Abstract
Rationale:
Angiogenesis is a key factor for tumor growth and metastasis both in cancer and sarcoma. Endostar, a novel safe and well-tolerated recombinant human endostatin, can suppress the expression of VEGF and the activation of ERK, MAPK, and AKT, and then inhibit tumor progression.
Patient concerns:
A pediatric osteosarcoma with pulmonary metastasis and malignant pleural effusion.
Diagnoses:
Osteosarcoma with pulmonary metastasis and malignant pleural effusion.
Interventions:
Considering the physical condition of patient, the patient underwent surgical resection of the right lung lesion after receiving endostar combined with chemotherapy and maintained endostar alone for 47 cycles.
Outcomes:
The patient obtained pathologic complete remission and had been in progression-free survival up to now.
Lessons:
Our experience could provide a treatment strategy for pediatric osteosarcoma patients with pulmonary metastasis and malignant pleural effusion.
Keywords: endostar, malignant pleural effusion, osteosarcoma, pulmonary metastasis
1. Introduction
Osteosarcoma is the most common primary malignant tumor in children and adolescents. Pulmonary metastasis from osteosarcoma is a devastating complication, resulting in reduced quality of life and shortened survival. To date, no salvage treatment is regarded standard for metastatic osteosarcoma. Here, we report a pediatric patient with osteosarcoma who developed lung metastasis and malignant pleural effusion and had a good disease control after treatment with endostar combined with chemotherapy.
2. Case report
In September 2008, an 8-year-old Chinese child presented to our hospital with pain and swelling of the left thigh for 1 month. The magnetic resonance imaging (MRI) in the Second Affiliated Hospital of Medical College of Zhejiang University showed a malignant bone tumor at the middle and inferior segment of left femur. The patient denied any other medical history. An incision biopsy was performed and histopathologic examination confirmed osteosarcoma. Before the planned curative resection, the patient received chemotherapy with methotrexate, Adriamycin, and cisplatin. The adjuvant treatment after surgery included 4 cycles chemotherapy with MAP regiment. In March 2012, a fellow-up chest computed tomography (CT) revealed a mass in the middle lobe of right lung measuring 1.9 × 1.1 cm (Fig. 1 A and B). Two months later, the patient was presented with cough, dyspnea, weakness, and fever, and a repeated chest CT showed the mass in the middle lobe of right lung enlarged measuring 10.1 × 6.7 cm (Fig. 1C and D). The possibility osteosarcoma with intrapulmonary metastasis was considered. In light of the patient's young age and poor situation with right lung mass, aggressive surgical treatment of the right lesion was not feasible. After obtaining the parents’ informed consent, the patient received to undergo chemotherapy with etoposide 0.1 g given intravenously on days 1 to 3, ifosfamide 1.0 g given intravenously on days 1 to 4, and endostar 15 mg given intravenously on days 1 to 14, every 3 to 4 weeks for a planned 7 cycles. During the chemotherapy, the patient was evaluated as partial remission (PR) with a significant reduction of effusion in the pleural and the right lung metastatic tumor shrinking dramatically (Fig. 2A–D). Later we organized multidisciplinary team (MDT) and decided metastasectomy of pulmonary metastasis in March 2013. Microscopically, histopathologic examination indicated the patient received pathologic complete remission (pCR). The mutation analysis of the right middle lobe lesion showed that mutation within vascular endothelial growth factor (VEGF) gene with no KRAS or PTEN gene mutation and immunohistochemical staining showed VEGFR1, VEGFR2, and VEGFR3 gene expression reached 46.5%, 85.9%, and 92.5%, respectively. The adjuvant treatment after surgery included 4 cycles of endostar combined with etoposide and ifosfamide. Later, the patient maintained endostar 15 mg alone every 3 weeks for 47 cycles and no recurrence or progression occurred with a progression-free survival(PFS) up to now (Fig. 2E and F).
Figure 1.
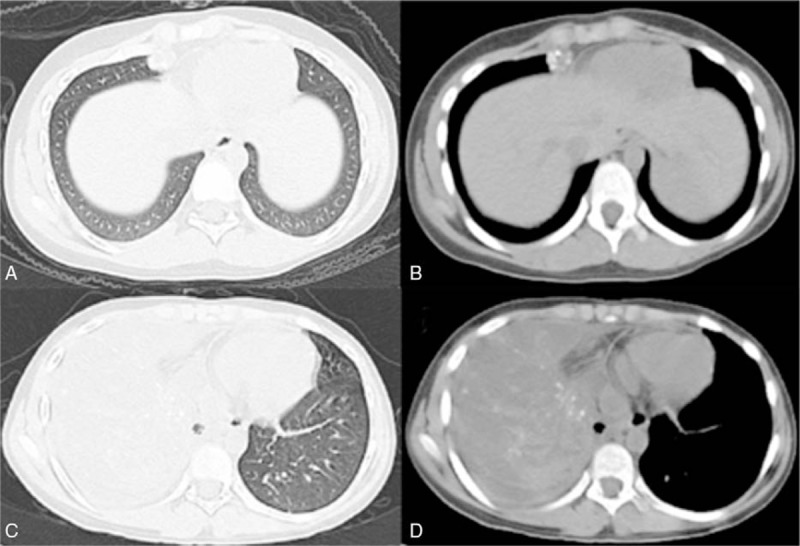
(A, B) Computed tomographic scan obtained in March 2012, showing a mass in the middle lobe of right lung measuring 1.9 × 1.1 cm. (C, D) Computed tomographic scan obtained May 2012, showing that the middle lobe of right lung had increased in size measured 10.1 × 6.7 cm (compared with the computed tomographic scan obtained in March 2012).
Figure 2.
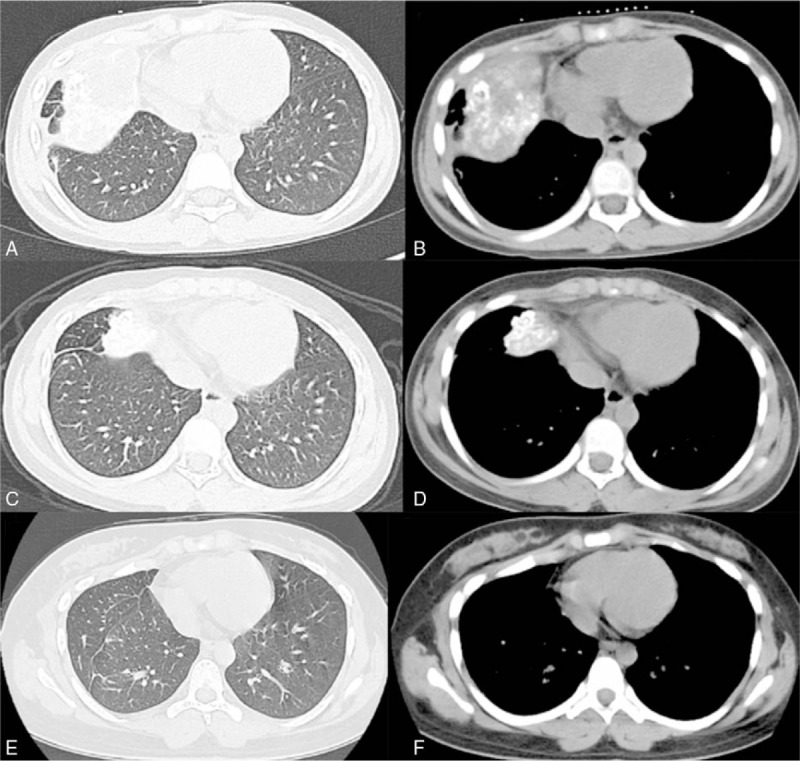
(A, B) Computed tomographic scan obtained in June 2012. (C, D) Computed tomographic scan obtained in March 2013, showing that the middle lobe of right lung had shrinked dramatically in size (compared with the computed tomographic scan obtained in May 2012). (E, F) Computed tomographic scan obtained in July 2017, showing no recurrence or progression in the lung.
3. Discussion
Osteosarcoma is the most common primary malignant tumor in children and adolescents, which exhibits a predilection to occur in the metaphysis of long bones.[1] The current osteosarcoma treatment regime consists of the combination of surgery and intensive multiagent chemotherapy. Several prognostic factors affecting overall survival have been identified in patients with osteosarcoma, such as tumor location and size, metastasis, surgical respectability, and degree of tumor necrosis after neoadjuvant chemotherapy. Advanced osteosarcoma patients are associated with pulmonary metastasis and relapse, which have significantly poor prognosis, with an overall 5-year survival rate is low.[2]
Angiogenesis is a key factor for tumor growth and metastasis both in cancer and sarcoma. Thus, antiangiogenesis therapy has become a vital part of the armamentarium against tumor.[3,4] Endostatin is an endogenous protein that potently inhibits VEGF expression and then inhibits tumor angiogenesis.[5] Endostar, a novel safe and well-tolerated recombinant human endostatin, is a multi-target tumor cell inhibitor which can suppress the expression of VEGF and the activation of ERK, MAPK, and AKT, and then inhibit tumor progression.[6] Endostar combined with chemotherapy has a broad spectrum of significant clinical benefits in several solid tumors such as lung cancer, breast cancer, cervical cancer, and hepatic cancer.[7–10]
VEGF is identified and isolated as an endothelial cell-specific mitogen that has the capacity to induce physiological and pathological angiogenesis.[11] In addition, VEGF has been widely proved to be involved in increasing permeability of the blood vessel contributes to the extravasation of proteins and thus mediates the development of a pleural effusion.[12] Our patient suffered malignant pleural effusion with pulmonary metastasis. Thus, we postulated that local administration of endostar might contribute to VEGF inhibition and block the production of effusion. After prescribing endostar combined with etoposide and ifosfamide, we saw a significant reduction of effusion in the pleural. Our patient attained PR with the right lung metastatic tumor reduced evidently. And, the postoperative pathological specimen was evaluated as pCR. Immunohistochemical staining showed VEGFR1, VEGFR2, and VEGFR3 gene expression of the right middle lobe lesion reached 46.5%, 85.9%, and 92.5%, respectively. The main toxicities observed in our patient included leukopenia and headache associated with nausea and vomiting. To our knowledge, the use of endostar combined with chemotherapy in pediatric osteosarcoma with pulmonary metastasis and malignant pleural effusion was first reported. There is still no typical study to investigate the role of endostar in pediatric osteosarcoma with pulmonary metastasis and malignant pleural effusion. This rare case obtained important clinical remissions in similar situation. Based on the mode of action of endostar, the anti-VEGF role may be an important reason for this effect. It may suggest that the combination of endostar with chemotherapy can synergistically enhance the response of osteosarcoma patients with pulmonary metastasis and provide an opportunity for metastasectomy. It may supply a new therapeutic strategy in this clinical setting.
4. Conclusion
In summary, we present an extremely rare case of a pediatric osteosarcoma with pulmonary metastasis and malignant pleural effusion which responded well to endostar combined with chemotherapy. The patient experienced a long-standing response to endostar with PFS up to now. This case provides insight into the efficacy of endostar combined with chemotherapy for pediatric osteosarcoma with pulmonary metastasis malignant pleural effusion. Doctors could consider this treatment strategy for patients with similar disease. To date, the patient is still alive with no disease progression.
Acknowledgments
The authors thank all the group members for helpful discussions.
Footnotes
Abbreviations: CT = chest computed tomography, MDT = multidisciplinary team, MRI = magnetic resonance imaging, pCR = pathologic complete remission, PFS = progression-free survival, PR = partial remission.
Author contributions: Sujing Jiang performed the data analyses and wrote the manuscript; Guannan Wang collected the information and fellowed up the patient; and Ying Dong contributed to the conception of the study and revised the manuscript.
Declaration: The patient provided written informed consent to publish their data and images in this report.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Tsuchiya H, Kanazawa Y, Abdel-Wanis ME, et al. Effect of timing of pulmonary metastases identification on prognosis of patients with osteosarcoma: the Japanese Musculoskeletal Oncology Group study. J Clin Oncol 2002;20:3470–7. [DOI] [PubMed] [Google Scholar]
- [2].Williams RF, Fernandez-Pineda I, Gosain A. Pediatric sarcomas. Surgical Clin N Am 2016;96:1107–25. [DOI] [PubMed] [Google Scholar]
- [3].Kim HS, Lim SJ, Park YK. Anti-angiogenic factor endostatin in osteosarcoma. APMIS 2009;117:716–23. [DOI] [PubMed] [Google Scholar]
- [4].Folkman J. Antiangiogenesis in cancer therapy—endostatin and its mechanisms of action. Exp Cell Res 2006;312:594–607. [DOI] [PubMed] [Google Scholar]
- [5].O’Reilly MS, Boehm T, Shing Y, et al. Endostatin: an endogenous inhibitor of angiogenesis and tumor growth. Cell 1997;88:277–85. [DOI] [PubMed] [Google Scholar]
- [6].Ling Y, Yang Y, Lu N, et al. Endostar, a novel recombinant human endostatin, exerts antiangiogenic effect via blocking VEGF-induced tyrosine phosphorylation of KDR/Flk-1 of endothelial cells. Biochem Biophys Res Commun 2007;361:79–84. [DOI] [PubMed] [Google Scholar]
- [7].Xing P, Hao X, Hu X, et al. recombinant human endostatin in the treatment of advanced lung squamous cell carcinoma. Chin J Lung Cancer 2016;19:670–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Chen J, Yao Q, Li D, et al. Neoadjuvant rh-endostatin, docetaxel and epirubicin for breast cancer: efficacy and safety in a prospective, randomized, phase II study. BMC Cancer 2013;13:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ke QH, Zhou SQ, Huang M, et al. Early efficacy of Endostar combined with chemoradiotherapy for advanced cervical cancers. Asian Pac J Cancer Prev 2012;13:923–6. [DOI] [PubMed] [Google Scholar]
- [10].Li Y. 739P Clinical study of endostar concomitant with chemotherapy in the treatment of patients with advanced primary hepatic cancer. Ann Oncol 2014;25(suppl 4):iv249. [Google Scholar]
- [11].Goel HL, Mercurio AM. VEGF targets the tumour cell. Nat Rev Cancer 2013;13:871–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Roberts WG, Palade GE. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci 1995;108(Pt 6):2369–79. [DOI] [PubMed] [Google Scholar]


