Abstract
Although it is clear that ventilated intensive care unit (ICU) patients have worse outcomes than those who are not, information about the risk factors of in-hospital mortality remains important for medical groups to target interventions for these patients.
The purpose of this study was to identify predictive factors of in-hospital mortality in ventilated ICU patients with an admission diagnosis of acute respiratory failure.
We conducted a prospective cohort study in 3 medical ICUs in a 3600-bed university hospital. Consecutive patients with acute respiratory failure who received mechanical ventilation (MV) for at least 96 hours without evidence of pre-existing neuromuscular diseases were followed until discharge. Upon inclusion, the following parameters were collected or evaluated: demographics, clinical history (admission body mass index [BMI], etiology of acute respiratory failure, comorbidity, Charlson comorbidity index, laboratory data), Acute Physiology and Chronic Health Evaluation (APACHE) II, and right and left quadriceps femoris muscle force. The days of MV before extubation, ICU length of stay, survival status at discharge, and hospital length of stay were recorded from the hospital discharge summary. The primary endpoint was in-hospital mortality.
In all, 113 patients (65.49% males) were recruited with a mean age of 69.78 years and mean APACHE II score of 22.63. The mean ICU length of stay was 14.88 ± 9.79 days. Overall in-hospital mortality was 25.66% (29 out of 113 patients). Multivariate analysis showed that the essential factors associated with increased in-hospital mortality were lower BMI (P = .013), and lower scores on the right or left quadriceps femoris muscle force (P = .002 and .010, respectively).
Our study suggests that lower BMI and lower scores on lower limb muscle force may be associated with increased in-hospital mortality in ventilated ICU patients.
Keywords: in-hospital mortality, intensive care unit, limb muscle force, mechanical ventilation
1. Introduction
Invasive ventilation using endotracheal intubation and mechanical ventilation (MV) is often required in patients with acute respiratory failure. Patients receiving MV may have an increased risk of respiratory muscle weakness,[1] sputum retention, atelectasis, pneumonia,[2] and malnutrition,[3] resulting in delayed extubation and prolonged ventilation, and also increased morbidity and mortality.
Several studies have evaluated short and long-term prognostic indicators in patients receiving MV and identified some significant risk factors for in-hospital mortality, including age, multiorgan system failure, human immunodeficiency virus infection, chronic liver disease, and cancer.[4] The factors may be present at the start of MV, emerge during the development of complications due to patient management in the intensive care unit (ICU),[5] or arise for patients with lower BMI.[6]
Patients requiring MV can experience limb muscle weakness resulting from prolonged periods of immobilization, and this weakness can increase with time. The incidence rate of limb muscle loss is 33% for patients receiving 3 days of MV, 50% for 4 days, and 80% for more than 7 days.[7] Limb muscle weakness is characterized by bilateral and symmetrical limb weakness, which leads to prolonged ICU stays, long-term disability in survivors, and increased mortality.[1,8,9]
Although it is clear that ventilated ICU patients have worse outcomes than those who are not, information about the risk factors of in-hospital mortality is important for medical groups to target interventions to this patient population. The aim of this study was to identify the predictive factors of in-hospital mortality in ventilated ICU patients with an admission diagnosis of acute respiratory failure. An analysis was performed on demographic and clinical history parameters, Acute Physiology and Chronic Health Evaluation (APACHE) II scores, muscle force, the number of days of MV before extubation, ICU length of stay, hospital length of stay, and survival status at discharge.
2. Methods
2.1. Study design and setting
This was a prospective cohort study to identify the predictive factors of in-hospital mortality in ventilated ICU patients with an admission diagnosis of acute respiratory failure. The study was conducted between April 2012 and December 2014 in 3 medical ICUs (MICUs) in a 3600-bed university hospital in northern Taiwan: MICU-1 is a 20-bed unit, MICU-3 is a 12-bed unit, and MICU-5 is a 24-bed unit; a total of 56 beds. The number of patients in these beds ranged from 90 to 120 patients per month during the study period. These 3 medical ICUs receive patients who have acute respiratory failure and need a breathing apparatus, or patients who have a serious medical disease (eg, currently using noninvasive respirator bilevel positive airway pressure, shock, hemodynamic instability). These ICUs use the same devices, and patient admission depends on which ICU has an empty bed for consecutive patients transferred by thoracic physicians. Among the 113 patients included in the analysis, 16 patients were from MICU-1, 27 from MICU-3, and 70 from MICU-5. Patients with hemodynamic, cardiovascular and gastrointestinal patients have their own exclusive ICU.
2.2. Patients
The study protocol was approved by the Chang Gung Medical Foundation Institution Review Board (IRB 100-4215A3). Each patient was recruited after obtaining the attending physician's permission and patient's written informed consent. A consecutive sample was drawn by reviewing the medical records of stable patients admitted to the hospital with acute respiratory failure and treated with invasive MV. Inclusion criteria for this study were: age 20 years or older; first-time intubation and ventilation support of at least 96 hours; and clear consciousness and capable of following commands. Exclusion criteria were as follows: neurologic injury or pathology (eg, acute stroke, Parkinsons disease, dementia); unstable vascular dysfunction (eg, concentration of blood platelets less than 50,000/mm3, disseminated intravascular coagulation, under Swan-Ganz catheterization, vasoactive medication use); muscular skeletal disease or inability to walk without assistance before ICU admission (use of a cane or walkers were not excluded); and were cared for in an ICU at another hospital for more than 96 hours or were treated at another hospital for a period of more than 30 days before ICU admission. All patients admitted to the study ICUs are evaluated by a dietician, and appropriate nutritional and energy requirements are determined. Patients were provided with enteral or parenteral nutrition according the severity of their disease and clinical condition.
2.3. Data collection
The primary outcome measure was in-hospital mortality. Secondary outcome measures included the days of MV before extubation, and lengths of stays in the ICU and hospital. Demographic information including age, sex, and smoking history was obtained, in part, directly from the hospital admission form and in part from the patient. The clinical history included the etiology of acute respiratory failure, the presence of comorbidity before hospitalization (from which the Charlson comorbidity index [CCI] score was calculated), weight (kg) and height (cm) (from which the body mass index [BMI] was calculated); laboratory values were obtained from the clinical documentation produced at the time of admission to the ICU. Gathered data were used to fill in the APACHE II form.
The APACHE II score is the sum of three scores: age points, chronic health points, and acute physiology score points.
Data regarding isometric right and left quadriceps femoris muscle force were evaluated by applying a handheld dynamometer (microFET2, Hoggan Health Industries, West Jordan, UT) at day 1 of the study. For testing isometric quadriceps femoris muscle force, the patient was placed in semi-Fowler position with knee extension and the transducer was placed on the anterior surface of the lower leg proximal to the ankle. Examiners demonstrated and verbally explained the task before testing. Instruction and encouragement were given to have the patient gradually apply maximum force against the transducer pad of the microFET2 over 3 seconds. At least 3 repetitions were performed until results were reproducible.
The days of MV before extubation were counted from the first day of admission with MV to the first time extubation was successful; this was determined by calendar days, not the total days of MV during hospitalization. The days of MV before extubation, ICU length of stay, survival status at discharge, and hospital length of stay were obtained from the hospital discharge summary. The primary endpoint was in-hospital mortality, and the secondary endpoint was length of hospitalization.
2.4. Sample size calculation
The purpose of this study was to determine the in-hospital mortality in ventilated ICU patients and to explore the risk factors of mortality. In 2013, a study by Zimmerman et al[10] showed that the in-hospital mortality rate in ventilated ICU patients was 20.0% from 2010 to 2012. To compare our results with those of Zimmerman et al, we tested for single proportions by determining the “difference from constant (binomial test, 1-sample case)” in G∗Power 3.1.9.2.[11,12] The input parameters were as follows: 2-sided, effect size = 0.125, α = 0.05, power = 0.8, and constant proportion = 0.20, which yielded a sample size of 97. Assuming a potential dropout rate of 15%, the minimum sample size needed was determined to be 112.
2.5. Statistical analysis
All statistical analyses were performed using SPSS software version 22 (SPSS for Windows, SPSS Inc., Chicago, IL). Descriptive statistics were expressed as mean ± standard deviation (SD) for continuous variables and number of patients (%) for categorical variables. The between-group comparisons were assessed, for simplicity, by using Mann-Whitney U test and Fisher exact test, respectively. Potential risk factors for in-hospital mortality were analyzed by using univariate logistic regression. For those risk factors with P value <.05 (statistically significant) in the univariate analysis, the multiple logistic regression was used to identify the variables associated with in-hospital mortality. All statistical tests were 2-tailed, and a P-value <.05 was considered to be statistically significant.
3. Results
3.1. Patient characteristics
During the study period, 3135 patients were admitted to 3 ICUs. Of these patients, 2868 were treated with invasive MV. After screening, 1547 met the inclusion criteria and 1424 patients were subsequently excluded: 329 were cared for by physicians who would not agree to permit participation, 396 required isolation (eg, protective, contact or respiratory tract isolation), 145 could not provide written consent because no surrogate was available, 459 needed hemodialysis requiring a double-lumen catheter in the groin, and 95 had no assessable lower limbs (eg, amputation or wound). Among the 123 remaining patients, 8 were not interested in participating and 2 experienced too much physical discomfort to participate (Fig. 1). The total number of patients included in the study (n = 113) provided written informed consent and completed the study protocol. Twenty-nine (25.66%) patients died in the hospital, including 20 (17.70%), who died in the ICU.
Figure 1.
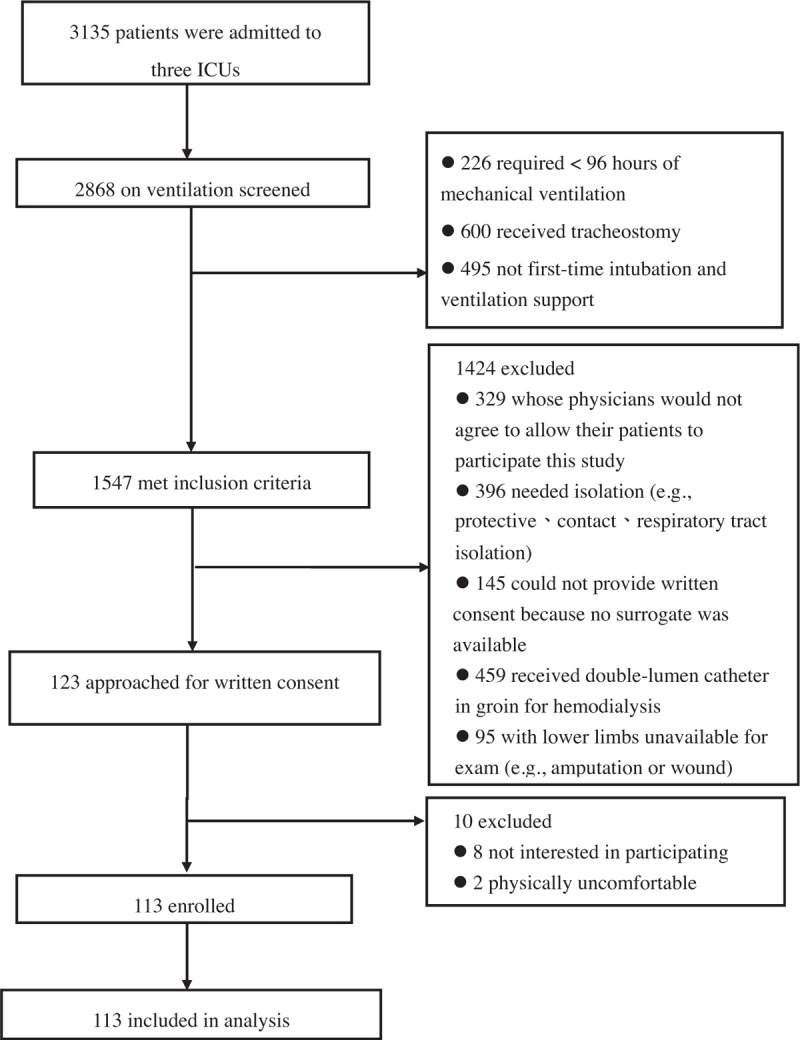
CONSORT enrollment diagram. CONSORT = consolidated standards of reporting trial.
Table 1 shows the characteristics of the study sample and the unadjusted differences between patients who survived to hospital discharge and those who did not. Of the total patients, there were 74 males (65.49%) and 39 females (34.51%); 90.30% had comorbidity (eg, hypertension, diabetes mellitus, heart disease, congestive heart failure, liver disease, asthma, chronic obstructive pulmonary disease, or renal disease). The patients with heart disease (n = 22) were diagnosed with atrial fibrillation, coronary artery disease, arrhythmia, aortic dissection, sick sinus syndrome, tricuspid regurgitation, dilated right atrium, and right ventricle. Diagnoses of liver disease (n = 9) included fatty liver, liver cirrhosis, hepatitis C, cholecystitis, abnormal liver function, and alcoholic liver disease; diagnoses of renal disease (n = 24) included chronic kidney disease, acute kidney disease, acute renal failure, chronic renal failure, right side renal stone, end stage renal disease, and glomerulonephritis. The average age was 69.78 ± 14.67 years, the median age was 74 years, BMI was 24.51 ± 5.78 kg/m2, and APACHE II score was 22.63 ± 7.35, BMI was 23.60 kg/m2, and APACHE II score was 22. The medians for length of stay in the ICU for ventilated survivors and nonsurvivors were 10 days (25th–75th percentiles 7–15 days) and 20 days (25th–75th percentiles 15–30 days), respectively. The medians of hospital length of stay for survivors and nonsurvivors were 26.5 days (25th–75th percentiles 18–46.8 days) and 39 days (25th–75th percentiles 25–57 days), respectively.
Table 1.
Characteristics of participants (N = 113) and differences between survivors and nonsurvivors.
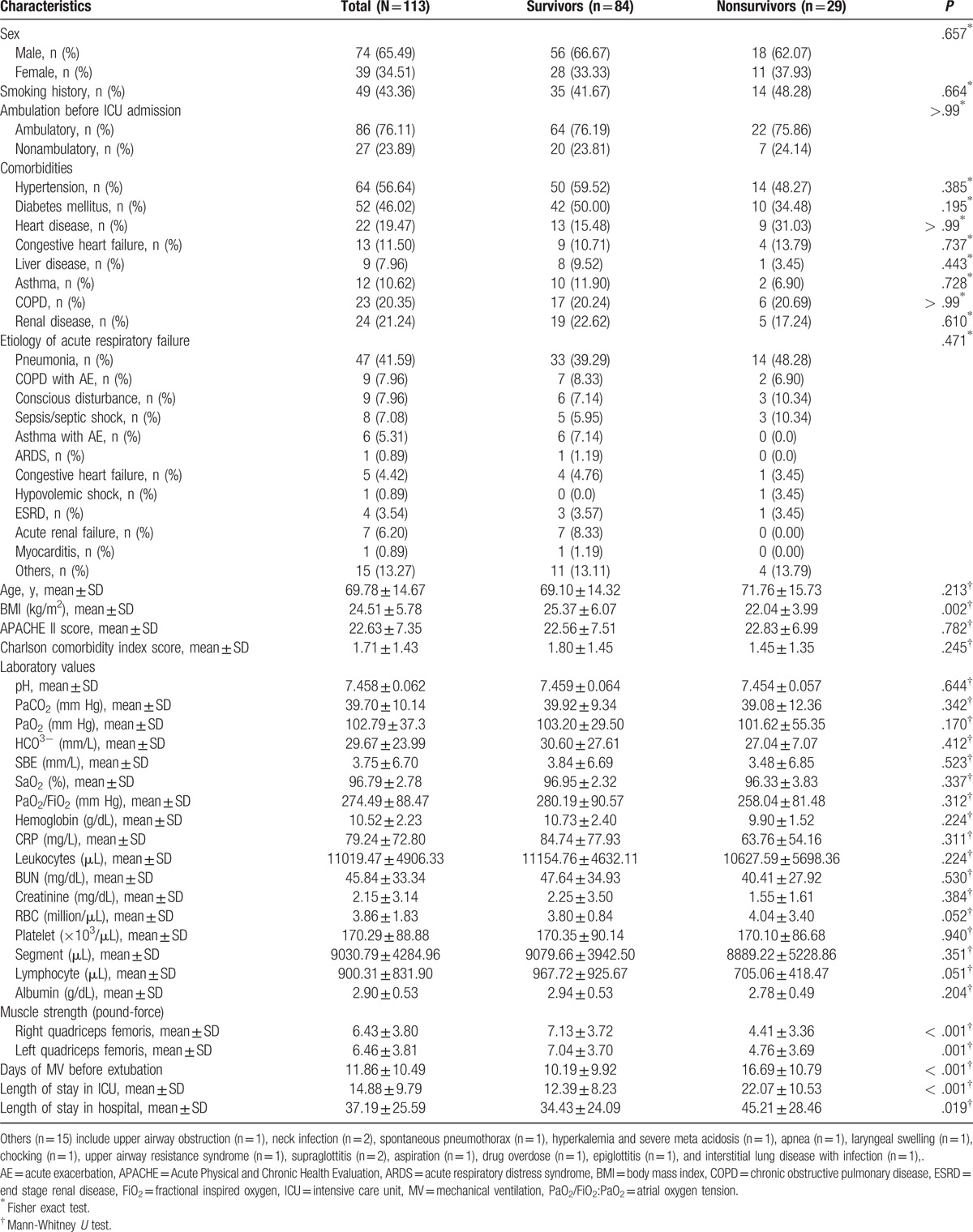
Univariate analysis indicated patients who died in hospital had a significantly lower mean BMI than survivors (22.04 ± 3.99 vs 25.37 ± 6.07; P = .002). Values for muscle strength were significantly different for nonsurvivors: mean right and left quadriceps femoris muscle force was lower (4.41 ± 3.36 vs 7.13 ± 3.72; P < .001; 4.76 ± 3.69 vs 7.04 ± 3.70; P < .001, respectively). Hospitalization variables were also significantly greater for nonsurvivors than survivors for mean number of days of MV before extubation (16.69 ± 10.79 vs 10.19 ± 9.92; P < .001), mean ICU lengths of stay (22.07 ± 10.53 vs 12.39 ± 8.23; P < .001), and mean hospital lengths of stay (45.21 ± 28.46 vs 34.43 ± 24.09; P = .019). Hospital nonsurvivors and survivors were similar with respect to sex, smoking history, comorbidities, etiology of acute respiratory failure, age, APACHE II score, CCI score, and laboratory values.
3.2. Univariate analysis for risk of in-hospital death
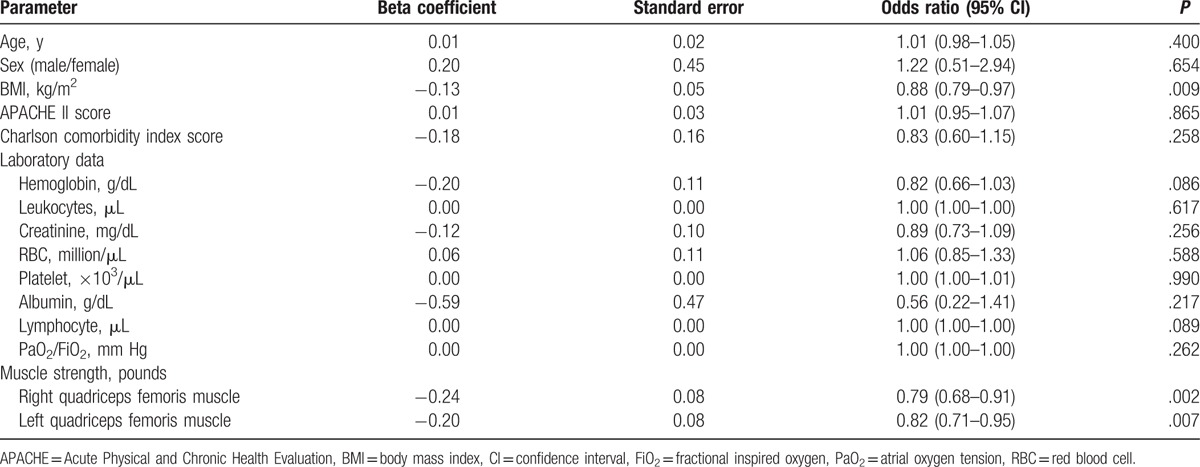
In the logistic regression analysis, we used nonsurvival (event) versus survival (nonevent) as the dependent variable, and the included potential risk factors were age, sex, BMI, CCI, APACHE II score, laboratory data, right quadriceps femoris muscle force, and left quadriceps femoris muscle force as the independent variables. We first used univariate logistic regression to assess the aforementioned factors to the risk (in terms of odds ratio [OR]) of in-hospital mortality. Table 2 shows the OR of in-hospital mortality according to the various predictors. In-hospital mortality was significantly associated with BMI, and right and left quadriceps femoris muscle force. Specifically, for each unit increase in BMI, the odds of in-hospital mortality significantly decreased by 12.0% (P = .009), disregarding the effects of other factors. Similarly, for each unit increase in the right or the left quadriceps femoris muscle force scores, the risks of in-hospital mortality significantly decreased by 21% and 18% (OR 0.79, 95% confidence interval [CI] 0.68–0.91; OR 0.82, 95% CI 0.71–0.95), respectively.
Table 2.
Univariate analysis of in-hospital mortality.
3.3. Multivariate analysis for risk of in-hospital death
The significant variables of BMI and right and left quadriceps femoris muscle force obtained from univariate analysis for risk of in-hospital death were examined in a multiple logistic regression model.
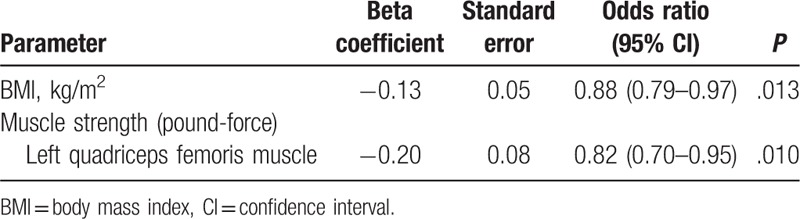
Because right and left quadriceps femoris muscle force were highly positively correlated (test for linear correlation using the Pearson product-moment correlation, r = 0.932, P < .001), attempting to use right and left quadriceps femoris muscle force in the same model to predict in-hospital death was inconvenient due to collinearity. We examined the association between the 2 factors (right and left quadriceps femoris muscle force) and in-hospital death separately with 2 multiple logistic regressions. After adjusting for the effects of right or left quadriceps femoris muscle force (Table 3, model I; and Table 4, model II, respectively), for every 1 kg/m2 increase in BMI at admission, the odds of in-hospital mortality significantly decreased by 12% (P = .013). After adjusting for the effects of BMI, for each unit increase in the right (Table 3) or the left (Table 4) quadriceps femoris muscle force, the odds of in-hospital mortality significantly decreased by 21% or 18% (P = .002 or .010, respectively). Therefore, 3 factors significantly contributed to the risk of in-hospital death, namely, BMI, right quadriceps femoris muscle force, and left quadriceps femoris muscle force.
Table 3.
Variables independently predictive of in-hospital mortality by logistic regression (model I).
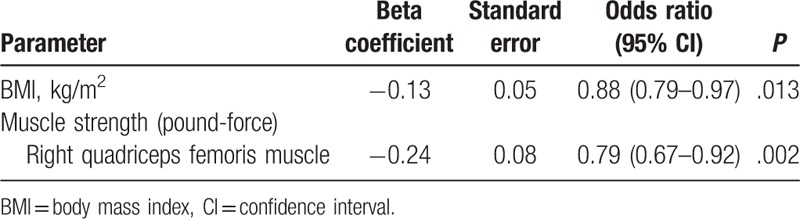
Table 4.
Variables independently predictive of in-hospital mortality by logistic regression (model II).
4. Discussion
The results of this study showed predictive factors for in-hospital mortality of ventilated ICU patients admitted for acute respiratory failure were BMI and scores for lower limb muscle force. Patients with lower BMI or lower scores on lower limb muscle force had a significantly lower survival rate. Other patient characteristics, such as age at admission, sex, CCI, or APACHE II score, were not associated with in-hospital death. This may, in part, reflect either the small size of the present study and therefore lower statistical power, or use of different study methodologies and definitions. Our study focused on in-hospital mortality as the primary outcome, but it must be recognized that this is only 1 of several potential outcomes of interest after prolonged ICU admission.
Age at admission was not a predictor of in-hospital mortality in our study. This finding differs from other studies of mortality in ICU patients that have found that older patients admitted to ICUs usually have more severe critical health conditions, greater physiological impairments, and higher mortality rates than patients in general medical wards.[13]
Our findings are consistent with previous studies that have shown lower BMI[6] and lower scores on limb muscle forces[1,8,9] are predictors of in-hospital mortality. Other studies also reported that low BMI,[14,15–17] low muscle strength,[18] or low skeletal muscle mass[19] increase the in-hospital mortality rate in ICU patients. However, these findings are in contrast with a retrospective study of 240 mechanically ventilated ICU patients by Weijs et al,[20] which showed that BMI is not an independent predictor of mortality when accounting for muscle area.
The strong association observed between low BMI and death might be a consequence of underlying disease, which leads to loss of weight and inadequate nutritional reserves to compensate for the stresses of critical illness. However, no firm conclusions could be drawn concerning the effect of BMI on the overall mortality rate. In fact, 1 study showed BMI did not accurately predict outcomes in older patients admitted to the ICU.[19] In addition, the association between BMI, percentage of body fat, and health risks for Asian populations differ from European populations.[17,21]
The present study shows that low scores on limb muscle forces is a potential predictor for in-hospital mortality. The exact underlying mechanisms of muscle mass losses are still unknown.[22] Studies that examined changes in body mass and composition in these patients observed a loss of lean tissue or protein loss from skeletal muscles.[22] Skeletal muscle is a major contributor to whole-body glucose and protein metabolism, especially glutamine, and helps maintain nutritional homeostasis and provides nutrients for the immune system and the rapid repair of tissue.[23] It is also important to note that hospitalization variables were also significantly greater for nonsurvivors than survivors such as days of MV before extubation, ICU lengths of stay, and hospital lengths of stay. This finding has been corroborated in several other studies.[1,8,18,19,22]
Some limitations of this study should be noted. Only 7.30% (113 out of 1547) patients were included in the analysis; the strict inclusion and exclusion criteria contributed to the small sample size. The study population consisted of stable ventilated patients admitted to 3 medical ICUs of a university teaching hospital and may not be generalized to patients hospitalized at other institutions. We did not collect data for subcutaneous fat levels, overall nutritional status, and electrolyte and water balance. In addition, weight and height data were not collected in the most stringent fashion, and fluid balance before weight measurement can affect the calculated BMI of many mechanically ventilated patients, potentially biasing the observed association between BMI and outcomes for these patients.[24] We did not formally establish inter-rater reliability among the clinicians who evaluated muscle strength, although there are studies showing handheld dynamometry strength has good inter-rater reliability.[25,26] The mechanisms underlying the higher mortality risk in ventilated ICU patients with lower BMI or poor muscle force remain to be elucidated. Limited duration of follow-up potentially underestimates the true impact of prolonged ICU admission.
5. Conclusions
Our findings suggest that lower BMI or lower scores on lower limb muscle force might contribute to an increase of in-hospital mortality rate in ventilated ICU patients. These findings reinforce the view that malnutrition and ICU acquired paresis are serious conditions with dramatic consequences that should be considered and tested for in ventilated ICU patients. New interventions and strategies to prevent or treat these conditions may also lead to reduction in short-term mortality. Additional research is needed to understand the contribution of subcutaneous fat levels, overall nutritional status, electrolyte and water balance, exact BMI range, and the appropriate threshold of quadriceps femoris muscle force at which ventilated ICU patients are at increased risk of death.
Acknowledgments
The authors would like to thank the nursing and medical staff of Chang Gung Memorial Hospital for their expert assistance.
Footnotes
Abbreviations: APACHE II = Acute Physiology and Chronic Health Evaluation II, BMI = body mass index, CCI = Charlson comorbidity index, ICU = intensive care unit, MV = mechanical ventilation.
Author contributions: C.-H. Wang and W.-R. Tang were responsible for the concept and design of the study, data collection, analysis and interpretation, and the writing of the paper. H.-C. Lin collected and interpreted the data, and revised the paper. Y.-C. Chang analyzed and interpreted the data, and critically reviewed the paper. S.-H. Maa and J.-S. Wang contributed to the concept and design of the study, interpretation of data, and revision of the paper.
The authors have no funding or conflicts of interest to disclose.
References
- [1].De Jonghe B, Bastuji-Garin S, Durand M-C, et al. Respiratory weakness is associated with limb weakness and delayed weaning in critical illness. Crit Care Med 2007;35:2007–15. [DOI] [PubMed] [Google Scholar]
- [2].Konrad F, Schreiber T, Brecht-Kraus D, et al. Mucociliary transport in ICU patients. Chest 1994;105:237–41. [DOI] [PubMed] [Google Scholar]
- [3].Pingleton SK. Complications of acute respiratory failure. Am Rev Respir Dis 1998;137:1463–93. [DOI] [PubMed] [Google Scholar]
- [4].Behrendt CE. Acute respiratory failure in the United States. Chest 2000;118:1100–5. [DOI] [PubMed] [Google Scholar]
- [5].Esteban A, Anzueto A, Frutos F, et al. Characteristics and outcomes in adults patients receiving mechanical ventilation, a 28-day international study. JAMA 2002;287:345–55. [DOI] [PubMed] [Google Scholar]
- [6].O’Brien MJ, Phillips GS, Ali NA, et al. Body mass index is independently associated with hospital mortality in mechanically ventilated adults with acute lung injury. Crit Care Med 2006;34:738–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Van Mook WN, Hulsewe-Evers RP. Critical illness polyneuropathy. Curr Opin Crit Care 2002;8:302–10. [DOI] [PubMed] [Google Scholar]
- [8].Ali NA, O’Brien JM, Hoffmann SP, et al. Acquired weakness, handgrip strength, and mortality in critically ill patients. Am J Respir Crit Care Med 2008;178:261–8. [DOI] [PubMed] [Google Scholar]
- [9].Sharshar T, Bastuji-Garin S, Stevens RD, et al. Presence and severity of intensive care unit-acquired paresis at time of awakening are associated with increased intensive care unit and hospital mortality. Crit Care Med 2009;37:3047–53. [DOI] [PubMed] [Google Scholar]
- [10].Zimmerman JE, Kramer AA, Knaus WA. Changes in hospital mortality for United States intensive care unit admissions from 1988 to 2012. Crit Care 2013;17:R81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Faul F, Erdfelder E, Buchner A, et al. Statistical power analyses using G∗Power 3.1: tests for correlation and regression analyses. Behav Res Methods 2009;41:1149–60. [DOI] [PubMed] [Google Scholar]
- [12].Faul F, Erdfelder E, Lang AG, et al. G∗Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods 2007;39:175–91. [DOI] [PubMed] [Google Scholar]
- [13].Bo M, Massaia M, Raspo S, et al. Predictive factors of in-hospital mortality in older patients admitted to a medical intensive care unit. J Am Geriatr Soc 2003;51:529–33. [DOI] [PubMed] [Google Scholar]
- [14].Pickker P, de Keizer N, Dusseljee J, et al. Body mass index is associated with hospital mortality in critically ill patients: an observational cohort study. Crit Care Med 2013;41:1878–83. [DOI] [PubMed] [Google Scholar]
- [15].Tremblay A, Bandi V. Impact of body mass index on outcomes following critical care. Chest 2003;123:1202–7. [DOI] [PubMed] [Google Scholar]
- [16].Garrouste-Orgeas M, Troche G, Azoulay E, et al. Body mass index: an additional prognostic factor in ICU patients. Intensive Care Med 2004;30:437–43. [DOI] [PubMed] [Google Scholar]
- [17].Yatabe T, Yamashita K, Yokoyama M. Lower body mass index is associated with hospital mortality in critically ill Japanese patients. Asia Pac J Clin Nutr 2016;25:534–7. [DOI] [PubMed] [Google Scholar]
- [18].Lee JJ, Waak K, Grosse-Sundrup M, et al. Global muscle strength but not grip strength predicts mortality and length of stay in a general population in a surgical intensive care unit. Phys Ther 2012;92:1546–55. [DOI] [PubMed] [Google Scholar]
- [19].Moisey L, Mourtzakis M, Cotton B, et al. Skeletal muscle predicts ventilator-free days, ICU-free days, and mortality in elderly ICU patients. Crit Care 2013;17:R206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Weijs PJM, Looijaard WGPM, Dekker IM, et al. Low skeletal muscle area is a risk factor for mortality in mechanically ventilated critically ill patients. Crit Care 2014;18:R12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004;363:157–63. [DOI] [PubMed] [Google Scholar]
- [22].Gruther W, Benesch T, Zorn C, et al. Muscle wasting in intensive care patients: ultrasound observation of the M. quadriceps femoris muscle layer. J Rehabil Med 2008;40:185–9. [DOI] [PubMed] [Google Scholar]
- [23].Lightfoot A, McArdle A, Griffiths RD. Muscle in defense. Crit Care Med 2009;37(suppl):S384–90. [DOI] [PubMed] [Google Scholar]
- [24].O’Brien JM, Jr, Philips GS, Ali NA, et al. The association between body mass index, processes of care, and outcomes from mechanical ventilation: A prospective cohort study. Crit Care Med 2012;40:1456–63. [DOI] [PubMed] [Google Scholar]
- [25].Vanpee G, Segers J, Mechelen HV, et al. The interobserver agreement of handheld dynamometry for muscle strength assessment in critically ill patients. Crit Care Med 2011;39:1929–34. [DOI] [PubMed] [Google Scholar]
- [26].Baldwin CE, Paratz JD, Bersten AD. Muscle strength assessment in critically ill patients with handheld dynamometry: an investigation of reliability, minimal detectable change, and time to peak force generation. J Crit Care 2013;28:77–86. [DOI] [PubMed] [Google Scholar]


