Abstract
Objective:
This meta-analysis aimed to illustrate the efficacy and safety of preganalin for pain management in patients with postherpetic neuralgia (PHN).
Methods:
In July 2017, a systematic computer-based search was conducted in PubMed, EMBASE, Web of Science, Cochrane Database of Systematic Reviews, and Google database. Data on patients with PHN that compared pregabalin versus placebo were retrieved. The endpoints were the visual analog scale (VAS) at 8 weeks, the percentage of 30% and 50% pain responders; sleep interference score and improvement in patient global impression of change (PGIC). After testing for publication bias and heterogeneity between studies, data were aggregated for random-effects models when necessary.
Results:
Seven clinical studies with 2192 patients (pregabalin group = 1381, control group = 811) were finally included in the meta-analysis. Pregabalin was associated with reduced pain scores at 8 weeks, corresponding to a reduction of 11.23 points (95% CI, −14.33, −8.13, P = .000) on a 100-point VAS. Pregabalin was also associated with a more percentage of 30% and 50% pain responders than controls (P < .05). Meanwhile, pregabalin can decrease sleep interference score and improvement in PGIC than control groups (P < .05).
Conclusions:
Pregabalin was efficacious in the reduction of postoperative pain and improvement the sleep quality in patients with PHN.
Keywords: meta-analysis, postherpetic neuralgia, pregabalin
1. Introduction
Postherpetic neuralgia (PHN) is the common types of neuropathic pain and the incidence of PHN rates increasing to 20% to 50% in the elderly.[1,2] Patients suffered with PHN experience intense pain and itching, as well as increased sensitivity to touch.[3] Tricyclic antidepressants were the first agents to control pain in PHN patients and were considered first-line therapy for many years, but their side-effect profiles may render their use in the elderly problematic.[4] Recently, anticonvulsant, opioid, and topical analgesics[5] have been found to have significant beneficial effects. However, their use is limited due to adverse effects such as nausea and vomiting.[6–8]
Pregabalin is an anticonvulsant agent that has an affinity to the alpha2delta subunit of voltage-dependent calcium channels and shows promising results in relieving chronic neuropathic pain.[9] Several randomized controlled trials (RCTs) have assess the efficacy of pregabalin in reducing pain in PNH patients.[10–12] Many of these trials contained relatively small samples and demonstrated inconsistent outcomes.[13,14] Additionally, more evidence is emerging, and it is necessary to reevaluate the efficacy and safety of pregabalin for pain control in PNH patients. This meta-analysis aimed to evaluate whether pregabalin can decrease pain intensity and whether high-dose pregabalin is superior to low-dose pregabalin.
2. Materials and methods
This systematic review was reported according to the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines.[15]
2.1. Search strategies
The following databases were searched in September 2016 without restrictions on location or publication types: PubMed (1950–July 2017), EMBASE (1974–July 2017), the Cochrane Library (July 2017 Issue 7), and the Google database (1950–July 2017). The Mesh terms and their combinations used in the search were as follows: “analgesia” OR “pain management” OR “anesthetic agents” OR “postherpetic neuralgia” OR “PNH” AND “pregabalin” [Mesh terms]. The reference lists of related reviews and original articles were searched for any relevant studies, including RCTs involving adult humans. Only articles originally written in English or translated into English were considered. When multiple reports describing the same sample were published, the most recent or complete report was used. This meta-analysis collected data from published articles and thus no ethic approval was necessary for this article.
2.2. Inclusion criteria and study selection
Patients: PNH patients that average pain scores of 4 or more on pain scale on the week before commencing study medication; Intervention: pregabalin used as a multimodal anesthetics as an intervention group; Comparison: placebo; Outcomes: visual analog scale (VAS) at 8 weeks, the percentage of 30% and 50% pain responders; sleep interference score and improvement in PGIC. Study design: RCTs. Two independent reviewers screened the title and abstracts of the identified studies after removing the duplicates from the search results. Any disagreements about the inclusion or exclusion of a study were solved by discussion or consultation with an expert. The reliability of the study selection was determined by Cohen kappa test, and the acceptable threshold value was set at 0.61.[6,7]
2.3. Data abstraction
A specific extraction was conducted to collect data in a pregenerated standard Microsoft Excel (Microsoft Corporation, Redmond, WA) file. The items extracted from relevant studies were as follows: first author and publication year, country, sample size of the intervention and control groups, preoperative and postoperative doses, timing and frequency and the total dose of gabapentin per number of days and follow-ups. Outcomes such as the VAS at 8 weeks, the percentage of 30% and 50% pain responders; sleep interference score and improvement in PGIC were abstracted and recorded in the spreadsheet. We define 30% and 50% pain responders as ≥30% and 50% decrease on the pain VAS at randomization compared with screening. Postoperative pain intensity was measured using a 110-point VAS (0 = no pain and 110 = extreme pain). When the numerical rating scale (NRS) was reported, it was converted to a VAS. Additionally, a 10-point VAS was converted to a 100-point VAS.[16] Data in other forms (i.e., median, interquartile range, and mean ± 95% confidence interval (CI)) were converted to the mean ± standard deviation (SD) according to the Cochrane Handbook.[17] If the data were not reported numerically, we extracted these data using the “GetData Graph Digitizer” software from the published figures. All the data were extracted by 2 independent reviewers, and disagreements were resolved by discussion.
2.4. Quality assessment
The quality of all included trials was independently assessed by 2 reviewers on the basis of the Cochrane Handbook for Systematic Reviews of Interventions, version 5.1.0 (http://handbook.cochrane.org/).[17] A total of 7 domains were used to assess the overall quality: random sequence generation, allocation concealment, blinding of participant and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, other bias, general characteristic balance, and intent to treat. Each domain was measured as low bias, unclear bias, or high bias under the guidance of Cochrane Handbook for Systematic Reviews of Interventions.[17]
2.5. Outcome measures and statistical analysis
Continuous outcomes (VAS at 8 weeks, sleep interference score and improvement in PGIC) were expressed as the weighted mean differences (WMD) with 95% CI. Dichotomous outcomes (the percentage of 30% and 50% pain responders) were expressed as a risk ratio (RR) with 95% CI. Statistical significance was set at P < .05 to summarize the findings across the trials. Variables in the meta-analysis were calculated using Stata software, version 12.0 (Stata Corp., College Station, TX). Statistical heterogeneity was evaluated using the Chi-square test and the I2 statistic. When there was no statistical evidence of heterogeneity (I2 < 50%, P > .1), a fixed-effects model was adopted; otherwise, a random-effects model was chosen. Publication bias was tested using funnel plots. Publication bias was visually assessed using funnel plots and was quantitatively assessed using Begg test.
Subgroup analysis was conducted according to the dose of pregabalin (<300 mg/d was identified as low dose, and ≥300 mg/d was identified as high dose). We considered there to be no publication bias if the funnel plot was symmetrical and the P-value was >.05. In addition, we calculated the number needed to harm (NNH) and the number need to treat (NNT) to examine the risks compared to the benefits of pregabalin therapy as it regarded complications.[18] The relationship between pregabalin dosage and the VAS at 8 weeks was explored using SPSS software (SPSS, Inc., Chicago, IL). The correlation coefficient (r) was used to evaluate the relationship between the dosage of gabapentin and the VAS at 8 weeks and the percentage of 30% and 50% pain responders.
3. Results
3.1. Search results
In the initial search, a total of 505 studies were identified from the electronic databases (PubMed = 107, EMBASE = 92, Web of Science = 160, Cochrane Library = 48, Google database = 98). Then, all papers were input into Endnote X7 (Thomson Reuters Corp., USA) software for the removal of duplicate papers. A total of 212 papers were reviewed and 205 papers were removed according to the inclusion criteria at abstract and title levels. Ultimately, 7 RCTs with 2192 patients (pregabalin group = 1381, control group = 811) were included in the meta-analysis.[10–21] The flow diagram for the included studies can be seen in Fig. 1.
Figure 1.

Flowchart of study search and inclusion criteria.
One study administered 4 different doses of gabapentin (300, 600, 900, and 1200 mg/d) versus placebo and the study was divided into 4 arms. One study adopted three different gabapentin doses (600, 900, and 1200 mg/d) and with different times of oral administration (preoperative and postoperative), this study was divided into 6 arms. The general characteristics of the included studies can be seen in Table 1.
Table 1.
The general characteristic of the included studies.
3.2. Quality assessment
The risk of bias summary and risk of bias graph are summarized in Figs. 2 and 3 respectively. Only 2 studies did not describe the random sequence generation procedure; the remaining 5 RCTs performed appropriate random sequence generation and listed as low risk of bias. Four studies did not describe allocation concealment and listed as unclear risk of bias. In addition, the risks of bias for blinding to the outcome assessment were unclear in 4 studies. The overall kappa value regarding the evaluation of the risk of bias of included RCTs was 0.805, indicating that the agreement between the 2 reviewers was acceptable.
Figure 2.
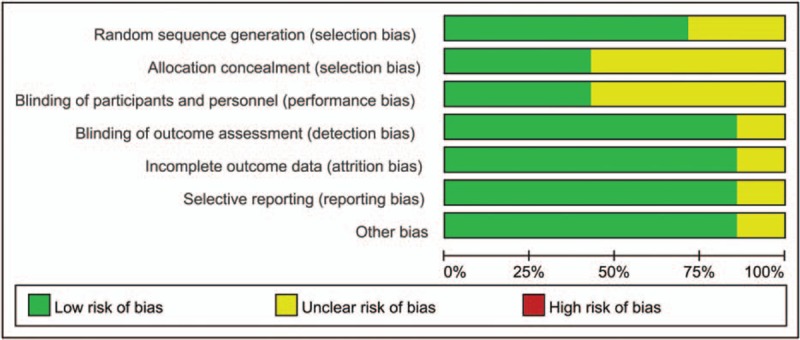
The risk of bias graph.
Figure 3.
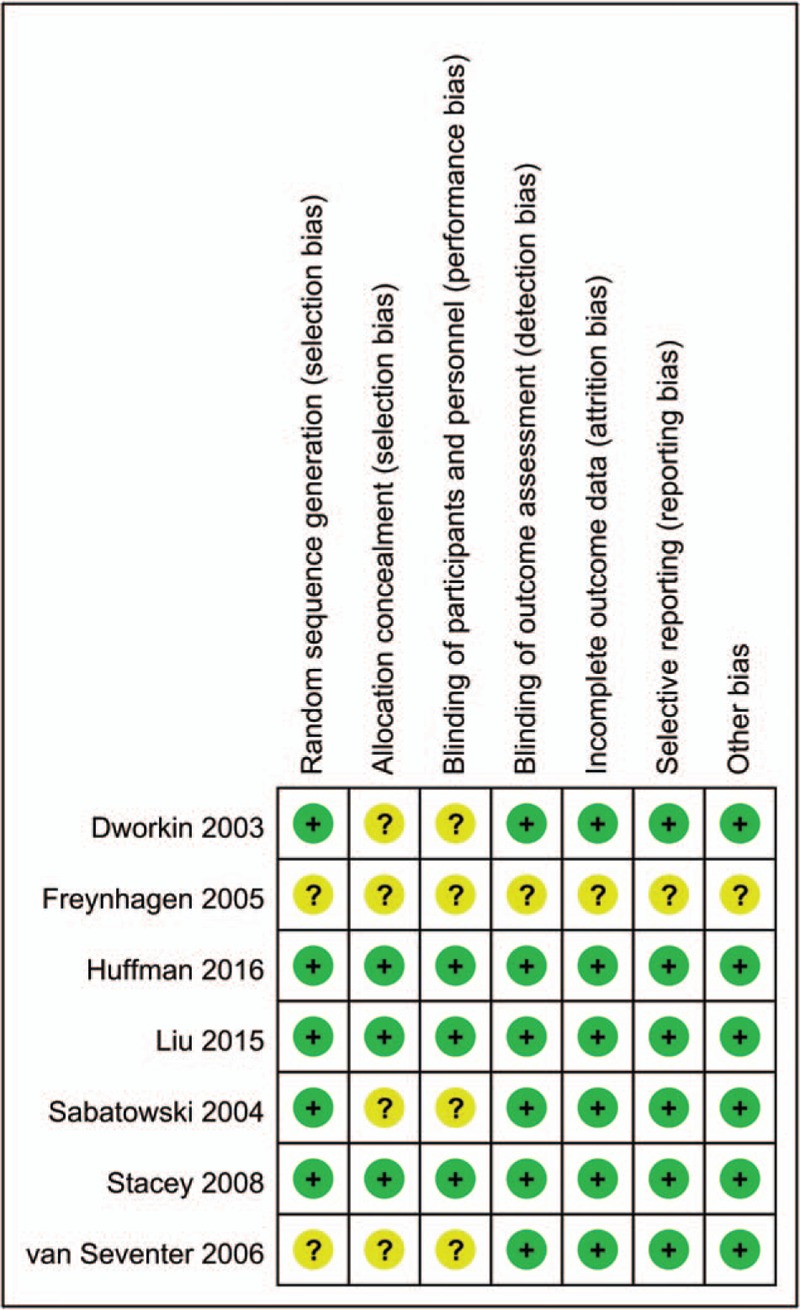
Risk of bias of included randomized controlled trials. +, no bias; –, bias; ?, bias unknown.
3.3. Results of the meta-analysis
3.3.1. VAS at 8 weeks
Postoperative VAS scores at 8 weeks were reported in 13 studies, and the pooled results indicated that administration of pregabalin can decrease VAS scores at 8 weeks (WMD = −11.23, 95% CI, −14.33, −8.13, P = .000, Fig. 4).
Figure 4.
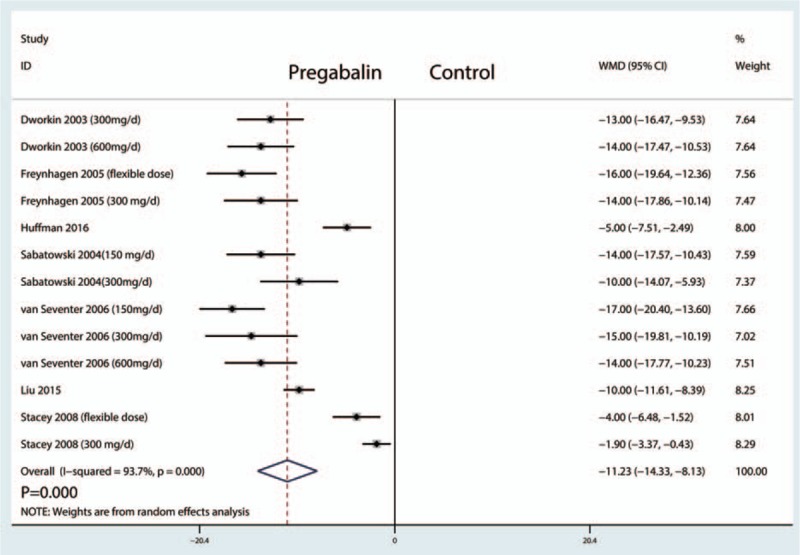
Forest plots of the included studies comparing the VAS at 8 weeks.
Funnel plots (Fig. 5 A) and Begg tests (P = .008, Fig. 5B) were performed, and the results indicated that there was publication bias between the included studies in terms of the VAS at 8 weeks. A sensitivity analysis was then conducted to analyze the source of heterogeneity between the studies, and the results indicated that none of the included studies affected the final results (Fig. 6).
Figure 5.
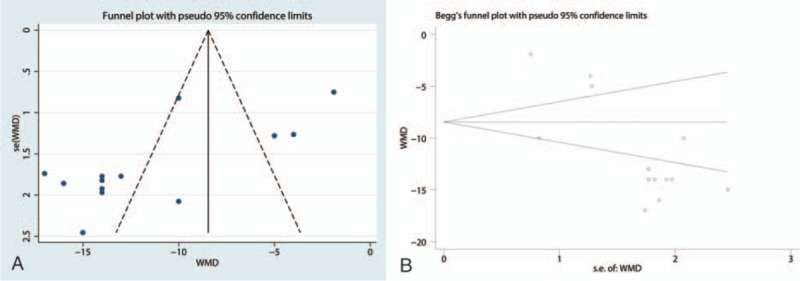
(A) Funnel plot of VAS at 8 weeks between pregabalin group and control group, (B) Begg test of VAS at 8 weeks.
Figure 6.
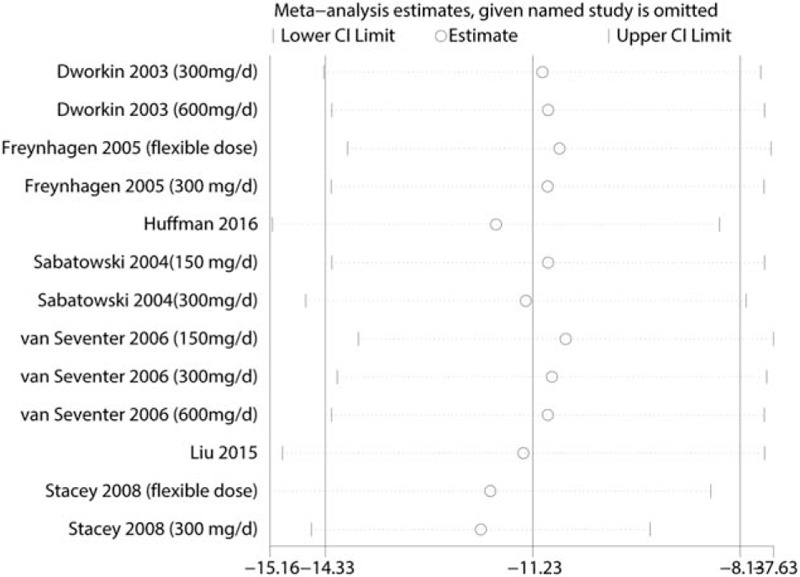
Sensitivity analysis of the VAS at 8 weeks.
3.3.2. Dose–effect relationship and meta-regression
We plotted the pregabalin dose on the abscissa, with the corresponding VAS scores at 8 weeks as the ordinate, to generate a scatterplot. In addition, the linear correlation coefficient (r) was also calculated. There was no negative correlation between the dosage of pregabalin and the VAS at 8 weeks (r = −0.503, P = .737; Fig. 7). The VAS at 8 weeks tended to decrease as the pregabalin dose increased. What's more, we performed a meta-regression about the dose of pregabalin on the VAS at 8 weeks.
Figure 7.
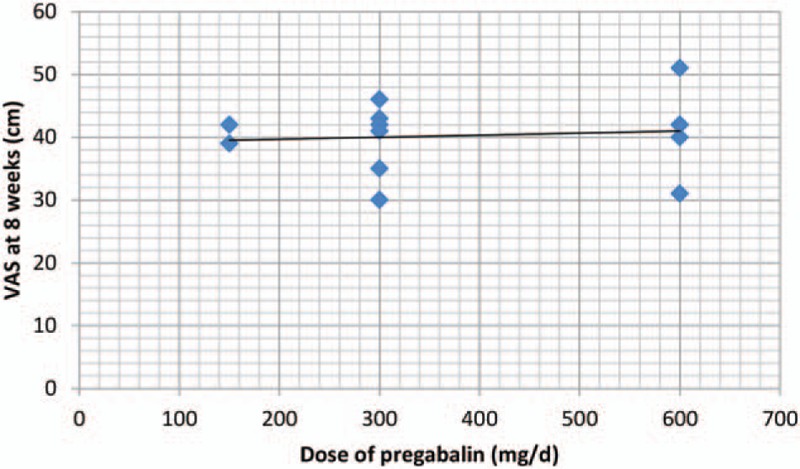
Scatter plot showing the relationship between the dose of pregabalin and the VAS at 8 weeks.
3.3.3. The percentage of 30% pain responders
The percentage of 30% pain responders were reported in 13 studies, and the pooled results indicated that administration of pregabalin has more percentage of 30% pain responders than control groups (RR = 1.09, 95% CI, 1.02, 1.18, P = .016, Fig. 8).
Figure 8.
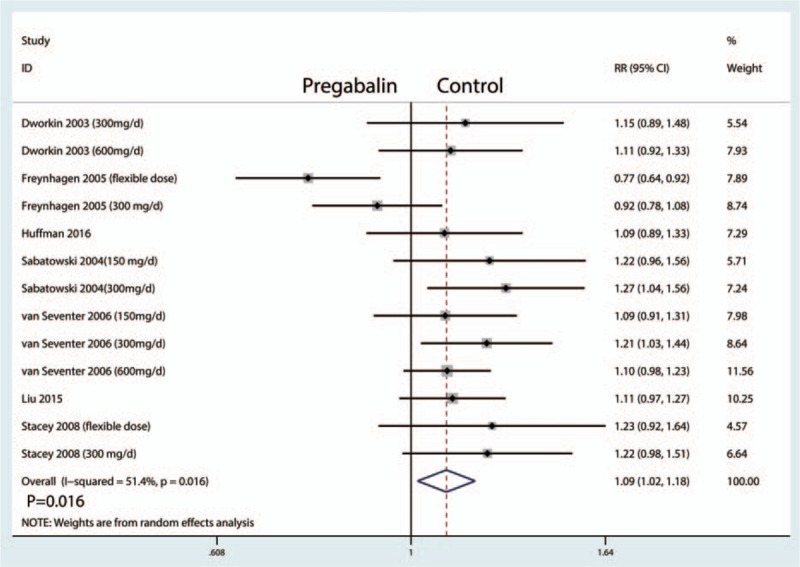
Forest plots of the included studies comparing the percentage of 30% pain responders.
3.3.4. The percentage of 50% pain responders
The percentage of 50% pain responders were reported in 10 studies, and the pooled results indicated that administration of pregabalin has more percentage of 50% pain responders than control groups (RR = 1.15, 95% CI, 1.03, 1.29, P = .010, Fig. 9) (Table 2).
Figure 9.
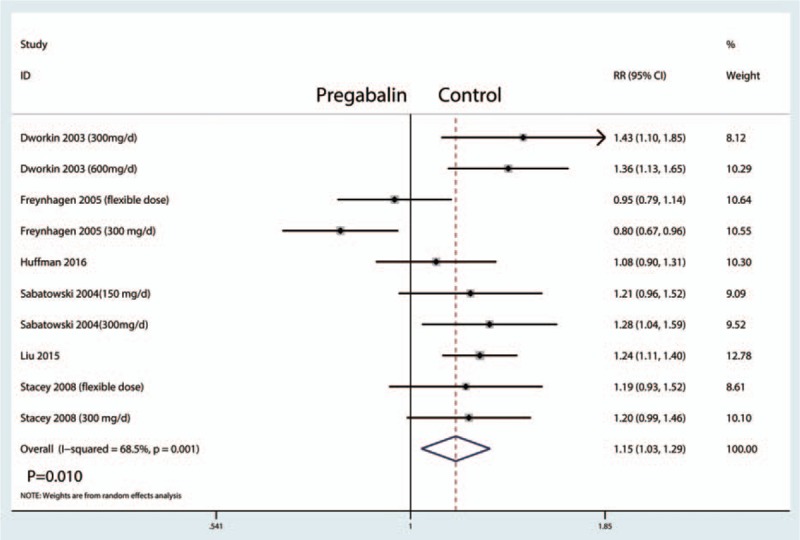
Forest plots of the included studies comparing the percentage of 50% pain responders.
Table 2.
Subgroup analysis of the VAS at 8 weeks, the percentage of 30% pain responders, the percentage of 50% pain responders, sleep interference score, and improvement in PGIC.

3.3.5. Sleep interference score
The sleep interference score were reported in 10 studies, and the pooled results indicated that administration of pregabalin can decrease sleep interference score than control groups (WMD = −10.71, 95% CI, −13.74, −7.68, P = .000, Fig. 10).
Figure 10.
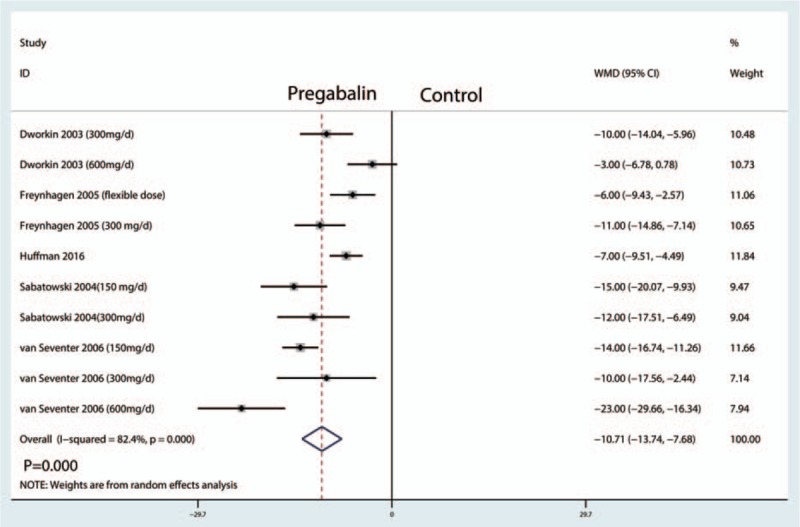
Forest plots of the included studies comparing the sleep interference score.
3.3.6. Improvement in PGIC
The improvement in PGIC were reported in 9 studies, and the pooled results indicated that administration of pregabalin can increase the improvement in PGIC than control groups (WMD = 14.20, 95% CI, 9.84, 18.55, P = .000, Fig. 11).
Figure 11.

Forest plots of the included studies comparing the improvement in PGIC.
4. Discussion
Current meta-analysis indicated that oral pregabalin was effective for the treatment of PHN patients with a significant decrease pain scores at 8 weeks, improvement in 30% and 50% pain responders, sleep interference scores, and PGIC than placebo groups. What's more important finding was that high dose of pregabalin was more effective than low dose of pregabalin.
A major strength of current meta-analysis was that we comprehensively searched the electronic databases and finally with strict statistical calculations. Another strength was that we performed a dose–effect relationship between the dose of pregabalin with the VAS at 8 weeks and the percentage of 30% and 50% pain responders. Results indicated that there was a negative correlation between the pregabalin dose with the VAS at 8 weeks and a positive correlation between the pregabalin dose with the percentage of 30% and 50% pain responders. Yin et al[22] performed a meta-analysis that compared pregabalin with placebo groups, however, they leave out 3 important studies, what's more, they did not perform the dose response relationship between the pregabalin dose and the VAS score and the percentage of 30% and 50% pain responders. Edelsberg et al[23] performed a systematic review of RCTs on the efficacy, safety, and tolerability of different drugs used to treat PHN patients and final results does not provide adequate guidance as to which agents are best to treat PNH.
Current-meta-analysis indicated that pregabalin can decrease VAS at 8 weeks by appropriately 11.23 points. Salah et al[24] reported that anticonvulsants can improve short-term pain intensity (standard mean difference (SMD) = −0.484, 95% CI, −0.622 to −0.346, P < .001). Nerve sensitization and afferent nerve block are the main factors that induce PHN. Based on these theories, pregabalin is recommended as the first-line treatment for PHN by the American Academy of Neurology and European Federation of Neurological Societies.
What's more, pregabalin can increase the percentage of 30% and 50% pain responders after 6 weeks treatment. If the percentage of pain responder was less than 30% and thus means the treatment was failed. At this situation, combined treatments of pregabalin and other medications should be considered. We then evaluated the effects of pregabalin for the sleep interference score. Meta-analysis indicated that pregabalin can decrease the sleep interference score by appropriately 10.71 points. And it can also increase the improvement in PGIC than control groups. Vinik et al[25] pregabalin treatment could not only improve sleep quality but also improve functional outcome.
We must consider the costs of pregabalin for the treatment of PNH. Wang et al[26] reported that pregabalin is an effective treatment for PHN and even for peripheral neuropathic pain extensively, but at increased cost. And total medical costs were similar at before approval and after approval.[27]
There were several limitations in this meta-analysis: only 7 RCTs were included, which might have affected the precision of the effect size estimations; follow-up in the included studies ranged from 8 to 13 weeks, and the relatively short-term follow-up may underestimate the cure effects; dosage and interval of pregabalin administration differed between the studies, and although a subgroup analysis was conducted to decrease the heterogeneity, that could affect the precision of the results; multiple analgesic approaches differed from each other, and consistent multiple analgesic approaches are needed to identify the most effective pain control method; and final results are presented with a high heterogeneity and will influence the precision of the outcomes.
5. Conclusion
In conclusion, pregabalin has a long-term analgesic efficacy and a significantly reduction of sleep interference score. What's more, pregabalin can increase increasing the percentage of 30% and 50% pain responders than control groups. Because the sample size and the number of included studies were limited, a multicenter RCT is needed to identify the effects of pregabalin in reducing acute pain for patients with PNH.
Footnotes
Abbreviations: CI = confidence interval, NNH = number needed to harm, NNT = number need to treat, NRS = numerical rating scale, PRISMA = preferred reporting items for systematic reviews and meta-analyses, RCTs = randomized controlled trials, RR = risk ratio, SD = standard deviation, SMD = standard mean difference, VAS = visual analog scale, WMD = weighted mean differences.
The authors have no conflicts of interest to disclose.
References
- [1].Kim HJ, Ahn HS, Lee JY, et al. Effects of applying nerve blocks to prevent postherpetic neuralgia in patients with acute herpes zoster: a systematic review and meta-analysis. Korean J Pain 2017;30:3–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Yong YL, Tan LT, Ming LC, et al. The effectiveness and safety of topical capsaicin in postherpetic neuralgia: a systematic review and meta-analysis. Front Pharmacol 2016;7:538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Dosenovic S, Jelicic Kadic A, Miljanovic M, et al. Interventions for neuropathic pain: an overview of systematic reviews. Anesth Analg 2017;125:643–52. [DOI] [PubMed] [Google Scholar]
- [4].Hadley GR, Gayle JA, Ripoll J, et al. Post-herpetic neuralgia: a review. Curr Pain Headache Rep 2016;20:17. [DOI] [PubMed] [Google Scholar]
- [5].Galer BS, Rowbotham MC, Perander J, et al. Topical lidocaine patch relieves postherpetic neuralgia more effectively than a vehicle topical patch: results of an enriched enrollment study. Pain 1999;80:533–8. [DOI] [PubMed] [Google Scholar]
- [6].Dolin SJ, Cashman JN. Tolerability of acute postoperative pain management: nausea, vomiting, sedation, pruritus, and urinary retention. Evidence from published data. Br J Anaesth 2005;95:584–91. [DOI] [PubMed] [Google Scholar]
- [7].Pavlin DJ, Chen C, Penaloza DA, et al. Pain as a factor complicating recovery and discharge after ambulatory surgery. Anesth Analg 2002;95:627–34. table of contents. [DOI] [PubMed] [Google Scholar]
- [8].Sun XL, Zhao ZH, Ma JX, et al. Continuous local infiltration analgesia for pain control after total knee arthroplasty: a meta-analysis of randomized controlled trials. Medicine (Baltimore) 2015;94:e2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Shahid M, Subhan F, Ahmad N, et al. Topical gabapentin gel alleviates allodynia and hyperalgesia in the chronic sciatic nerve constriction injury neuropathic pain model. Eur J Pain 2016. [DOI] [PubMed] [Google Scholar]
- [10].Dworkin RH, Corbin AE, Young JP, Jr, et al. Pregabalin for the treatment of postherpetic neuralgia: a randomized, placebo-controlled trial. Neurology 2003;60:1274–83. [DOI] [PubMed] [Google Scholar]
- [11].Sabatowski R, Galvez R, Cherry DA, et al. Pregabalin reduces pain and improves sleep and mood disturbances in patients with post-herpetic neuralgia: results of a randomised, placebo-controlled clinical trial. Pain 2004;109:26–35. [DOI] [PubMed] [Google Scholar]
- [12].van Seventer R, Feister HA, Young JP, Jr, et al. Efficacy and tolerability of twice-daily pregabalin for treating pain and related sleep interference in postherpetic neuralgia: a 13-week, randomized trial. Curr Med Res Opin 2006;22:375–84. [DOI] [PubMed] [Google Scholar]
- [13].Huffman CL, Goldenberg JN, Weintraub J, et al. Efficacy and safety of once-daily controlled-release pregabalin for the treatment of patients with postherpetic neuralgia: a double-blind, enriched enrollment randomized withdrawal, placebo-controlled trial. Clin J Pain 2017;33:569–78. [DOI] [PubMed] [Google Scholar]
- [14].Liu Q, Chen H, Xi L, et al. A randomized, double-blind, placebo-controlled trial to evaluate the efficacy and safety of pregabalin for postherpetic neuralgia in a population of Chinese patients. Pain Pract 2017;17:62–9. [DOI] [PubMed] [Google Scholar]
- [15].Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wang C, Cai X-Z, Yan S-G. Comparison of periarticular multimodal drug injection and femoral nerve block for postoperative pain management in total knee arthroplasty: a systematic review and meta-analysis. J Arthroplasty 2015;30:1281–6. [DOI] [PubMed] [Google Scholar]
- [17].Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions version 5.1.0. Available at: http://handbook.cochrane.org/. 2011. [Google Scholar]
- [18].Laupacis A, Sackett DL, Roberts RS. An assessment of clinically useful measures of the consequences of treatment. N Engl J Med 1988;318:1728–33. [DOI] [PubMed] [Google Scholar]
- [19].Freynhagen R, Strojek K, Griesing T, et al. Efficacy of pregabalin in neuropathic pain evaluated in a 12-week, randomised, double-blind, multicentre, placebo-controlled trial of flexible- and fixed-dose regimens. Pain 2005;115:254–63. [DOI] [PubMed] [Google Scholar]
- [20].Huffman CL, Goldenberg JN, Weintraub J, et al. Efficacy and safety of once-daily controlled-release pregabalin for the treatment of patients with postherpetic neuralgia: a double-blind, randomized withdrawal, placebo-controlled trial. Clin J Pain 2016. [DOI] [PubMed] [Google Scholar]
- [21].Ogawa S, Suzuki M, Arakawa A, et al. [Long-term efficacy and safety of pregabalin in patients with postherpetic neuralgia: results of a 52-week, open-label, flexible-dose study]. Masui 2010;59:961–70. [PubMed] [Google Scholar]
- [22].Yin J, Pan Y, Zeng Z, et al. Efficacy of pregabalin in the treatment of postherpetic neuralgia: a meta-analysis of randomized controlled trials. Int J Clin Exp Med 2016;9:20693–701. [Google Scholar]
- [23].Edelsberg JS, Lord C, Oster G. Systematic review and meta-analysis of efficacy, safety, and tolerability data from randomized controlled trials of drugs used to treat postherpetic neuralgia. Ann Pharmacother 2011;45:1483–90. [DOI] [PubMed] [Google Scholar]
- [24].Salah S, Thomas L, Ram S, et al. Systematic review and meta-analysis of the efficacy of oral medications compared with placebo treatment in the management of postherpetic neuralgia. J Oral Facial Pain Headache, 30: 2016, 255–266. [DOI] [PubMed] [Google Scholar]
- [25].Vinik A, Emir B, Cheung R, et al. Relationship between pain relief and improvements in patient function/quality of life in patients with painful diabetic peripheral neuropathy or postherpetic neuralgia treated with pregabalin. Clin Ther 2013;35:612–23. [DOI] [PubMed] [Google Scholar]
- [26].Wang BC, Liu D, Furnback WE, et al. The cost-effectiveness of pregabalin versus gabapentin for peripheral neuropathic pain (pNeP) and postherpetic neuralgia (PHN) in China. Pain Ther 2016;5:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Honda M, Murata T, Ebata N, et al. Treatment patterns of postherpetic neuralgia patients before and after the launch of pregabalin and its effect on medical costs: analysis of Japanese claims data provided by Japan Medical Data Center. J Dermatol 2017;44:767–73. [DOI] [PubMed] [Google Scholar]


