Abstract
Background:
We conducted a meta-analysis to assess the efficacy and safety of ketamine for reducing pain and narcotic use for patients undergoing laparoscopic cholecystectomy (LC).
Methods:
PubMed, Embase, Web of science, Medline, and Cochrane library databases were systematically searched. Randomized controlled trials (RCTs) were regarded as eligible in our study. After testing the heterogeneity across RCTs, data were aggregated for fixed/random effect model according to the I2 statistic. The meta-analysis was conducted using Stata 11.0 software.
Results:
Five studies were included, with a total sample size of 212 patients. Current meta-analysis revealed that there were significant differences regarding postoperative pain score at 12 hours [standard mean difference (SMD) = −0.322, 95% confidence interval (95% CI): −0.594 to −0.050, P = .020], 24 hours (SMD = −0.332, 95% CI: −0.605 to −0.059, P = .017), and 48 hours (SMD = −0.340, 95% CI: −0.612 to −0.068, P = .014). Ketamine intervention was found to significantly decrease narcotic use at 12 hours (SMD = −0.296, 95% CI: −0.567 to −0.025, P = .033), 24 hours (SMD = −0.310, 95% CI: −0.581 to −0.039, P = .025), and 48 hours (SMD = −0.338, 95% CI: −0.609 to −0.066, P = .015).
Conclusion:
Ketamine appeared to significantly reduce postoperative pain and narcotic use following LC. On the basis of the current evidence available, higher quality RCTs are still required for further research.
Keywords: ketamine, laparoscopic cholecystectomy, meta-analysis, pain management
1. Introduction
Laparoscopic cholecystectomy (LC) was first performed in 1987 and now it has become a successful surgical procedure for the treatment of cholelithiasis, cholecystitis, and biliary colic.[1] It has shown improved outcomes compared with conventional open procedures and replaced open cholecystectomy as the first-choice.[2] It was reported that more than 600,000 LCs were performed in the US annually, which predict an increasing trend and requirement for the next few years.[3]
Pain control after general surgery has become a huge challenge for surgeon and anesthetist. Many strategies have been applied to reduce postoperative pain following LC, including nonsteroid anti-inflammatory drug and patient-controlled analgesia[4–6]; however, it was still insufficient to achieve expected results. Expert consensus has proposed that multimodal analgesia regime was effective for pain control following laparoscopic operation.[7] Ketamine is a noncompetitive N-methyl-D-aspartate (NMDA) receptor antagonist, which is commonly used as dissociative general anesthetic. Recently, it is also used for pain control in surgical procedure.[8,9] Published articles showed that ketamine added to multimodal analgesia demonstrated improved analgesia. Buvanendran et al[10] indicated that oral ketamine provided improved postoperative analgesia after amputation surgery without increased risk of adverse effects. Tuchscherer et al[11] found that adding subcutaneous ketamine to standard analgesic regime resulted in a reduction of postoperative pain scores.
Although some conclusions have been made, its role in postoperative pain relief after LC has not been investigated in meta-analysis. Thus, there is still controversy regarding the efficacy and safety of ketamine for pain management. Therefore, we conducted a meta-analysis from random controlled trials, to assess the efficacy and safety of ketamine for reducing pain and narcotic use for patients undergoing LC.
2. Methods
No ethical approval was required because this was a meta-analysis that was based on the previous articles.
2.1. Search strategy
Potentially relevant studies were identified from electronic databases including Medline (1966–2017.10), PubMed (1966–2017.10), Embase (1980–2017.10), ScienceDirect (1985–2017.10), and the Web of Science (1950–2017.10). The following key words were used on combination with Boolean operators AND or OR: “laparoscopic cholecystectomy”, “ketamine,” and “pain control OR pain management OR analgesia.” No restrictions were imposed on language. The bibliographies of retrieved trials and other relevant publications were cross-referenced to identify additional articles. The search process was performed as presented in Fig. 1.
Figure 1.
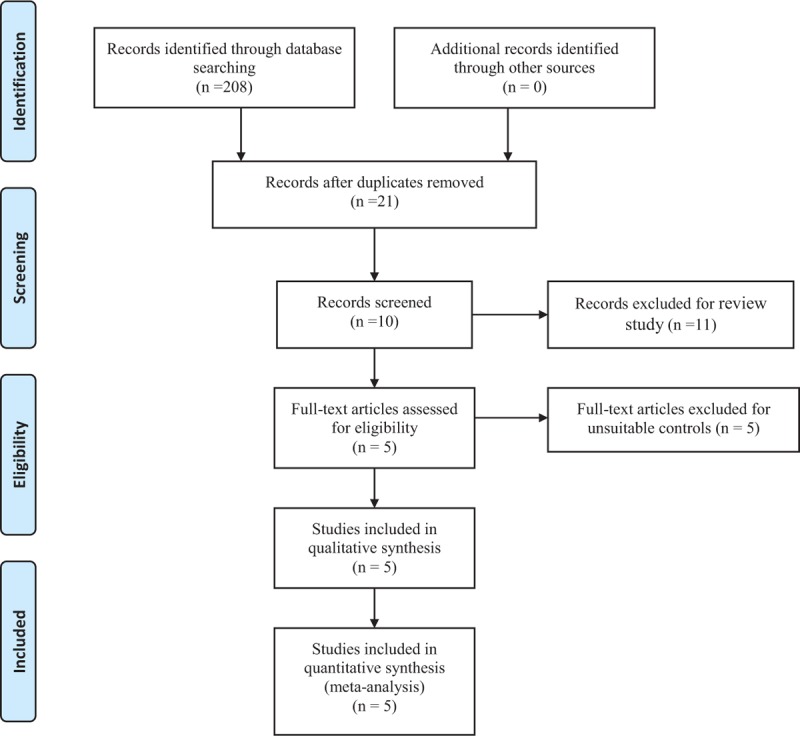
Flow diagram.
2.2. Inclusion criteria and study selection
Inclusion criteria included participants: Patients suffering symptomatic gallstones or cholecystitis and prepared for LC were included in our study; Interventions: Intravenous ketamine; Comparisons: Placebo; Outcomes: Postoperative pain measured by visual analog scale (VAS), narcotic use, and postoperative complications (nausea, vomiting, ileus, and pruritus); and Study design: Randomized controlled trials were eligible for inclusion. Exclusion criteria included articles would be excluded from the present meta-analysis for case reports, conference abstract, or review articles.
2.3. Selection criteria
Two reviewers independently scanned the abstracts of the potential articles identified by the above searches. Subsequently, the full text of the studies that met the inclusion criteria was screened, and a final decision was made. A senior author had the final decision in any case of disagreement regarding which studies to include.
2.4. Data extraction
A standard form for date extraction is printed for date extraction. Two of the authors independently extracted data from the included studies: first author names, publication year, samples size, baseline characteristics, intervention procedures, and outcome parameters. Other relevant data were also extracted from individual studies. Outcomes are postoperative pain measured by VAS, narcotic use, and postoperative complications (nausea, vomiting, ileus, and pruritus). Corresponding authors were consulted for details of data, which were incomplete. Any disagreements were resolved through discussion.
2.5. Assessment of methodological quality
Quality assessment of the included RCTs is assessed by 2 authors independently according to the Cochrane Handbook for Systematic Reviews of Interventions 5.0. We apply the assessing the “risk of bias” table, which includes the following key domains: adequate sequence generation, allocation of concealment, blinding, incomplete outcome data, free of selective reporting, and free of other bias.
2.6. Evidence synthesis
The evidence grade for the main outcomes was assessed using the guidelines of the Recommendations Assessment, Development, and Evaluation (GRADE) system including the following items: risk of bias, inconsistency, indirectness, imprecision, and publication bias. The recommendation level of evidence is divided into the following categories: high, which means that further research is unlikely to change confidence in the effect estimate; moderate, which means that further research is likely to significantly change confidence in the effect estimate but may change the estimate; low, which means that further research is likely to significantly change confidence in the effect estimate and to change the estimate; and very low, which means that any effect estimate is uncertain. The evidence quality was graded using the GRADEpro Version 3.6 software.
2.7. Statistical analysis
Stata 11.0 software (The Cochrane Collaboration, Oxford, UK) is used to perform the meta-analyses. The standard mean difference (SMD) is recommended to assess continuous variable outcomes with a 95% confidence interval (95% CI). For dichotomous outcomes, the results are presented as risk difference (RD) with a 95% CI. The Chi-squared test and I2 statistic are used to test for the presence of statistical heterogeneity. P < .05 and I2 > 50% are defined as having significant heterogeneity and a random-effects model is adopted. A fixed-effects model is applied when no significant heterogeneity is found.
3. Results
3.1. Search result
A total of 208 studies were identified through an initial search. By scanning the abstracts, 187 articles were removed for duplication and 11 records were removed as they were review articles only. After scanning the full papers, 2 articles were removed due to unsuitable controls. Five studies[12–16] published between 2004 and 2016 were included in the present meta-analysis. These articles involved a total of 106 patients in the ketamine group and 106 patients in the control group.
3.2. Study characteristics
All included RCTs assessed the analgesic efficiencies between ketamine and placebo for patients undergoing LC. The sample size of the included studies ranged from 40 to 56. There are variations in dosage of ketamine among the groups. All articles highlighted that LCs were performed by the same surgical teams. The characteristics of the included studies are summarized in Table 1. Statistically similar baseline characteristics were found among studies.
Table 1.
Characteristics of included studies.
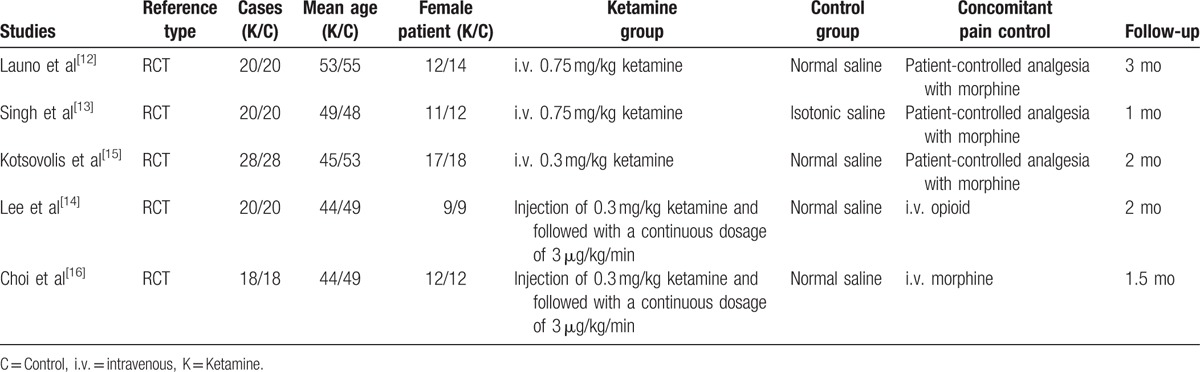
3.3. Risk of bias
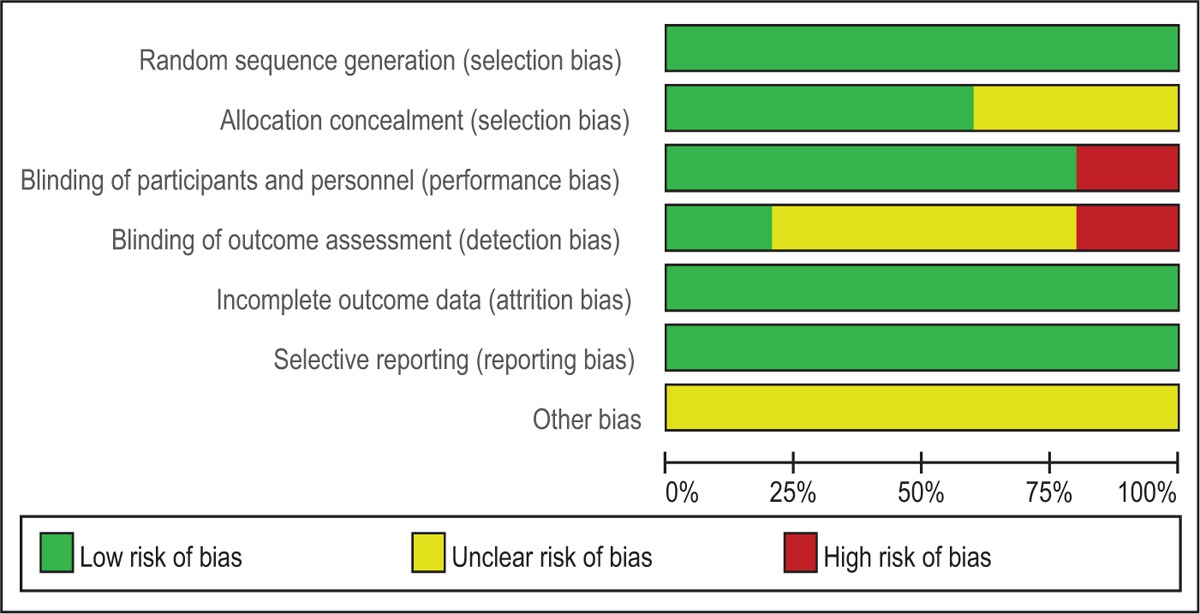
Quality assessment of the RCTs was based on the Cochrane Collaboration's tool. Clear inclusion and exclusion criteria were described in all studies and all of them reported that eligible participants were randomized with a computerized random number generator. Three RCTs[13,14,16] provided that sealed envelope was selected to make sure allocate concealment. Four included articles[12,13,15,16] confirmed double blinding and only 1 study[14] applied blinding for the assessors. The methodological quality of the included studies is presented in Table 2. Each risk of bias item was shown as the percentage across all included RCTs (Table 3).
Table 2.
Methodological quality of the randomized controlled trials.
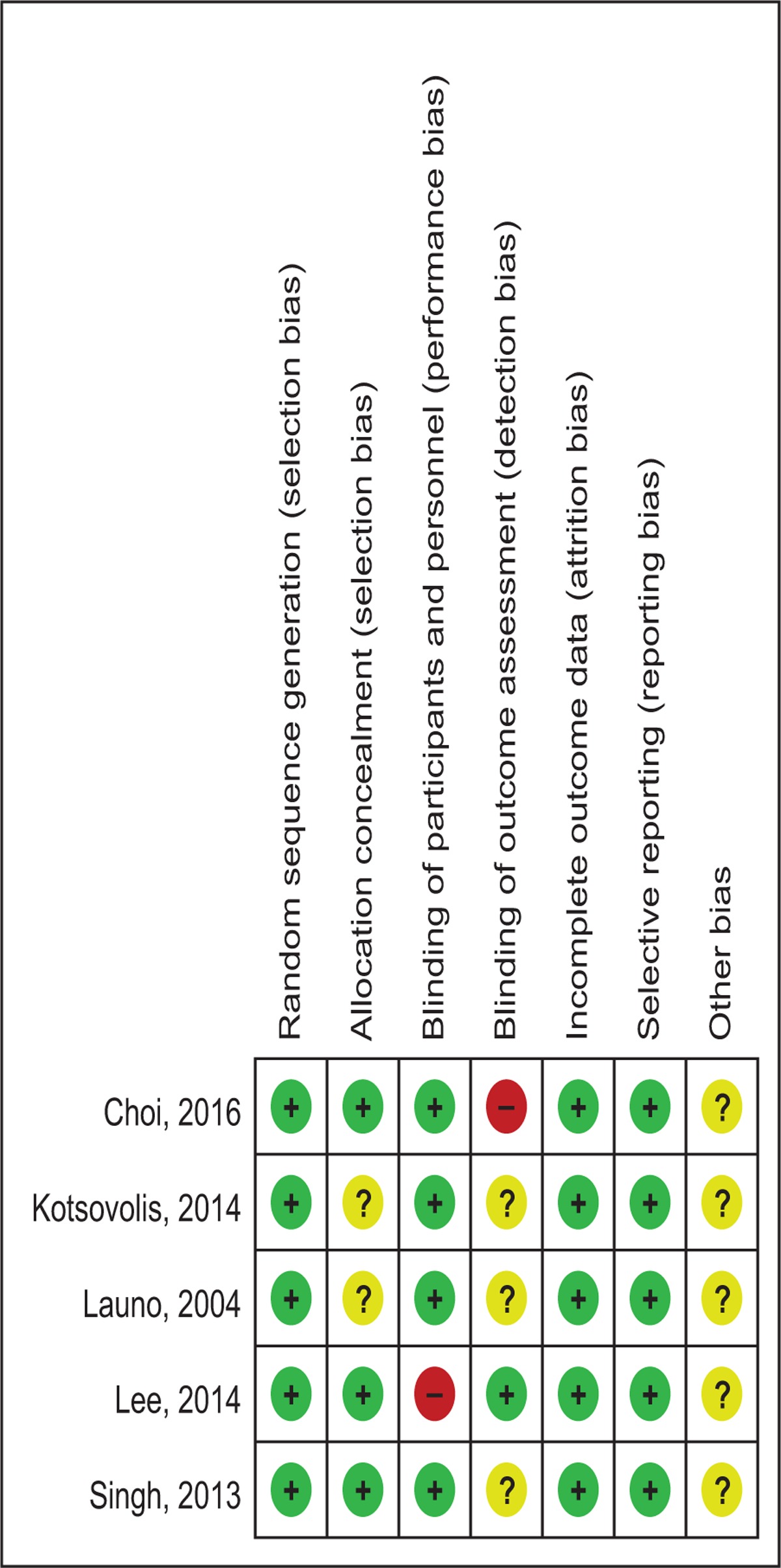
Table 3.
Risk of bias.
3.4. Outcomes for meta-analysis
3.4.1. Postoperative pain at 12 hours
Five articles[12–16] showed the postoperative pain at 12 hours measured by VAS scores. A fixed-effects model was adopted because no significant heterogeneity was found among the articles (χ2 = 1.41, df = 4, I2 = 0%, P = .843). The pooled results indicated that there was significant difference between groups regarding the postoperative pain at 12 hours (SMD = −0.322, 95% CI: −0.594 to −0.050, P = .020, Fig. 2).
Figure 2.
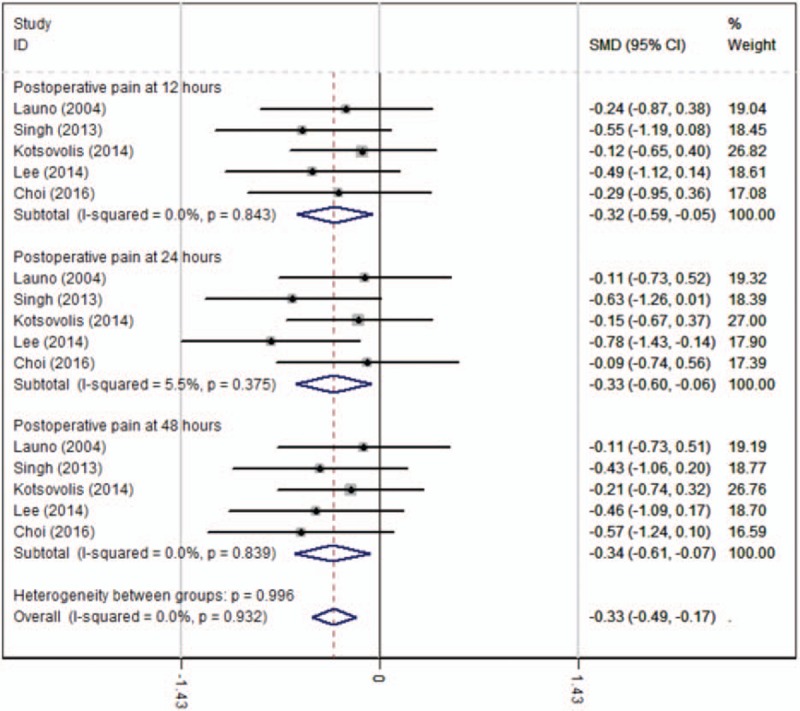
Forest plot diagram showing postoperative pain scores after LC.
3.4.2. Postoperative pain at 24 hours
Five articles[12–16] with 212 participants provided the outcome of postoperative pain at 24 hours measured by VAS scores. A fixed-effects model was used because no significant heterogeneity was found among the studies (χ2 = 4.23, df = 4, I2 = 5.5%, P = .375). The pooled results demonstrated that a significant difference in postoperative pain at 24 hours was identified between groups (SMD = −0.332, 95% CI: −0.605 to −0.059, P = .017, Fig. 2).
3.4.3. Postoperative pain at 48 hours
Five studies[12–16] with 212 patients showed the outcome of postoperative pain at 48 hours measured by VAS scores. A fixed-effects model was used because no significant heterogeneity was found among the studies (χ2 = 1.43, df = 4, I2 = 0%, P = .839). There was a significant difference in postoperative pain scores at 48 hours between groups (SMD = −0.340, 95% CI: −0.612 to −0.068, P = .014, Fig. 2).
3.4.4. Narcotic use at 12 hours
Narcotic use at 12 hours was reported in 5 RCTs.[12–16] No significant heterogeneity was found among these studies (χ2 = 0.57, df = 4, I2 = 0%, P = .967) and a fixed-effects model was used. Significant difference was detected in narcotic use at 12 hours between the 2 groups (SMD = −0.296, 95% CI: −0.567 to −0.025, P = .033, Fig. 3).
Figure 3.
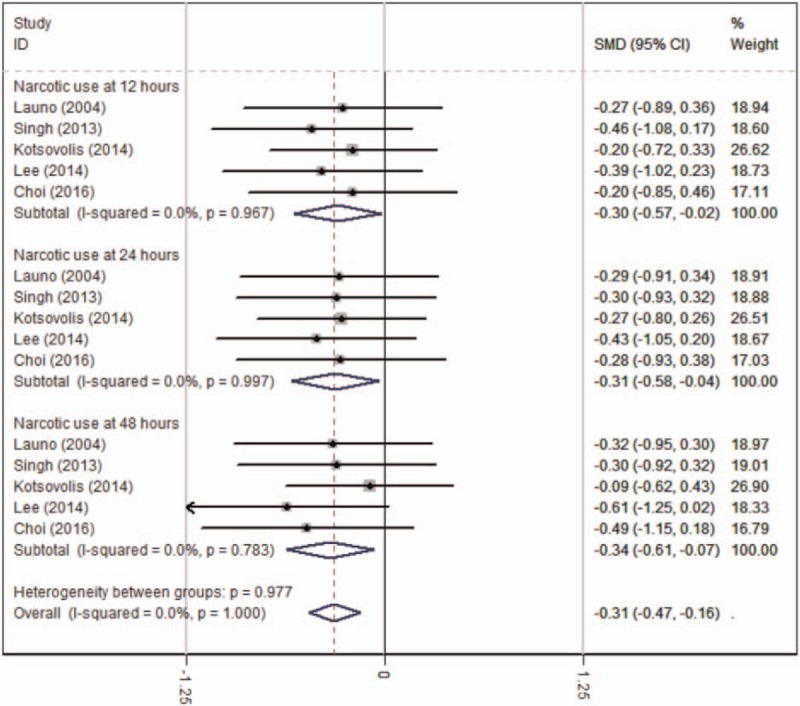
Forest plot diagram showing narcotic use after LC.
3.4.5. Narcotic use at 24 hours
Five RCTs[12–16] reported the outcome of narcotic use at 24 hours. A fixed-effects model was adopted because no significant heterogeneity was found s (χ2 = 0.17, df = 4, I2 = 0%, P = .997). There was a significant difference in narcotic use at 24 hours between groups (SMD = −0.310, 95% CI: −0.581 to −0.039, P = .025, Fig. 3).
3.4.6. Narcotic use at 48 hours
Five studies[12–16] reported narcotic use at 48 hours. A fixed-effects model was adopted (χ2 = 1.74, df = 4, I2 = 0.0%, P = .783). There was a significant difference in narcotic use at 48 hours between groups (SMD = −0.338, 95% CI: −0.609 to −0.066, P = .015, Fig. 3).
3.4.7. Nausea and vomiting
Five studies[12–16] reported the postoperative complications of nausea and vomiting after LC. A fixed-effects model was adopted because no significant heterogeneity was found among the articles (χ2 = 0.49, df = 4, I2 = 0%, P = .974). Significant difference in the incidence of nausea was found between the 2 groups (RD = −0.163, 95% CI: −0.291 to −0.036, P = .012; Fig. 4).
Figure 4.
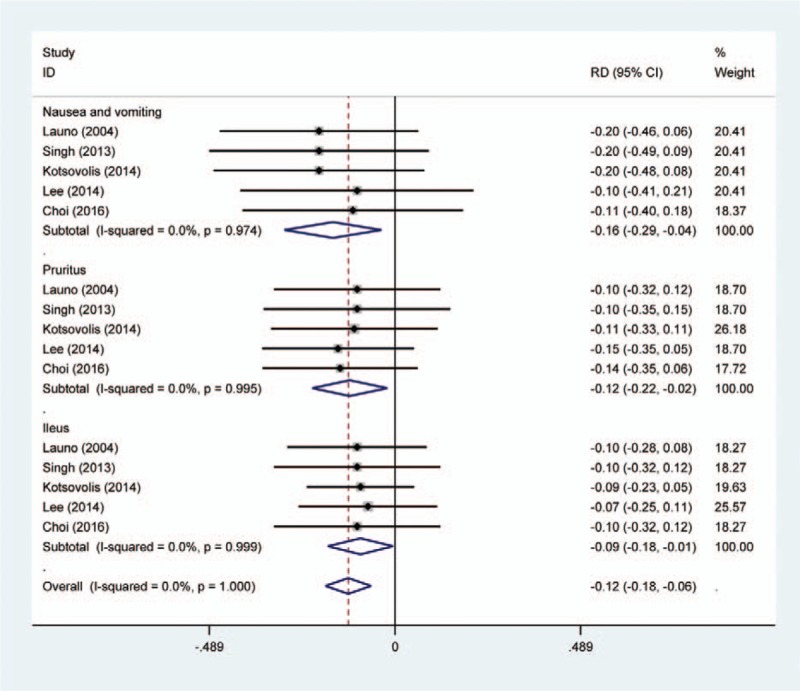
Forest plot diagram showing the postoperative complications.
3.4.8. Pruritus
Five articles[12–16] showed the postoperative complications of pruritus. A fixed-effects model was used (χ2 = 0.21, df = 4, I2 = 0%, P = .995). The pooled results demonstrated that there was an increased risk of pruritus in control groups (RD = −0.119, 95% CI: −0.218 to −0.020, P = .018; Fig. 4).
3.4.9. Ileus
All RCTs[12–16] showed the incidence of ileus between groups. There was a significant difference regarding the incidence of ileus (RD = −0.091, 95% CI: −0.177 to −0.005, P = .038; Fig. 4).
3.4.10. Publication bias
Publication bias is a tendency on average to produce results that appear significant, because negative or near neutral results are almost never published. Funnel plot was used to assess the publication bias of the main outcomes. The funnel plot diagrams of postoperative pain and narcotic use at 12 hours are symmetrical, indicating a low risk of publication bias (Figs. 5 and 6).
Figure 5.
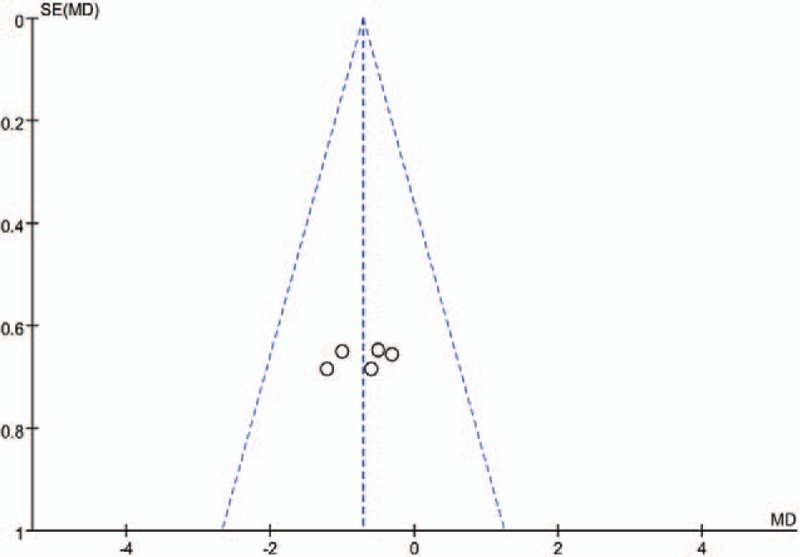
A funnel plot of VAS scores at 12 hours after LC.
Figure 6.
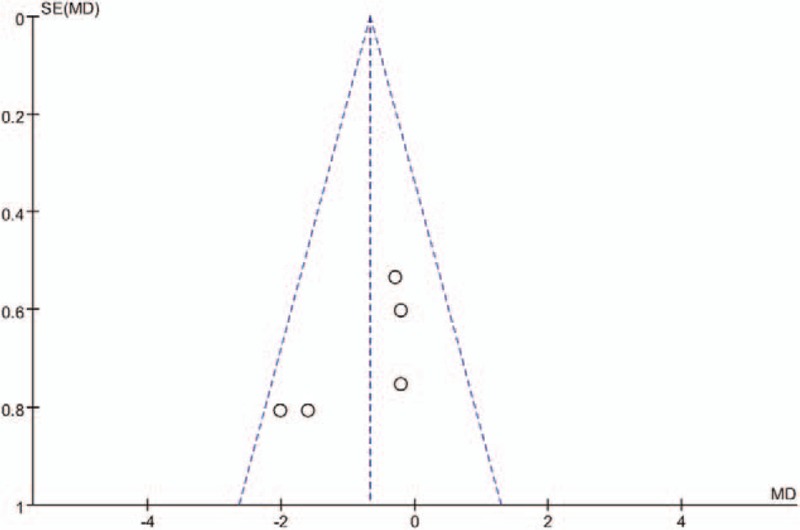
A funnel plot of opioid consumption at 12 hours after LC.
3.4.11. Quality evidence
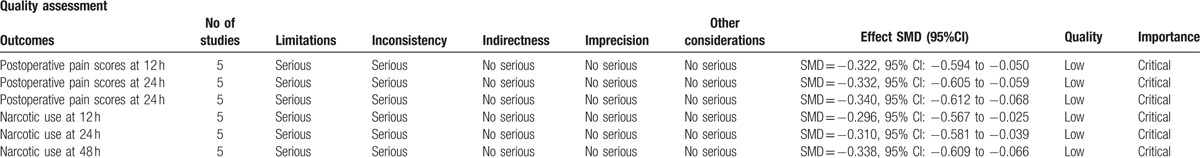
Main outcomes in this meta-analysis were evaluated using the GRADE system. The evidence quality for each outcome was low, which means that further research was likely to significantly change confidence in the effect estimate and to change the estimate (Table 4).
Table 4.
The GRADE evidence quality for main outcome.
4. Discussion
The most important finding of the present meta-analysis was that intravenous ketamine was associated with a significant reduction of postoperative pain and narcotic use following LC. The evidence quality for each outcome was low, which means that further research was likely to significantly change confidence in the effect estimate and to change the estimate.
It was reported that approximately 50% to 70% of LC patients experienced moderate to severe postoperative pain.[17,18] The incidence of postsurgical pain depends upon multiple factors, such as psychological, emotional, surgical, and anesthetic factors.[19] Consensus has been reached that effective pain control following major surgery is important for functional recovery and reducing postoperative complications. Although various analgesic methods have been applied, the optimal analgesic methods remain controversial. Any single-mode analgesia is not enough to provide satisfactory results. The management of pain after LC is often directed at the reduction of pain and reducing morphine requirements by multimodal analgesia techniques. As an NMDA receptor antagonist, ketamine has been increasingly applied as part of the multimodal analgesia in the setting of perioperative pain. Cogan et al[20] reported that low-dose intravenous ketamine infusions during the postoperative period may help to decrease acute and chronic postoperative pain after cardiac surgery. Othman et al[21] showed that the intravenous ketamine could prolong the time to first request of analgesia and reduce total opioid consumption without serious side effects in patients who underwent a modified radical mastectomy. On the basis of the previous research, it is thus plausible that intravenous ketamine may be adequate for minimally invasive surgery such as LC, which causes less tissue trauma. The VAS is a psychometric response scale that can be used in questionnaires. It is a measurement instrument for subjective characteristics or attitudes that cannot be directly measured. Five articles with 212 participants provided the outcome of postoperative pain measured by VAS scores. The present meta-analysis showed that intravenous ketamine was associated with a significant reduction of VAS scores compared with control groups.
Narcotic consumption was also an important indicator for assessing the analgesic effect of liposomal bupivacaine. It was normally used as an adjunct to a multimodal analgesia protocol. Also, the analgesic effect of the additional opioids provides a long postoperative period without any pain experienced by the participants. Narcotic consumption is also considered as an objective method of measuring pain. However, previous studies have frequently reported that patients have experienced drug-related side effects, such as nausea, vomiting, headache, pruritus, and respiratory depression.[22,23] Moreover, long-term opioid use may result in drug dependence, which is an important issue that should be considered.[24] Effective analgesia protocol is crucial to reduce the consumption of opioids. Although some published articles have shown that the intravenous ketamine was associated with opioid-sparing effect in major abdominal surgery, there was a lack of reliable evidence in LC. Therefore, we conducted the present meta-analysis and indicated that intravenous ketamine would significantly reduce total narcotic consumption.
Drug-related side effects were major concerns following additional opioid. Gastrointestinal events are well-known side effects that are related to systemic use of opioid. Ileus was frequently occurred after general anesthesia following abdominal surgery. The present meta-analysis showed that intravenous ketamine could significantly decrease the incidence of postoperative complications. Thrombosis events have been considered as a common postoperative complication and there was no significant difference between groups regarding the incidence of deep venous thrombosis or pulmonary embolism. Considering that only 5 studies were included in our study, large sample sizes from high-quality studies should be conducted in the future. A risk of bias should also be considered when interpreting the findings.
Several potential limitations of this study should be noted. First, only 5 studies were included, and the sample size was relatively small; second, some important outcome parameters such as range of motion were not fully described and could not be included in the meta-analysis; Third, subgroups analysis was not performed, thus we could not determine the source of heterogeneity; Fourth, the evidence quality for each outcome was low that may influence the results of the meta-analysis; Fifth, short-term follow-ups may lead to the underestimation of complications, such as neurotoxicity and cardiotoxicity.
5. Conclusion
Ketamine appeared to significantly reduce postoperative pain and narcotic use following LC. On the basis of the current evidence available, higher quality RCTs are still required for further research.
Footnotes
Abbreviations: LC = laparoscopic cholecystectomy, LOS= length of stay, RCT= randomized controlled trials, VAS = visual analog scale.
Authorship: CLZ conceived the design of the study. YYW performed and collected the data and contributed to the design of the study. FY finished the manuscript. All authors read and approved the final manuscript.
The authors declare that they have no competing interests.
References
- [1].Reynolds W., Jr The first laparoscopic cholecystectomy. JSLS 2001;5:89–94. [PMC free article] [PubMed] [Google Scholar]
- [2].Hendolin HI, Paakonen ME, Alhava EM, et al. Laparoscopic or open cholecystectomy: a prospective randomised trial to compare postoperative pain, pulmonary function, and stress response. Eur J Surg 2000;166:394–9. [DOI] [PubMed] [Google Scholar]
- [3].Tucker JJ, Grim R, Bell T, et al. Changing demographics in laparoscopic cholecystectomy performed in the United States: hospitalizations from 1998 to 2010. Am Surg 2014;80:652–8. [PubMed] [Google Scholar]
- [4].Fanelli G, Ghisi D, Berti M, et al. Preoperative administration of controlled-release oxycodone as a transition opioid for total intravenous anaesthesia in pain control after laparoscopic cholecystectomy. Surg Endosc 2008;22:2220–8. [DOI] [PubMed] [Google Scholar]
- [5].Jimenez Fuertes M, Costa Navarro D. Outpatient laparoscopic cholecystectomy and pain control: a series of 100 cases. Cir Esp 2015;93:181–6. [DOI] [PubMed] [Google Scholar]
- [6].Papagiannopoulou P, Argiriadou H, Georgiou M, et al. Preincisional local infiltration of levobupivacaine vs ropivacaine for pain control after laparoscopic cholecystectomy. Surg Endosc 2003;17:1961–4. [DOI] [PubMed] [Google Scholar]
- [7].Nicolau AE, Merlan V, Grecu I, et al. [Multimodal analgesia in elective laparoscopic cholecystectomy. A double-blind randomized controlled trial]. Chirurgia (Bucur) 2008;103:547–51. [PubMed] [Google Scholar]
- [8].Guillou N, Tanguy M, Seguin P, et al. The effects of small-dose ketamine on morphine consumption in surgical intensive care unit patients after major abdominal surgery. Anesth Analg 2003;97:843–7. [DOI] [PubMed] [Google Scholar]
- [9].McNicol ED, Schumann R, Haroutounian S. A systematic review and meta-analysis of ketamine for the prevention of persistent post-surgical pain. Acta Anaesthesiol Scand 2014;58:1199–213. [DOI] [PubMed] [Google Scholar]
- [10].Buvanendran A, Kroin JS, Rajagopal A, et al. Oral ketamine for acute pain management after amputation surgery. Pain Med 2017;[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [11].Tuchscherer J, McKay WP, Twagirumugabe T. Low-dose subcutaneous ketamine for postoperative pain management in Rwanda: a dose-finding study. Can J Anaesth 2017;[Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- [12].Launo C, Bassi C, Spagnolo L, et al. Preemptive ketamine during general anesthesia for postoperative analgesia in patients undergoing laparoscopic cholecystectomy. Minerva Anestesiol 2004;70:727–34. 734–728. [PubMed] [Google Scholar]
- [13].Singh H, Kundra S, Singh RM, et al. Preemptive analgesia with ketamine for laparoscopic cholecystectomy. J Anaesthesiol Clin Pharmacol 2013;29:478–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Lee MH, Chung MH, Han CS, et al. Comparison of effects of intraoperative esmolol and ketamine infusion on acute postoperative pain after remifentanil-based anesthesia in patients undergoing laparoscopic cholecystectomy. Korean J Anesthesiol 2014;66:222–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Kotsovolis G, Karakoulas K, Grosomanidis V, et al. Comparison between the combination of gabapentin, ketamine, lornoxicam, and local ropivacaine and each of these drugs alone for pain after laparoscopic cholecystectomy: a randomized trial. Pain Pract 2015;15:355–63. [DOI] [PubMed] [Google Scholar]
- [16].Choi SK, Yoon MH, Choi JI, et al. Comparison of effects of intraoperative nefopam and ketamine infusion on managing postoperative pain after laparoscopic cholecystectomy administered remifentanil. Korean J Anesthesiol 2016;69:480–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lepner U, Goroshina J, Samarutel J. Postoperative pain relief after laparoscopic cholecystectomy: a randomised prospective double-blind clinical trial. Scand J Surg 2003;92:121–4. [PubMed] [Google Scholar]
- [18].Saadati K, Razavi MR, Nazemi Salman D, et al. Postoperative pain relief after laparoscopic cholecystectomy: intraperitoneal sodium bicarbonate versus normal saline. Gastroenterol Hepatol Bed Bench 2016;9:189–96. [PMC free article] [PubMed] [Google Scholar]
- [19].Macrae WA. Chronic post-surgical pain: 10 years on. Br J Anaesth 2008;101:77–86. [DOI] [PubMed] [Google Scholar]
- [20].Cogan J, Lalumiere G, Vargas-Schaffer G, et al. Low-dose intravenous ketamine for postcardiac surgery pain: effect on opioid consumption and the incidence of chronic pain. Ann Card Anaesth 2017;20:395–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Othman AH, El-Rahman AM, El Sherif F. Efficacy and safety of ketamine added to local anesthetic in modified pectoral block for management of postoperative pain in patients undergoing modified radical mastectomy. Pain Physician 2016;19:485–94. [PubMed] [Google Scholar]
- [22].Crofford LJ. Adverse effects of chronic opioid therapy for chronic musculoskeletal pain. Nat Rev Rheumatol 2010;6:191–7. [DOI] [PubMed] [Google Scholar]
- [23].Lema MJ. Opioid effects and adverse effects. Reg Anesth 1996;21:38–42. [PubMed] [Google Scholar]
- [24].Harris AC, Gewirtz JC. Acute opioid dependence: characterizing the early adaptations underlying drug withdrawal. Psychopharmacology (Berl) 2005;178:353–66. [DOI] [PubMed] [Google Scholar]


