorder (the average NIHSS score at admission was 3). The NIHSS score represents the degree of neurological deficits. The NIHSS score at admission may be an important predictor of END in the acute phase.
CRP level is an important prognostic indicator for ischemic stroke.[31,32] Nevertheless, Seo et al[33] found that CRP level at admission was significantly associated with END in acute ischemic stroke. The increasing of CRP level may be related to END. Our results also support these findings.
In this study, we found that elevated HCY levels at admission are also an important predictor of END, but are not associated with poor prognosis. Many researchers found that HCY levels were associated with END and prognosis.[19,24] The reason of springy structure damage, endothelial dys-function, and basal lamina injury of microvessels was elevated HCY levels. This potential mechanism may be the impairment of vessel wall integrity and break of cerebrovascular permeability.[34] That acute hyperhomocysteinemia causes endothelial dysfunction, which might affect cerebrovascular reactivity and promote atheroma development.[19,35] However, the mechanisms underlying these associations are not yet completely understood and require further study.
To the best of our knowledge, this is the first report of END in acute ischemic stroke as an independent predictor of poor outcomes during a follow-up period of approximately 18 months. Previous studies only reported that END was associated with short-term poor functional outcomes.[8–10,11–15] In our study, 29.8% of patients had poor outcomes, a lower rate than those reported in other studies.[23,36,37] The results of our study also revealed that END was associated with long-term death, similar to the results reported by Kim et al.[22] The underlying mechanism may be explained by decreased perfusion and/or decreased potential for rapid development during adequate collateral blood flow to ischemic zones.[38,39] Furthermore, the platelets are activated and formed blood clots, and then they further develop the thrombus, thus causing ischemic stroke symptoms to become worse.[40] However, we found no association of END in acute ischemic stroke and recurrence. The reason for this observation is unclear at present. Finally, older age and high BMI were independent predictors of long-term outcome in our study. Similarly, Helbok et al[41] and Wang et al[42] also reported older age is an independent predictor of disability and death. However, Tanaka et al[27] observed no relationship between BMI and outcome. This may be partly related to their relatively small sample size (n = 242), and shorter time to assess outcome (30 days).
In this study, we found an important connection between coronary heart disease and poor outcomes. A previous study[43] also showed that coronary heart disease was associated with poor long-term outcomes. These data suggest that prevention and early assessment of medical complications are essential to prevent poor functional outcomes.
This study also have several limitations. First, this is a retrospective study. Unknown but potentially important factors might have confounded our results. Retrospective identification of patients might cause a selection bias. However, to minimize this error, we restricted the inclusion and exclusion criteria to avoid potential confounding factors. Second, we did not record the cause and time of death. It remains unclear whether the outcomes are affected after adjusting for cause and time of death. Finally, we defined END as an increment of at least one point in motor power or total NIHSS score ≥2 points. However, an increment of NIHSS ≤1 is easy to occur in clinical. The score may have changed because of uncertainty factors rather than neurological impairment. Therefore, further prospective studies are required.
5. Conclusions
Our study demonstrated that diabetes, NIHSS score at admission, CRP, and HCY are independent predictors of END. During the follow-up period, it showed that END was an important predictor of poor functional outcome and was associated with death. In addition, we also revealed that older age, high BMI, coronary heart disease, and high CRP levels were also associated with poor functional outcomes. Further research into this topic is warranted to reduce the burden of unfavorable outcomes.
Footnotes
Abbreviations: BMI = body mass index, CI = confidence interval, CRP = C-reactive protein, END = early neurological deterioration,, HCY = homocysteine, HDL = high-density lipoprotein, HOD = hospitalization day, LDL = low-density lipoprotein, MRS = modified Rankin scale, NIHSS = National Institute of Health Stroke Scale, OR = odds ratio, SD = standard deviation.
Author Contributions: All authors contributed to the planning of the study and the refinement of its theoretic framework. HHG, QW, BL, BBC, RLF, and PXW supervised the data collection and quality control. HHG, BBC, RLF, QZ, JJW, and YPJ conducted data analyses, and HHG drafted the manuscript. All authors contributed to the critical revision of the article for content.
This study was supported by a research grant from the Department of Neurology of Huai-He Hospital, the School of Nursing of Henan University, and the Department of Science and Technology, Henan Province (Grant number 142300410008), and the Guangzhou 121 Talents Program (GZRSH-2014-2048). We thank San-San Wang and Yan-Xia Zhu from Huai-He Hospital and Qiang Wang from Henan University for their research assistance.
The authors have no conflicts of interest to disclose.
References
- [1].Kim AS, Johnston SC. Global variation in the relative burden of stroke and ischemic heart disease. Circulation 2011;124:314–23. [DOI] [PubMed] [Google Scholar]
- [2].Zhao D, Liu J, Wang W, et al. Epidemiological transition of stroke in China: twenty-one-year observational study from the Sino-MONICA-Beijing Project. Stroke 2008;39:1668–74. [DOI] [PubMed] [Google Scholar]
- [3].Ferrari J, Knoflach M, Kiechl S, et al. Early clinical worsening in patients with TIA or minor stroke: the Austrian Stroke Unit Registry. Neurology 2010;74:136–41. [DOI] [PubMed] [Google Scholar]
- [4].Thanvi B, Treadwell S, Robinson T. Early neurological deterioration in acute ischaemic stroke: predictors, mechanisms and management. Postgrad Med J 2008;994:412–7. [DOI] [PubMed] [Google Scholar]
- [5].Roden-Jullig A. Progressing stroke: epidemiology. Cerebrovasc Dis 1997;7(suppl 5):2–5. [Google Scholar]
- [6].Davalos A, Cendra E, Teruel J, et al. Deteriorating ischemic stroke: risk factors and prognosis. Neurology 1990;40:1865–9. [DOI] [PubMed] [Google Scholar]
- [7].Dávalos A, Toni D, Iweins FL, et al. Neurological deterioiation in acute ischemic stroke: potential predictors and associated factors in the European Cooperative Acute Stroke Study (ECASS) I. Stroke 1999;30:2631–6. [DOI] [PubMed] [Google Scholar]
- [8].Kwan J, Hand P. Early neurological deterioration in acute stroke: clinical characteristics and impact on outcome. Q J Med 2006;99:625–33. [DOI] [PubMed] [Google Scholar]
- [9].Birschel P, Ellul J, Barer D. Progressing stroke: towards an internationally agreed definition. Cerebrovasc Dis 2004;17:242–52. [DOI] [PubMed] [Google Scholar]
- [10].Toni D, Fiorelli M, Gentile M, et al. Progressing neurological deficit secondary to acute ischemic stroke: a study on predictability, pathogenesis, and prognosis. Arch Neurol 1995;52:670–5. [DOI] [PubMed] [Google Scholar]
- [11].Sumer M, Ozdemir I, Erturk O. Progression in acute ischemic stroke: frequency, risk factors and prognosis. J Clin Neurosci 2003;10:177–80. [DOI] [PubMed] [Google Scholar]
- [12].Castillo J. Deteriorating stroke: diagnostic criteria, predictors, mechanisms and treatment. Cerebrovasc Dis 1999;9(suppl 3):1–8. [DOI] [PubMed] [Google Scholar]
- [13].Roquer J, Rodriguez-Campello A, Gomis M, et al. Acute stroke unit care and early neurological deterioration in ischemic stroke. J Neurol 2008;255:1012–7. [DOI] [PubMed] [Google Scholar]
- [14].Cuadrado-Godia E. Early neurological deterioration, easy methods to detect it. Indian J Med Res 2015;141:266–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Helleberg BH, Ellekjær H, Rohweder G, et al. Mechanisms, predictors and clinical impact of early neurological deterioration: the protocol of the Trondheim early neurological deterioration study. BMC Neurol 2014;14:201–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Millikan CH, Siekert RG. Studies in cerebrovascular disease. IV. The syndrome of intermittent insufficiency of the carotid arterial system. Mayo Clin Proc 1955;30:186–91. [PubMed] [Google Scholar]
- [17].Kwon HM, Lim JS, Park HK, et al. Hypertriglyceridemia as a possible predictor of early neurological deterioration in acute lacunar stroke. J Neurol Sci 2011;309:128–30. [DOI] [PubMed] [Google Scholar]
- [18].The neuroscience society. The nerve surgery to learn. All kinds of cerebrovascular disease diagnosis points. Chin J Neurol 1996;29:379. [Google Scholar]
- [19].Tanne D, Haim M, Goldbourt U, et al. Prospective study of serum homocysteine and risk of ischemic stroke among patients with preexisting coronary heart disease. Stroke 2003;34:632–6. [DOI] [PubMed] [Google Scholar]
- [20].Wityke RJ, Pessin MS, Kaplan RF, et al. Serial assessment of acute stroke using the NIH Stroke Scale. Stroke 1994;25:362–5. [DOI] [PubMed] [Google Scholar]
- [21].Zhang X, Sun Z, Ding C, et al. Metabolic syndrome augments the risk of early neurological deterioration in acute ischemic stroke patients independent of inflammatory mediators: a hospital-based prospective study. Oxid Med Cell Longev 2016;2016:8346301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kim YD, Song D, Kim EH, et al. Long-term mortality according to the characteristics of early neurological deterioration in ischemic stroke patients. Yonsei Med J 2014;55:669–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kim JT, Heo SH, Yoon W, et al. Clinical outcomes of patients with acute minor stroke receiving rescue IA therapy following early neurological deterioration. J NeuroIntervent Surg 2016;8:461–5. [DOI] [PubMed] [Google Scholar]
- [24].Kwon HM, Lee YS, Bae HJ, et al. Homocysteine as a predictor of early neurological deterioration in acute ischemic stroke. Stroke 2014;45:871–3. [DOI] [PubMed] [Google Scholar]
- [25].Siegler JE, Boehme AK, Kumar AD, et al. What change in the national institutes of health stroke scale should define neurologic deterioration in acute ischemic stroke? J Stroke Cerebrovasc Dis 2013;22:675–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Siegler JE, Martin-Schild S. Early neurological deterioration (END) after stroke: the END depends on the definition. Int J Stroke 2011;6:211–2. [DOI] [PubMed] [Google Scholar]
- [27].Tanaka R, Ueno Y, Miyamoto N, et al. Impact of diabetes and prediabetes on the short-term prognosis in patients with acute ischemic stroke. J Neurol Sci 2013;332:45–50. [DOI] [PubMed] [Google Scholar]
- [28].Jorgensen HS, Nakayama H, Raaschou HO, et al. Effect of blood pressure and diabetes on stroke in progression. Lancet 1994;344:156–9. [DOI] [PubMed] [Google Scholar]
- [29].Suda S, Katsumata T, Okubo S, et al. Low serum n–3 polyunsaturated fatty acid/n–6 polyunsaturated fatty acid ratio predicts neurological deterioration in Japanese patients with acute ischemic stroke. Cerebrovasc Dis 2013;36:388–93. [DOI] [PubMed] [Google Scholar]
- [30].Kim JP, Kim SJ, Lee JJ, et al. Diffusion-perfusion mismatch in single subcortical infarction: a predictor of early neurological deterioration and poor functional outcome. Eur Neurol 2015;73:353–9. [DOI] [PubMed] [Google Scholar]
- [31].Abubakar SA, Okubadejo NU, Ojo OO, et al. Relationship between admission serum C-reactive protein and short-term outcome following acute ischemic stroke at a tertiary health institution in Nigeria. Nigerian J Clin Pract 2013;16:320–4. [DOI] [PubMed] [Google Scholar]
- [32].Di Napoli M, Papa F, Bocola V. C-reactive protein in ischemic stroke: an independent prognostic factor. Stroke 2001;32:917–24. [DOI] [PubMed] [Google Scholar]
- [33].Seo WK, Seok HY, Kim JH, et al. C-reactive protein is a predictor of early neurologic deterioration in acute ischemic stroke. J Stroke Cerebrovasc Dis 2012;21:181–6. [DOI] [PubMed] [Google Scholar]
- [34].Rosell A, Ortega-Aznar A, Alvarez-Sabín J, et al. Increased brain expression of matrix metalloproteinase-9 after ischemic and hemorrhagic human stroke. Stroke 2006;37:1399–406. [DOI] [PubMed] [Google Scholar]
- [35].Boldyrev AA. Molecularmechanisms of homocysteine toxicity. Biochemistry 2009;74:588–98. [DOI] [PubMed] [Google Scholar]
- [36].Dharmasaroja PA, Muengtaweepongsa S, Dharmasaroja P. Early outcome after intravenous thrombolysis in patients with acute ischemic stroke. Neurol India 2011;59:351–4. [DOI] [PubMed] [Google Scholar]
- [37].Mori M, Naganuma M, Okada Y, et al. Early neurological deterioration within 24 h after intravenous rt-PA therapy for stroke patients: the stroke acute management with urgent risk factor assessment and improvement rt-PA Registry. Cerebrovasc Dis 2012;34:140–6. [DOI] [PubMed] [Google Scholar]
- [38].Karepov VG, Gur AY, Bova L, et al. Stroke in-evolution: infarct-inheren mechanisms versus systemic causes. Cerebrovasc Dis 2006;21:42–6. [DOI] [PubMed] [Google Scholar]
- [39].Caplan LR. Worsening in ischemic stroke patients is it time for a new strategy. Stroke 2002;33:1443–5. [DOI] [PubMed] [Google Scholar]
- [40].Wang Q, Zhao W, Bai S. Association between plasma soluble P-selection elements and progressive ischemic stroke. Exp Ther Med 2013;5:1427–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Helbok R, Kurtz P, Vibbert M, et al. Early neurological deterioration after subarachnoid haemorrhage: risk factors and impact on outcome. J Neurol Neurosurg Psychiatry 2013;84:266–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Wang D, Hao Z, Tao W, et al. Acute ischemic stroke in the very elderly Chinese: risk factors, hospital management and one-year outcome. Clin Neurol Neurosurg 2011;113:442–6. [DOI] [PubMed] [Google Scholar]
- [43].Bassetti CL, Milanova M, Gugger M. Sleep-disordered breathing and acute ischemic stroke: diagnosis, risk factors, treatment, evolution, and long-term clinical outcome. Stroke 2006;37:967–72. [DOI] [PubMed] [Google Scholar]


