Abstract
Rationale:
Low-grade myofibroblastic sarcoma (LGMS) is a malignant lesion composed of myofibroblasts. It is an uncommon tumor of unknown etiology that mainly develops in the bone or soft tissue and is most often reported in the head and neck, particularly in the tongue and oral cavity.
Patient concerns:
A 2-year-old girl, previously well and with no significant medical history or family history of other diseases, presented with a 2-week painless swelling of the right orbit.
Diagnoses:
Preoperative computed tomography (CT) revealed a large homogeneous enhanced mass, 21 × 13 mm in size, located on lateral wall of the right orbit with bone absorption. The mass was resected and histopathological examination revealed LGMS of the orbit.
Interventions:
On May 2016, she underwent surgery without the additional postoperative treatment.
Outcomes:
The patient's postoperative course was uneventful, and was discharged on the 6th day after surgery. During a year follow-up period, there was no recurrence of the postoperative CT. The patient and her family were satisfied with the result of the surgery.
Lessons:
Based on clinical characteristics and postoperative CT, we considered the mass may be a benign tumor. We completely resected along the capsule without an extensive surgical margin. However, postoperative histopathology diagnose LGMS, which shows a strong potential for local recurrence and vascular invasion. So we should close observation of the patient's symptoms and sign. If the tumor has invaded adjacent tissues, we will use adjuvant chemotherapy or radiotherapy.
Keywords: clinical characteristics, low-grade myofibroblastic sarcoma, mesenchymal tumor, orbit
1. Introduction
Myofibroblastic sarcoma was reported as a distinct entity in 1998 by Mentzel.[1] Low-grade myofibroblastic sarcoma (LGMS) is an uncommon solid tumor of mesenchymal origin that usually develops in the head and neck.[2] LGMS consists mainly of myofibroblasts, which are spindle-shaped mesenchymal cells with ultrastructural features of both fibroblasts and smooth muscle cells.[3] Some myofibroblastic tumors have relatively benign or only low-grade malignant characteristics.[4] LGMS has been most frequently reported in adult men,[5] and has been found in a wide variety of tissues, including skin, breast, vulva, salivary glands, parapharyngeal space, jaw, larynx, nasal cavity/paranasal sinuses, soft tissue of the cheek, piriform fossa, soft palate, and tongue.[6–18] Reports of LGMS of the orbit are extremely rare. Here, the authors report a case of a 2-year-old girl with LGMS of the orbit and discuss its clinical manifestations, pathological characteristics, diagnosis, therapy, and prognosis. The previous literature is reviewed.
2. Case report
This study was approved by the Ethics Committee of Shengjing Hospital of China Medical University. Written informed consent was obtained from the patient's parent. A 2-year-old girl, previously well and with no significant medical history or family history of other diseases, presented with a 2-week painless swelling of the right orbit (Fig. 1A and B). On physical examination a palpable mass about the size of a quail egg was found to be located at the right orbit. The mass had unclear boundaries, was fairly immobile, and was not tender. Bilateral vision and eye movements were unaffected. Computed tomography (CT) showed a large homogeneous enhanced mass, 21 × 13 mm in size, located on lateral wall of the right orbit with bone absorption (Fig. 2A–E).
Figure 1.
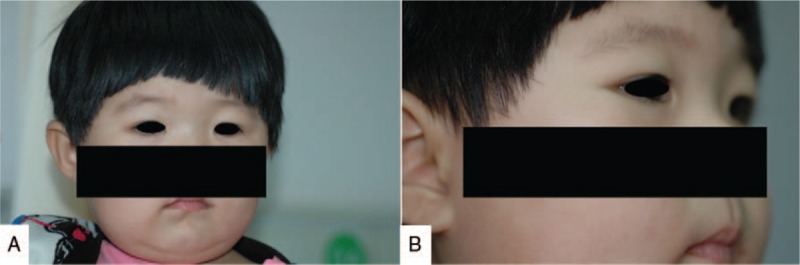
Preoperative photographs. The mass is located at the lateral aspect of the right orbit. A, Front view. B, Lateral view.
Figure 2.
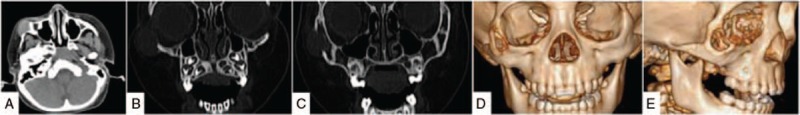
Three-dimensional computed tomography showing the soft tissue mass on the lateral wall of the right orbit. The density is uniform and the size is approximately 21 × 13 mm. There is bony destruction extending to the lateral wall of the right orbit from the zygomatic bone. A, Axial view. B and C, Coronal view. D and E, Three-dimensional reconstruction.
The patient underwent open surgery under general anesthesia for excision of the mass, which was exposed via a right lower eyelid approach. The mass had an intact capsule measuring 2.5 × 2.5 × 1.5 cm and was completely resected along the capsule. Macroscopically, the tumor was round, firm, smooth, and well circumscribed, measured approximately 2.0 × 1.5 × 1.5 cm, and had a yellowish-gray and homogeneous surface on transection (Fig. 3A–D).
Figure 3.
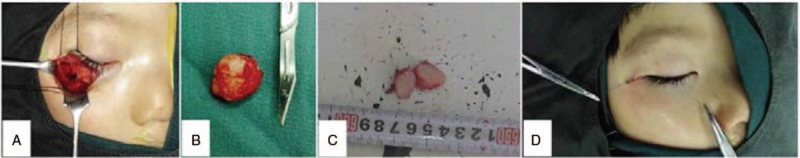
Intraoperative findings: (A) incision was made via the lower eyelid. The mass was located on the lateral right orbit. There was visible destruction of the bony wall of the right orbit. B, The mass was completely encapsulated and measured 2.0 × 1.5 × 1.5 cm. C, The tumor was uniform on transection and exhibited a yellowish and gray surface with a firm and rubbery texture. D, Suturing the incision.
Histopathologic examination confirmed the diagnosis of LGMS. Microscopic examination showed spindle-shaped tumor cells that were arranged in a fascicular or storiform pattern infiltrating the adjacent bone trabecula. The cells were demonstrated nuclear atypia with mitotic figures. The cytoplasm was acidic and the stroma consisted of a small number of collagen fibers. On immunohistochemical staining, the spindle-shaped cells were strongly and diffusely positive for smooth muscle actin (SMA) and vimentin (Fig. 4C and D). The Ki-67 index was >5% (Fig. 4E), and staining for calponin, NP, and S-100 were negative (Fig. 4F–H).
Figure 4.
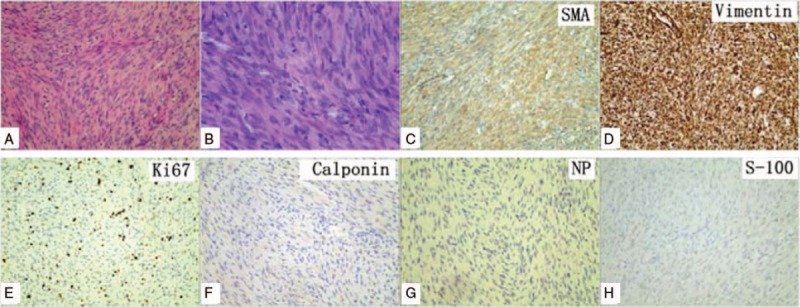
A and B, At lower magnifications, the tumor was composed of loosely arranged cells in a myxoid background (hematoxylin and eosin). A, Magnification × 100. B, Magnification ×400. C–H, On immunostaining, cells were strongly positive for smooth muscle actin (SMA) and vimentin, the Ki-67 index was >5%, and calponin, NP, and S-100 were negative.
The postoperative course was uneventful and the patient did not receive adjunctive therapy. The patient was discharged on the 14th day after surgery. No additional treatment was necessary, and there was no recurrence or metastasis of the postoperative CT during a year follow-up period (Figs. 5 and 6).
Figure 5.
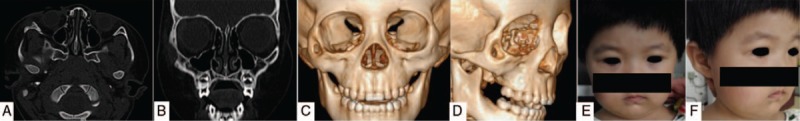
Follow-up evaluation at 3 months postoperatively. There is no evidence of recurrence and the affected area of bone is healing. A, Axial view. B, Coronal view. C and D, Three-dimensional reconstruction. E and F, Frontal and right lateral photographs of the patient.
Figure 6.
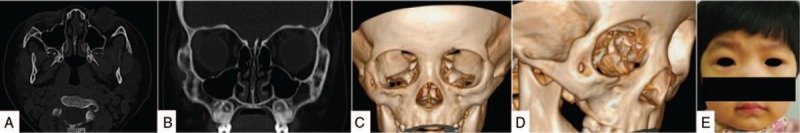
The patient remains recurrence-free at a year postoperatively, with additional healing of the affected bone. A, Axial view. B, Coronal view. C and D, Three-dimensional reconstructions. E, Frontal-view photograph of the patient.
3. Discussion
The World Health Organization classification of soft-tissue tumors describes LGMS as an atypical myofibroblastic proliferation with fibromatosis-like features that most commonly develop in the head and neck.[19] LGMS is a low-grade mesenchymal malignancy composed of myofibroblasts that can occur in submucosal and subcutaneous tissues, deep soft tissues, and intraosseous areas.[20] Myofibroblasts are modified fibroblasts that are morphologically and functionally similar to both fibroblasts and smooth muscle cells.[21] They were first reported in granulation tissue and can also arise in the stroma of normal, inflammatory, neoplastic, or reactive soft tissue lesions.[3,22] LGMS shows a strong potential for local recurrence, while vascular invasion and malignant transformation occur only occasionally.[23] In some cases, LGMS has an incomplete capsule and may infiltrate adjacent fibrous tissue, fat, or skeletal muscle.[21]
While LGMS is most commonly found in the head and neck, particularly in the tongue and oral cavity, it has also been reported in almost every other organ.[24] The mean age of affected patients is 40 years, ranging from 4 to 85 years, and a preponderance of adult men is reported.[25] The tumors generally present as a painless swelling or a slow-growing mass with a relatively indolent course.[26] Some patients may present with pyrexia, chills, leukocytosis, or meningeal irritation.[23]
Although preoperative CT or magnetic resonance imaging is essential for diagnosis, LGMS is hardly diagnosed definitely by them. In vivo molecular imaging is an innovative and cornerstone method for clinical diagnosis for tumors. The new method collects information at the molecular and cellular levels in humans.[27] Therefore, new molecular imaging may be an effective method for preoperative diagnosis, therapy as well as prognosis in malignant tumors, especially occurred in the head and neck regions. Of molecular imaging methodologies, 3 main techniques are intriguing. The first one is nuclear medical imaging, in which radioactive molecular probe is utilized and its distribution and kinetics are measured by positron emission tomography (PET) and single-photon emission computed tomography.[27] Probes for nuclear molecular imaging were developed to image intact function difference between wild-type and the transduced type F98 gliomas in cat brain. 18F-labeled amino acids for PET/CT imaging were utilized to show elevated amino acid metabolism in gliomas, neuroendocrine tumors, prostate cancer, and breast cancer.[27–29] A number of amino acid PET radiotracers were helpful in pretreatment evaluation of pediatric low-grade gliomas.[30] The second one is optical molecular imaging, in which a fluorescent probe is used to provide real-time imaging of distribution and kinetics. Although high spatial resolution could be obtained inexpensively, the imaging region is limited to the body surface.[31] The third one is photoacoustic imaging, which could detect highly sensitive and high-resolution photoacoustic signals in deep tissues.[32] Thus, we speculated that new molecular imaging techniques could be helpful for preoperative diagnosis, therapy, and prognosis estimate in LGMS. Unfortunately, due to the lack of the new molecular imaging system, this patient was not performed these specific examinations before surgery. Because cytogenetic and molecular genetic alterations of LGMS are presently obscure, in vivo molecular imaging could be tested in this disease in the future.
A definitive diagnosis of LGMS is made mainly on the basis of histopathological findings and immunohistochemical analyses.[33] Histologically, LGMS is mainly composed of slender spindle cells showing low mitotic activity and variable nuclear pleomorphism.[24] The nucleus has been described as fusiform, slender, and undulate.[6] The tumor cells are arranged in sheet-like interlacing fascicles or storiform patterns, and have eosinophilic cytoplasm with sparse inflammatory lymphocytic and plasmacytic infiltration.[4] The interstitial tissue may consist of hyalinized collagen fibers.[34] Immunohistochemical staining may be positive, to some degree, for SMA, muscle specific antigen, fibronectin, desmin, calponin, and vimentin. CD34 and CD99 are also positive in a small fraction, while anaplastic lymphoma kinase (ALK), S-100, and epithelial markers, including cytokeratin and epithelial membrane antigen, and laminin are generally negative.[4,6,35]
Surgery is the primary treatment modality for LGMS.[36] Because LGMS may have a tendency for invasive growth and local recurrence, an extensive surgical margin should be recommended,[37] although in our case, the mass was completely encapsulated. Complete resection of the primary lesion and local recurrent lesions is essential, but the optimal extent of resection has yet to be determined. Some authors have recommended adjuvant chemotherapy and radiotherapy, particularly if the tumor has invaded adjacent tissues or the lymphatic or hematological metastasis is evidenced.[38] However, other reports have indicated that myofibrosarcoma is poorly responsive to radiation therapy, and that the cure rate with chemotherapy is uncertain.[39] Thus, the best method of treatment requires further prospective investigation. We did not recommend postoperative chemo- or radiotherapy for the present patient because of concerns regarding side effects and preservation of her eyesight.
The differential diagnosis of LGMS includes a variety of malignant or benign lesions, including fibrosarcoma, leiomyosarcoma, inflammatory myofibroblastic tumor (IMT), nodular fasciitis, and fibromatosis.[40] Fibrosarcomas are composed of malignant spindle cells showing fibroblastic differentiation, which are distinguished from myofibroblasts by the absence of immunohistochemical evidence of fibronectin, SMA, and calponin.[41] Leiomyosarcoma is characterized by alternate fascicles of cells with marked cytological pleomorphism that show immunoreactivity for desmin, h-caldesmon, and occasionally keratin, without evidence of immunoreactivity for fibronectin.[42] Nodular fasciitis is less cellular and uniform than LGMS, and has a heterogeneous appearance with cellular, myxoid, and fibrous areas.[37] Fibromatosis may display a prominent nodularity, and tends to infiltrate adjacent tissues.[37] The tumor cells are lacking of atypia and mitotic figures, and are positive only for vimentin.[20] Finally, LGMS is most easily confused with IMT. IMT is mainly composed of myofibroblastic spindle cells mixed with prominently lympho- and plasmacytic infiltration, and is positive for ALK or cytokeratin.[43] while LGMS has a more uniform histological pattern, with a greater degree of nuclear atypia and more mitotic figures, and it is negative for ALK and cytokeratin at immunohistochemistry.[8]
4. Conclusion
In the present report, we describe a rare case of LGMS in the wall of the right orbit in a 2-year-old girl. The tumor was completely encapsulated and followed a relatively indolent course. Complete resection was performed. The prognosis was good, and at a year after surgery the patient remained recurrence-free. LGMS presents many diagnostic challenges, particularly in the differentiation of a low-grade malignant process from an intermediate-grade malignant transformation. In vivo molecular imaging may be an effective method for diagnosis and postoperational follow-up for LGMS, and should be further evaluated.
Footnotes
Abbreviations: CT = computed tomography, IMT = inflammatory myofibroblastic tumor, LGMS = low-grade myofibroblastic sarcoma.
This study was supported by Natural Science Foundation of Liaoning Province (No. 20170541042).
The authors have no conflicts of interest to disclose.
References
- [1].Agaimy A, Wünsch PH, Schroeder J, et al. Low-grade abdominopelvic sarcoma with myofibroblastic features (low-grade myofibroblastic sarcoma): clinicopathological, immunohistochemical, molecular genetic and ultrastructural study of two cases with literature review. J Clin Pathol 2008;61:301–6. [DOI] [PubMed] [Google Scholar]
- [2].Arora R, Gupta R, Sharma A, et al. A rare case of low-grade myofibroblastic sarcoma of the femur in a 38-year-old woman: a case report. J Med Case Rep 2010;28:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Taccagni G, Rovere E, Masullo M, et al. Myofibrosarcoma of the breast: review of the literature on myofibroblastic tumors and criteria for defining myofibroblastic differentiation. Am J Surg Pathol 1997;21:489–96. [DOI] [PubMed] [Google Scholar]
- [4].Montgomery E, Goldblum JR, Fisher C. Myofibrosarcoma: a clinicopathologic study. Am J Surg Pathol 2001;25:219–28. [DOI] [PubMed] [Google Scholar]
- [5].Laco J, Simáková E, Slezák R, et al. Low grade myofibroblastic sarcoma of tongue: a case report. Cesk Patol 2006;42:150–3. [PubMed] [Google Scholar]
- [6].Mentzel T, Dry S, Katenkamp D, et al. Low-grade myofibroblastic sarcoma: analysis of 18 cases in the spectrum of myofibroblastic tumors. Am J Surg Pathol 1998;22:1228–38. [DOI] [PubMed] [Google Scholar]
- [7].Diaz-Cascajo C, Borghi S, Weyers W, et al. Fibroblastic/myofibroblastic sarcoma of the skin: a report of five cases. J Cutan Pathol 2003;30:128–34. [DOI] [PubMed] [Google Scholar]
- [8].Morgan PB, Chundru S, Hatch SS, et al. Uncommon malignancies: case 1. Low-grade myofibroblastic sarcoma of the breast. J Clin Oncol 2005;23:6249–51. [DOI] [PubMed] [Google Scholar]
- [9].Roth TM, Fratkin J, Woodring TC, et al. Low-grade myofibroblastic sarcoma of the vulva. Gynecol Oncol 2004;92:361–4. [DOI] [PubMed] [Google Scholar]
- [10].Bisceglia M, Magro G. Low-grade myofibroblastic sarcoma of the salivary gland. Am J Surg Pathol 1999;23:1435–6. [DOI] [PubMed] [Google Scholar]
- [11].Takahama A, Jr, Nascimento AG, Brum MC, et al. Low-grade myofibroblastic sarcoma of the parapharyngeal space. Int J Oral Maxillofac Surg 2006;35:965–8. [DOI] [PubMed] [Google Scholar]
- [12].Bisceglia M, Tricarico N, Minenna P, et al. Myofibrosarcoma of the upper jawbones: a clinicopathologic and ultrastructural study of two cases. Ultrastruct Pathol 2001;25:385–97. [DOI] [PubMed] [Google Scholar]
- [13].Vlad D, Albu S. Low-grade myofibroblastic sarcoma of the larynx. J Craniofac Surg 2016;27:e270–1. [DOI] [PubMed] [Google Scholar]
- [14].Kondo S, Yoshizaki T, Minato H, et al. Myofibrosarcoma of the nasal cavity and paranasal sinus. Histopathology 2001;38:482–4. [DOI] [PubMed] [Google Scholar]
- [15].Eyden BP, Christensen L, Tagore V, et al. Myofibrosarcoma of subcutaneous soft tissue of the cheek. J Submicrosc Cytol Pathol 1992;24:307–13. [PubMed] [Google Scholar]
- [16].Coyne JD. Low-grade myofibroblastic sarcoma of the piriform fossa: a case report with a literature review of a tumour with a predilection for the head and neck. Br J Oral Maxillofac Surg 2007;45:335–7. [DOI] [PubMed] [Google Scholar]
- [17].Lee DH, Williams B, Taylor SM, et al. Low-grade myofibroblastic sarcomas of the soft palate and oral tongue. J Otolaryngol Head Neck Surg 2011;40:E31–34. [PubMed] [Google Scholar]
- [18].Jay A, Piper K, Farthing PM, et al. Low-grade myofibroblastic sarcoma of the tongue. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;104:e52–8. [DOI] [PubMed] [Google Scholar]
- [19].Cai C, Dehner LP, El-Mofty SK. In myofibroblastic sarcomas of the head and neck, mitotic activity and necrosis define grade: a case study and literature review. Virchows Arch 2013;463:827–36. [DOI] [PubMed] [Google Scholar]
- [20].Morii T, Mochizuki K, Sano H, et al. Occult myofibroblastic sarcoma detected on FDG-PET performed for cancer screening. Ann Nucl Med 2008;22:811–5. [DOI] [PubMed] [Google Scholar]
- [21].Fisher C. Myofibrosarcoma. Virchows Arch 2004;445:215–23. [DOI] [PubMed] [Google Scholar]
- [22].Gabbiani G, Ryan GB, Majne G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia 1971;27:549–50. [DOI] [PubMed] [Google Scholar]
- [23].Niedzielska I, Janic T, Mrowiec B. Low-grade myofibroblastic sarcoma of the mandible: a case report. J Med Case Rep 2009;3:8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yamada T, Yoshimura T, Kitamura N, et al. Low-grade myofibroblastic sarcoma of the palate. Int J Oral Sci 2012;4:170–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Qiu JY, Liu P, Shi C, et al. Low-grade myofibroblastic sarcomas of the maxilla. Oncol Lett 2015;9:619–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Watanabe K, Ogura G, Tajino T, et al. Myofibrosarcoma of the bone: a clinicopathologic study. Am J Surg Pathol 2001;25:1501–7. [DOI] [PubMed] [Google Scholar]
- [27].Schaller BJ, Cornelius JF, Sandu N, et al. Molecular imaging of brain tumors personal experience and review of the literature. Curr Mol Med 2008;8:711–26. [DOI] [PubMed] [Google Scholar]
- [28].McConathy J, Yu W, Jarkas N, et al. Radiohalogenated nonnatural amino acids as PET and SPECT tumor imaging agents. Med Res Rev 2012;32:868–905. [DOI] [PubMed] [Google Scholar]
- [29].Ulaner GA, Goldman DA, Corben A, et al. A prospective clinical trial of 18F-Fluciclovine PET/CT neoadjuvant therapy response in invasive ductal and invasive lobular breast cancers. J Nucl Med 2016;58:1037–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Juhász C, Bosnyák E. PET and SPECT studies in children with hemispheric low-grade gliomas. Childs Nerv Syst 2016;32:1823–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Saji H. In vivo molecular imaging. Biol Pharm Bull 2017;40:1605–15. [DOI] [PubMed] [Google Scholar]
- [32].Kanazaki K, Sano K, Makino A, et al. Polyoxazoline multivalently conjugated with indocyanine green for sensitive in vivo photoacoustic imaging of tumors. Sci Rep 2016;6:33798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Demarosi F, Bay A, Moneghini L, et al. Low-grade myofibroblastic sarcoma of the oral cavity. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2009;108:248–54. [DOI] [PubMed] [Google Scholar]
- [34].Miyazawa M, Naritaka Y, Miyaki A, et al. A low-grade myofibroblastic sarcoma in the abdominal cavity. Anticancer Res 2011;31:2989–94. [PubMed] [Google Scholar]
- [35].Qiu X, Montgomery E, Sun B. Inflammatory myofibroblastic tumor and low-grade myofibroblastic sarcoma: a comparative study of clinicopathologic features and further observations on the immunohistochemical profile of myofibroblasts. Hum Pathol 2008;39:846–56. [DOI] [PubMed] [Google Scholar]
- [36].Keller C, Gibbs CN, Kelly SM, et al. Low-grade myofibrosarcoma of the head and neck: importance of surgical therapy. J Pediatr Hematol Oncol 2004;26:119–20. [DOI] [PubMed] [Google Scholar]
- [37].Kordač P, Nikolov DH, Smatanová K, et al. Low-grade myofibroblastic sarcoma of the larynx: case report and review of literature. Acta Medica (Hradec Kralove) 2014;57:162–4. [DOI] [PubMed] [Google Scholar]
- [38].Meng GZ, Zhang HY, Bu H, et al. Myofibroblastic sarcoma of the nasal cavity and paranasal sinus: a clinicopathologic study of 6 cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 2007;104:530–9. [DOI] [PubMed] [Google Scholar]
- [39].Chan JY, Gooi Z, Wong EW, et al. Low-grade myofibroblastic sarcoma: a population-based study. Laryngoscope 2017;127:116–21. [DOI] [PubMed] [Google Scholar]
- [40].Thompson LD, Wieneke JA, Miettinen M, et al. Spindle cell (sarcomatoid) carcinomas of the larynx: a clinicopathologic study of 187 cases. Am J Surg Pathol 2002;26:153–70. [DOI] [PubMed] [Google Scholar]
- [41].Myong NH, Min JW. Low-grade myofibroblastic sarcoma arising in fibroadenoma of the breast—a case report. Diagn Pathol 2016;11:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Perez-Montiel MD, Plaza JA, Dominguez-Malagon H, et al. Differential expression of smooth muscle myosin, smooth muscle actin, h-caldesmon, and calponin in the diagnosis of myofibroblastic and smooth muscle lesions of skin and soft tissue. Am J Dermatopathol 2006;28:105–11. [DOI] [PubMed] [Google Scholar]
- [43].Ni C, Xu YY, Zhou SH, et al. Differential diagnosis of inflammatory myofibroblastic tumour and low-grade myofibroblastic sarcoma: two case reports with a literature review. J Int Med Res 2011;39:311–20. [DOI] [PubMed] [Google Scholar]


