Abstract
Rationale:
An acute presentation with diffuse magnetic resonance imaging lesions can have a broad differential. Demyelination and malignancy are important considerations. Therefore, sometimes it is hard to differentiate glioma from some demyelinating diseases solely on imaging because of the similar clinical presentation and imaging features. Detection of highly specific serum autoantibody marker aquaporin-4 (AQP4)-IgG positivity has helped to define a category of neuromyelitis optica spectrum disorders (NMOSD), but the test of AQP4 antibody has not been reported in patients with glioma.
Patients concerns and diagnoses:
We report a case of a 56-year-old woman with cerebrospinal fluid (CSF) positive aquaporin-4 antibodies with initial response to immune therapy and secondary deterioration. A surgical biopsy revealed an anaplastic astrocytoma.
Interventions and outcomes:
After the admission the patient was treated with a short course of intravenous steroid agents. After anaplastic astrocytoma was diagnosed, she began to receive a radiation treatment and soon later experienced a clinical deterioration with frequent epilepsy seizure and disturbance of consciousness within a few months.
Lesson:
This case indicates that tumors could lead to polyclonal antibody responses as in this case with aquaporin-4 and myelin oligodendrocyte glycoprotein antibodies. The absence of a typical clinical phenotype and lack of sustained response to immunotherapy should alert the clinical suspicion of an alternative diagnosis. When AQP4 antibody was detected positive in CSF of a patient but negative in serum, differential diagnosis should especially be considered.
Keywords: anaplastic astrocytoma, AQP4-IgG, CSF
1. Introduction
Anaplastic astrocytoma is a highly devastating disease, and any mass-like lesions can raise suspicion of this diagnosis. Therefore, sometimes it is hard to differentiate glioma from some demyelinating diseases solely on imaging because of the similar clinical presentation and imaging features. We report a case of a woman with cerebrospinal fluid (CSF) positive aquaporin-4 antibodies with initial response to immune therapy and secondary deterioration. The patient was presented to outpatient department complaining of transient loss of consciousness 1 month ago. The neuroimaging studies and positive test of aquaporin 4 (AQP4)-immunoglobulin G (IgG) initially support the AQP4-IgG-related demyelinating diseases. However, a sudden clinical deterioration and expansion of lesions disclosed by magnetic resonance imaging (MRI) re-examination suggest it to be a malignant disease. Finally, a surgical biopsy revealed an anaplastic astrocytoma.
2. Case report
History and presentation: A 56-year-old women was admitted to our department with main complaint of transient loss of consciousness 1 month ago. She suddenly experienced a transient loss of consciousness and recovered in 2 minutes, with her left front tooth and left knee broken. Four days after admission, she suddenly began to feel dizzy and soon be clouding of consciousness for 4 hours. During this period, she could answer some simple questions but when recovered, she could not recall the conversations. One week later, she developed to have some seizure-like symptoms presenting occasionally trance or dizziness. The patient has not suffered any recent special febrile illness.
Examination, initial diagnosis, and treatment: Physical examination showed no abnormal findings. We undertook a brain MRI that are shown in Fig. 1. There was only a mild focal Gadolinium enhancement in the left occipital lobe (Fig. 2). MRI of bilateral optic nerves and cervical spinal cord showed no abnormal findings. The electroencephalography study showed complex δ waves, sharp-and-wave complexes, and sharp waves discharge in left front-mid temporal regions and left sphenoidal electrode. CSF and blood serological routine tests revealed no abnormal findings. The pressure of CSF was 120 mm H2O, and cell count (4×106/L), protein (0.26 g/L), glucose (3.91 mmol/L), and chlorine (117.90 mmol/L) were normal. Oligoclonal bands were negative in both CSF and serum. The immunology study of CSF showed that AQP4-IgG was significantly positive by the cell-based assay method but in serum the antibody was negative (Fig. 3). Anri-myelin oligodendrocyte glycoprotein (MOG) antibodies were found a slightly elevated in both CSF (0.875 μg/L, normal value <0.560) and serum (0.802 μg/L, normal value <0.640). Meanwhile, some other antibodies such as anti- N-methyl D-aspartate (NMDA), anti-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA1), anti-AMPA2, anti-voltage-gated potassium channel (VGKC), and anti-gamma-aminobutyric acid (GABA) antibodies were also tested but there were no positive findings. The patients responded well to a short course treatment of intravenous steroid agents and mannitol. She was discharged with a regimen of oral prednisone and sodium valproate administration.
Figure 1.
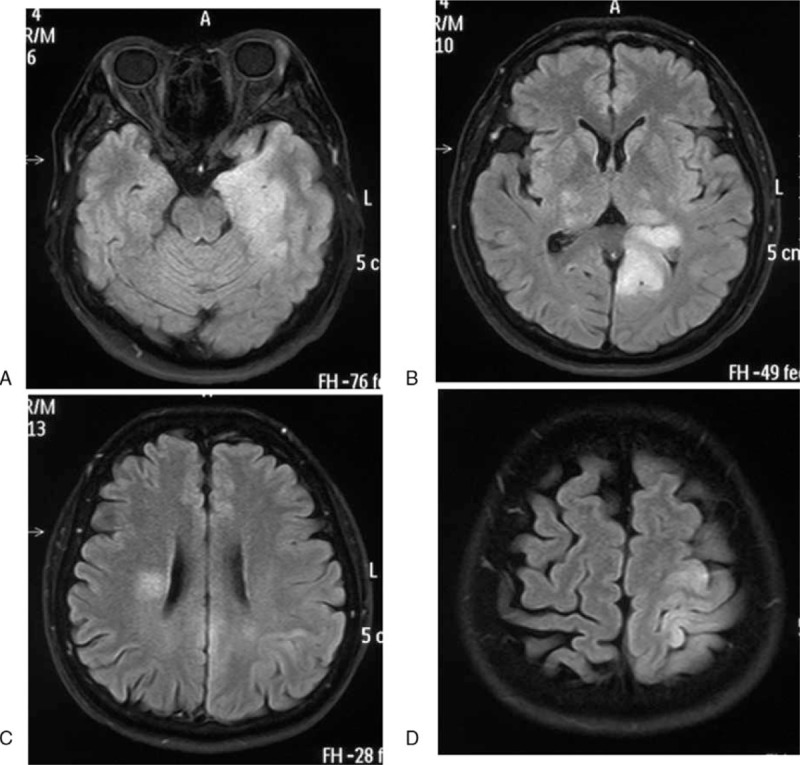
MR images T2 FLAIR images obtained 2 weeks before admission. It revealed diffuse hyperintensity in bilateral thalamus, periventricular white matter, left hippocampus, occipital, parietal lobe. MR = magnetic resonance.
Figure 2.
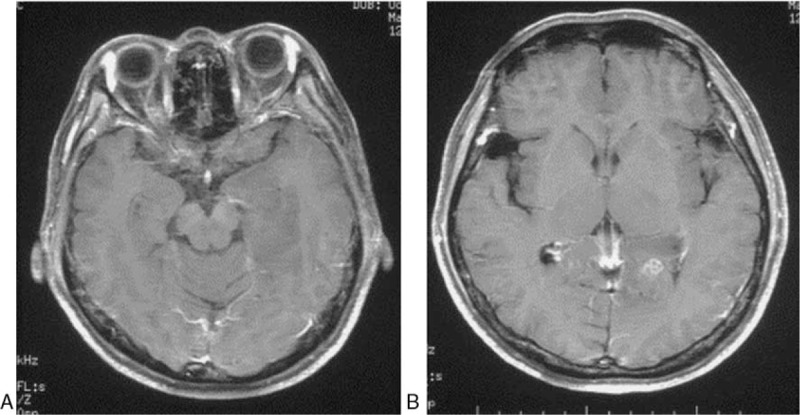
Gadolinium-enhanced T1-wighted MR images obtained at admission. It showed a mildly focal Gadolinium-enhancement in left occipital lobe. MR = magnetic resonance.
Figure 3.
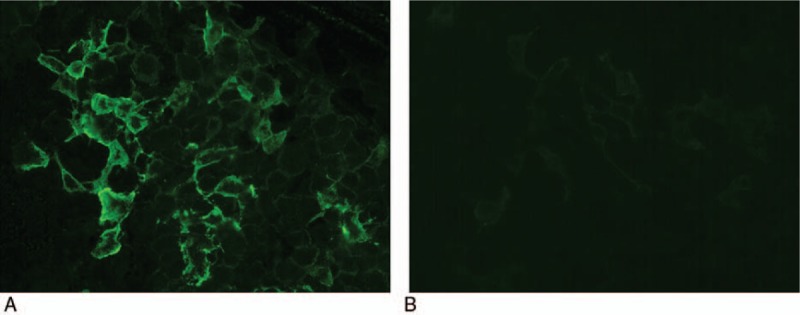
AQP4 antibody detection. A, The anti-AQP4 antibody was positive in the CSF of this reported patient. B, No fluorescence was observed in negative control. AQP4 = aquaporin 4, CSF = cerebrospinal fluid.
Despite transient clinical improvement, she soon experienced a sudden deterioration and MRI re-examination performed 2 months later disclosed an expansion of the lesions. It suggested to be a malignant entity and the patient finally approved to receive a surgical biopsy. Pathological examination of the biopsy tissue from lesions in left occipital lobe revealed a proliferation of astrocytes with mitotic activity. Immunohistochemical staining of the glial fibrillary acidic protein (GFAP), alpha thalassemia/mental retardation syndrome (ATRX), and epidermal growth factor receptor (EGFR) was positive, and the synaptophysin and p53 were negative. The percentage of the ki-67 positive neoplastic cells in the total of the tumor cells was about 10%. The histopathological diagnosis was an anaplastic astrocytoma (WHO-grade III) (Fig. 4). The patient began to receive a radiation treatment and soon later experienced a clinical deterioration with frequent epilepsy seizure and disturbance of consciousness within a few months.
Figure 4.
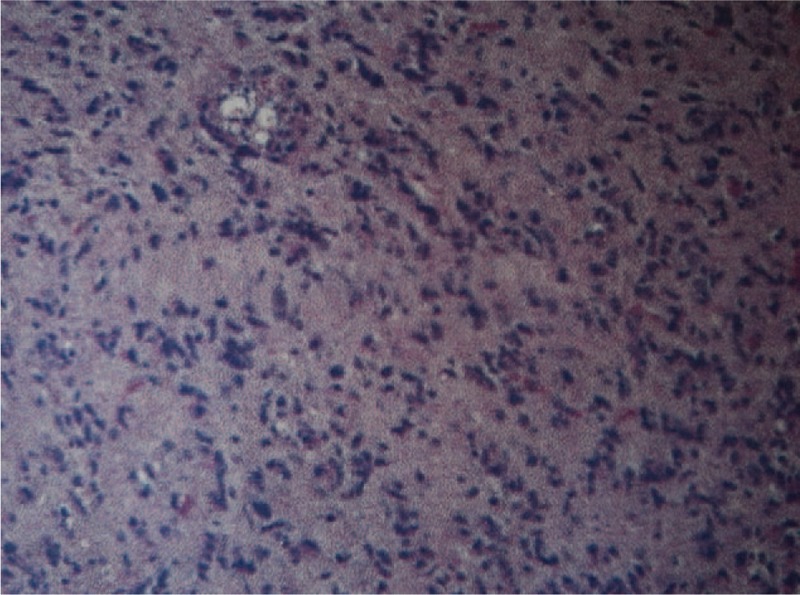
Photomicrographs of the lesions: astrocytes with nuclear atipia and mitosis (hematoxylin and eosin, ×40).
3. Discussion
The patient suffered a sudden transient loss of consciousness 1 month before admission. During hospitalization, she developed to have seizure-like symptoms presenting occasionally trance or dizziness, which could be associated with lesions of temporal lobe. Neuroimaging revealed a diffuse, widespread white matter as well as cortical lesions involving the bilateral thalamus, parietal lobe, periventricular white matter, and left hippocampus with mild focal Gadolinium-enhancement. Such image findings suggested a broad differential diagnosis such as demyelinating lesions, neoplastic lesions, and CNS infection. Because of the high specificity of AQP4 IgG for diagnosing neuromyelitis optica spectrum disorder (NMOSD), we initially diagnosed the patient with AQP4-related demyelinating diseases.
NMO-IgG, which targets aquaporin-4, a water channel protein mainly expressed in the astrocytic foot process, has been identified as a highly specific biomarker to distinct neuromyelitis optica (NMO) from other demyelinating diseases in recent years.[1] Therefore, a category of NMOSD has been defined that the diagnosis criteria were not restricted by longitudinally extensive myelitis or optic neuritis due to a highly specific autoantibody marker AQP4-IgG.[2–5] There have been several reports that serum is a more sensitive specimen for AQP4-IgG.[6,7] A recent study reported that there was no case among their 616 paired specimens with a positive test of AQP4-IgG solely in CSF but negative serum.[8] Other studies also demonstrated that the amount of AQP4 antibody present directly in CSF strongly correlates with astrocyte damage and blood–brain barrier disruption.[9] They found that AQP4 antibody titers in the CSF were remarkably higher in patients during attacks compared with those during remission and AQP4-IgG was produced in peripheral lymphoid tissues and targeted to attack AQP4 on the astrocytic foot process which resulted in pathological damage in brain or spinal cord tissue of patients with NMOSD. In our case, AQP4-IgG was positivity detected solely in CSF other than serum, which suggested a different pathogenesis from that of AQP4-related demyelinating disease. Moreover, AQP4 has been widely described to be upregulated and redistributed in human glioma.[10,11] The AQP4 was restrictively expressed on the perivascular endfoot in normal astrocytes, whereas in the astrocytoma, the whole glioma cell was strongly AQP4-immunopositive.[12] However, positive test of AQP4 antibody in CSF of patients with glioma has not yet been reported. It was assumed to be a secondary phenomenon for the diffusion of the glioma and disruption of cerebrospinal fluid barrier. Further investigations should be performed to confirm this speculation.
4. Conclusions
Our report indicates that tumors could lead to polyclonal antibody responses as in this case with aquaporin-4 and MOG antibodies. The absence of a typical clinical phenotype and lack of sustained response to immunotherapy should alert the clinical suspicion of an alternative diagnosis. When AQP4 antibody was detected solely positive in CSF of a patient other than in serum, differential diagnosis should especially be concerned. It was thought that the discrepant origin of AQP4-IgG could help differentiate between them in the future.
Acknowledgments
The authors thank the patient and her family for participating in this study.
Footnotes
Abbreviations: anti-AMPA = anti-α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid, anti-GABA = gamma-aminobutyric acid, anti-MOG = anti-myelin oligodendrocyte glycoprotein, anti-NMDA = anti-N-methyl D-aspartate, anti-VGKC = anti-voltage-gated potassium channel antibodies, AQP4 = aquaporin 4, ATRX = alpha thalassemia/mental retardation syndrome, CSF = cerebrospinal fluid, EGFR = epidermal growth factor receptor, GFAP = glial fibrillary acidic protein, IgG = immunoglobulin G, MRI = magnetic resonance imaging, NMOSD = neuromyelitis optica spectrum disorder.
YL recruited the patients, performed the clinical evaluation, and wrote the manuscript. WS, FG, and HH contributed to data analysis. YH conducted and supervised this study, evaluated the data, and wrote the manuscript.
This study was supported by a grant from the Beijing Science Committee (No. D111107003111008).
Patient consent: Obtained.
The authors have no conflicts of interest to disclose.
References
- [1].Jacob A, McKeon A, Nakashima I, et al. Current concept of neuromyelitis optica (NMO) and NMO spectrum disorders. J Neurol Neurosurg Psychiatry 2013;84:922–30. [DOI] [PubMed] [Google Scholar]
- [2].Wingerchuk DM, Lennon VA, Lucchinetti CF, et al. The spectrum of neuromyelitis optica. Lancet Neurol 2007;6:805–15. [DOI] [PubMed] [Google Scholar]
- [3].Wingerchuk DM, Lennon VA, Pittock SJ, et al. Revised diagnostic criteria for neuromyelitis optica. Neurology 2006;66:1485–9. [DOI] [PubMed] [Google Scholar]
- [4].Tan CT, Mao Z, Qiu W, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2016;86:491–2. [DOI] [PubMed] [Google Scholar]
- [5].Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015;85:177–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dujmovic I, Mader S, Schanda K, et al. Temporal dynamics of cerebrospinal fluid anti-aquaporin-4 antibodies in patients with neuromyelitis optica spectrum disorders. J Neuroimmunol 2011;234:124–30. [DOI] [PubMed] [Google Scholar]
- [7].Jarius S, Franciotta D, Paul F, et al. Cerebrospinal fluid antibodies to aquaporin-4 in neuromyelitis optica and related disorders: frequency, origin, and diagnostic relevance. J Neuroinflammation 2010;7:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Majed M, Fryer JP, McKeon A, et al. Clinical utility of testing AQP4-IgG in CSF: guidance for physicians. Neurol Neuroimmunol Neuroinflamm 2016;3:e231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Sato DK, Callegaro D, de Haidar Jorge FM, et al. Cerebrospinal fluid aquaporin-4 antibody levels in neuromyelitis optica attacks. Ann Neurol 2014;76:305–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Saadoun S, Papadopoulos MC, Davies DC, et al. Aquaporin-4 expression is increased in oedematous human brain tumours. J Neurol Neurosurg Psychiatry 2002;72:262–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Warth A, Kroger S, Wolburg H. Redistribution of aquaporin-4 in human glioblastoma correlates with loss of agrin immunoreactivity from brain capillary basal laminae. Acta Neuropathol 2004;107:311–8. [DOI] [PubMed] [Google Scholar]
- [12].Warth A, Simon P, Capper D, et al. Expression pattern of the water channel aquaporin-4 in human gliomas is associated with blood-brain barrier disturbance but not with patient survival. J Neurosci Res 2007;85:1336–46. [DOI] [PubMed] [Google Scholar]


