Abstract
Rationale:
Diabetic foot ulcer (DFU) is a chronic complication of diabetes characterized by continuity, repeatability, and nonhealing. In recent years, mesenchymal stem cells hydrogel complex has been a new emerging technique in the treatment of DFU. The placenta-derived mesenchymal stem cells (PDMSCs) hydrogel is multipotent, and can secrete growth factors, cytokines, and immunomodulatory substances which could accelerate wound healing.
Patient concerns:
In this case report, we present a 57-year-old female with type 2 diabetes mellitus and a 20-day DFU.A wound bed located at the dorsalis pedis of the right foot, and conventional therapies had no effect on the foot.
Diagnoses:
The patient was confirmed a diagnosis of type 2 DM with diabetic foot (Wagner classification III).
Interventions:
To assess the efficacy and safety of PDMSCs hydrogel in wound repair and to improve the rate of wound healing, we administered PDMSCs hydrogel (cell number: 1 × 106/cells/cm2) topically into the wound with the patient's permission.
Outcomes:
The patient's foot ulcer was almost healed, and foot function in walking was well preserved. No complications were observed. No recurrence occurred in the subsequent 6 months.
Lessons:
To the best of our knowledge, this is the first patient globally to receive PDMSCs hydrogel to treat DFU. The present case study suggests that PDMSCs hydrogel may provide a new approach to DFU treatment. Clinical Trial Registration-URL: http://www.chictr.org.cn/searchproj.aspx:chiCRT-ONC-16008732.
Keywords: case report, diabetic foot ulcer, hydrogel complex, placenta-derived mesenchymal stem cells
1. Introduction
The International Diabetes Federation reported that, at present, there are 415 million subjects worldwide with diabetes mellitus (DM) aged between 20 and 79 years, with a global prevalence of 8.8%,[1] which by 2030 will increase to >360 million.[2] The country with the highest number of DM patients is China (109.6 million).[3,4] The prevalence of complications in DM is approximately 70%, among these being diabetic foot, with a high amputation rate and cost. Diabetic foot ulcers (DFUs) are projected to occur in 25% of all diabetes patients in 2030.[5,6] Furthermore, foot ulcers have high treatment costs (approximately US$17,500–27,987 [UK£9533–15,246]).[7] Therefore, the diabetic foot has become a major burden globally to health systems.
DFU is a chronic, complex, and universal disease, which requires continuous medical care, and has now become a leading global cause of nontraumatic amputation, accounting for 85% of such amputations.[5] The amputation rate in DM was 19.03% in China in 2015.[8] Many factors cause nonhealing DFU, including infection, microvascular disease, peripheral neuropathy, foot trauma, and impaired angiogenesis of wounds, with angiogenesis disorders above all playing a vital role in wound-repair problems.[4] Conventional therapies for DFU include medicine, physical therapies, and surgical treatments, which require a long-term hospital stay with high costs, but it remains difficult for the wound to recover completely.
Recent studies have identified that regenerative approaches using growth factors and various cell therapies are particularly applied in clinical therapies for DFU.[9] Mesenchymal stem cells (MSCs) that are present in normal skin are known to participate in wound repair.[10] Consequently, the application of other tissue-derived MSCs to heal wounds has been investigated. Placenta-derived mesenchymal stem cells (PDMSCs) were extracted from human placental tissue. Placental tissue was processed through washing, centrifugation, culturing, and expansion to finally obtain PDMSCs. These cells were activated and mixed with a given percentage of sodium alginate powder to generate a gelatinous PDMSCs complex. PDMSCs can be obtained in greater numbers and their acquisition is noninvasion compared with bone-marrow-derived stem cells and those from other tissues. In addition, because PDMSCs are of fetal origin, they display lower immunogenicity.[9–11] Most importantly, PDMSCs hydrogel can secrete different cytokines and growth factors, which is vital to wound repair.[12,13] In particular, its secretory activity led to decreased levels of proinflammatory cytokines, including tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-8, IL-1, and intercellular adhesion molecule 1 (ICAM-1), and increased anti-inflammatory cytokine IL-10. PDMSCs hydrogel might mediate the inflammatory response by activating nuclear factor kappa-light-chain enhancer of activated B cells signaling in fibroblasts. Therefore, the mechanism by which PDMSCs hydrogel promotes wound repair, at least in part, is by inhibiting the proinflammatory response and by promoting increased expression of the anti-inflammatory factor IL-10, which is important during the early inflammatory stage of wound healing.[14] Furthermore, PDMSCs hydrogel releases growth factors, cytokines, and chemokines, specifically vascular endothelial growth factor (VEGF), platelet-derived growth factor, basic fibroblast growth factor (bFGF), epidermal growth factor, keratinocyte growth factor, and transforming growth factor-β to accelerate wound healing. These unique characteristics make PDMSCs hydrogel a potential form of cell therapy for DFU.
PDMSCs were first identified in mature placental leaflets by Fukuchi et al[10] in 2004. Currently, its application in ischemic diseases is being investigated. Kinzer et al[13] applied human PDMSCs in the revascularization of ischemic tissues, demonstrating a positive role of these cells on neovascularization in vivo. Furthermore, Kranz et al[15] successfully demonstrated that PDMSCs had the potential to improve revascularization of the cerebral artery. Han[16] in a pilot study treated 2 subjects with diabetic foot by PDMSCs transplantation, whereby the lower extremity lesions displayed abundant collateral vessel formation following transplantation. Numerous studies have demonstrated that MSCs play a role in the prevention and treatment of DFU,[10,12,15–17] which suggests a new treatment method of DFU. We report here a case of DFU treated with PDMSCs hydrogel, with the purpose of assessing the safety and clinical feasibility of PDMSCs hydrogel.
2. Materials and methods
2.1. Study design
The Ethical Committee of the First Affiliated Hospital of Nanchang University, Jiangxi Province, China approved the study. The patient signed an informed consent form before treatment.
2.2. Patient
A 57-year-old Chinese female who had suffered from foot ulcer for more than 20 days was presented to the First Affiliated Hospital of Nanchang University on October 18, 2016. The patient complained of thirst, asthenia, foot pains when walking, and a right dorsum pedis ulcer. The patient had a fasting glucose level of 8.1 mmol/L. A physician at a local clinic advised treatment by dressing on alternate days for 7 days. However, this had no effect on the foot. The patient did not report having fever or chills. There was no family history of DM or hypertension.
2.2.1. Physical examination
The patient weighed 73 kg, was of height 155 cm, with a body mass index of 31.21 kg/m2, a blood pressure of 151/88 mm Hg, a pulse rate of 84 beats/min, and an oxygen saturation of 99%. A wound bed located at the dorsalis pedis of the right foot was approximately 8.0 × 4.0 × 3.0 cm. Periwound callus and rolled wound edges were clearly present. The wound had a slightly foul discharge with no malodor (Fig. 1).
Figure 1.
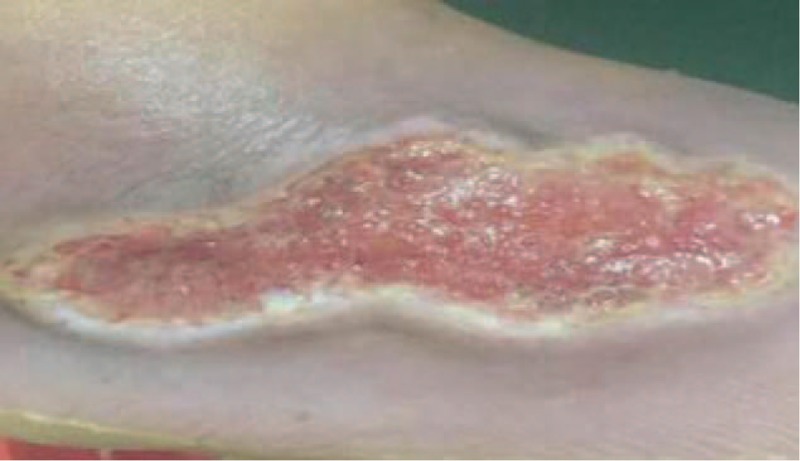
Nonhealing wound at the right dorsum pedis of a 57-year-old female with type 2 diabetes mellitus.
2.2.2. Laboratory examination
The peripheral blood displayed the following properties: white blood cells, 4.67 × 109/L; total red blood cell count, 4.22 × 1012/L, hemoglobin, 125 g/L; granulocytes, 56.7%; fasting glucose, 6.65 mmol/L; postprandial glucose, 14.33 mmol/L; HbA1c, 6.8%. Inflammatory indicators: erythrocyte sedimentation rate, 35 mm/h; high-sensitivity C-reactive protein, 8.15 mg/L; procalcitonin, normal (<0.25 ng/mL). Pus Germiculture and Drug Sensitivity Test were Candida glabrata, sensitive to cefoperazone and sulbactam. Radiographs showed the presence of low-density shadows together with soft-tissue edema around the right foot first toe and fifth phalanges, but without osseous impairment. A slack and powerless radial artery pulse was palpated. Ankle/brachial index: left, 0.85; right, 0.80. Vascular magnetic resonance of the lower limbs indicated that in both lower extremities vasculars were patency.
2.2.3. Diagnosis
Based on these results, we confirmed a diagnosis of type 2 DM with diabetic foot (Wagner classification III).
2.3. Reagents
PDMSCs hydrogel (cell number: 1 × 106/cells/cm2) was kindly provided by the Stem-cell Engineering Research Center of Jiangxi province (Shangrao, China).
2.4. PDMSCs hydrogel
Human placentas were obtained from healthy mothers (38–40 weeks gestation), each having signed an informed consent form prior to delivery. Disposal of placenta tissue and isolation of PDMSCs were performed in the good manufacturing practice (GMP) laboratory of Beijing Health-biotech Co. Ltd using a method described previously.[12,17–19] In summary, the placentas were washed several times with phosphate-buffered saline and then pieces were excised. To this was added 0.1% collagenase (Sigma–Aldrich, St Louis, MO) and 10% fetal bovine serum (FBS) (Hyclone, South Logan, UT) to digest the tissue at 37°C for 1 h. The digested mixture was passed through 70-lm filters by centrifugation (2000 rpm, 10 min) to obtain cell suspensions. The cells were cultured in 30 mL Dulbecco Modified Eagle's Medium media (37°C, 5% CO2, humidified incubator) and plated in a T75 cell-culture flask (Corning Glass Works, Corning, NY) with 10% FBS, 100 U/mL penicillin, 100 lg/mL streptomycin, 2 mmol/ l-glutamine, and 1% nonessential amino acids. The adherent MSCs obtained from the second passage were harvested for cell banking followed by a series of safety evaluations. The PDMSCs released from the cell banking were cultured and expanded in the GMP laboratory for 5 passages to prepare final cell products, which should be sterile and all shown to be free from mycoplasma, hepatitis B virus, hepatitis C virus, human immunodeficiency virus, Epstein–Barr virus, cytomegalovirus, syphilis, and endotoxins. The PDMSCs highly expressed nestin, CD151, CD105, CD73, CD166, Oct4, and Sox2, but not CD34, CD31, CD45, CD184, or HLA-DR. The final PDMSCs were activated and mixed with sodium alginate powder, resulting in a gelatinous PDMSCs complex.
2.5. Treatment
The patient received PDMSCs (cell number: 1 × 106/cells/cm2) for topical wound treatment and the dressings changed everyday lasting 3 weeks. The entire wound surface was filled with this gel and covered with dressings, and the wound was debrided daily. Cefoperazone and sulbactam (both 4 g/d) were given intravenously for 7 days until the edematous and erythematous of the left dorsalis pedis had returned to normal and the inflammatory indicators returned to normal levels. Insulin was given to maintain a fasting glucose level <6.0 mmol/L, a postprandial glucose level <7.5 mmol/L, and an HbA1c level <6.3%. The patient was followed up after treatment for 6 months.
2.6. Results
Wound healing was monitored daily. At 48 h after wound debridement, foot pain was relieved and the skin temperature improved markedly. There were no skin allergies or other local abnormal symptoms or signs, and blood, urine, stool tests, and biochemical examinations (including liver and renal function tests) were normal. After 72 h, the purulent secretions from the wound had clearly decreased, local swelling alleviated, the wound bed filled with granulation tissue, and the wound had shrunk (Fig. 2). After 3 weeks, the wound had completely healed (Fig. 3). Therefore, on topical PDMSCs hydrogel application, the wound healed rapidly, and the patient was discharged from hospital.
Figure 2.
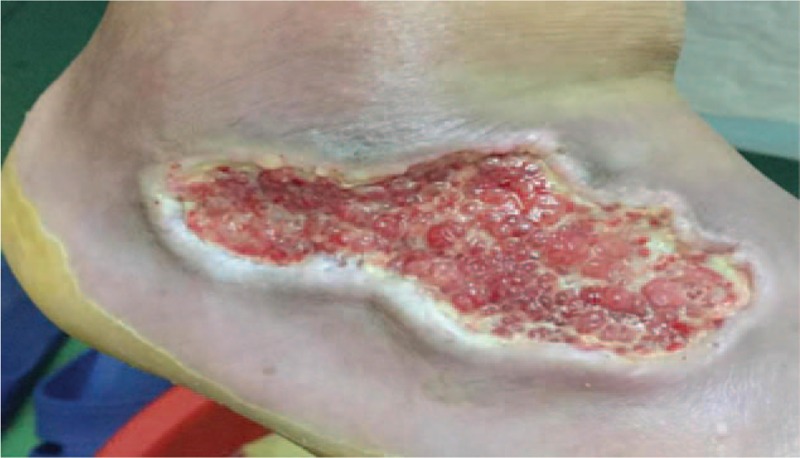
Right dorsum pedis wound 72 h after treatment with placenta-derived mesenchymal stem cells hydrogel, showing clearly decreased purulent secretions and wound size.
Figure 3.
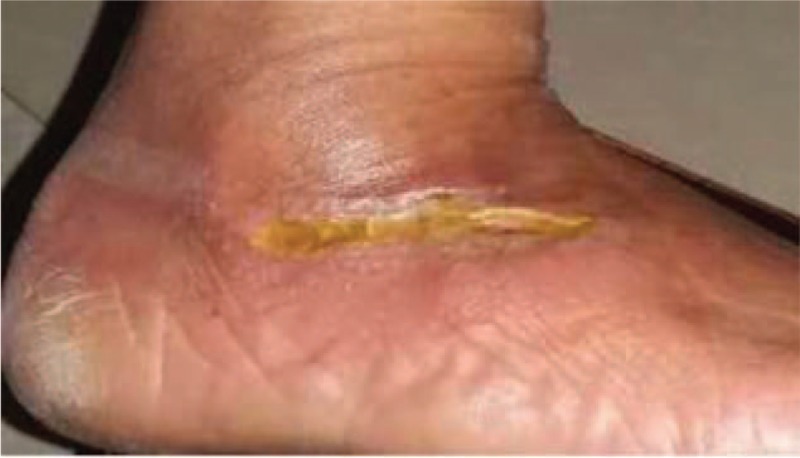
Wound almost healed 3 weeks after treatment with placenta-derived mesenchymal stem cells hydrogel.
2.7. Follow-up visit
During the follow-up visit in the subsequent 6 months, the patient had no relapse. The patient was advised on a special diabetic diet, and a record was made of regular treatment and investigation for diabetes. A pair of soft cotton shoes was made for the patient's feet. Health education included on DM and diabetic foot, including among others avoiding force on or wounding of the impaired limb, foot care in diabetes, and reducing physical activities.
3. Discussion
In this clinical study, we investigated the effect of PDMSCs hydrogel on DFU healing. Furthermore, our results demonstrated for the first time that PDMSCs hydrogel accelerated wound healing and ameliorated the condition of the wound in a diabetic patient within only 3 weeks. The mechanism of PMSCs hydrogel in diabetic wound healing may be associated with its secretion of paracrine factors and stimulation of vascular differentiation. Therefore, our findings for the first time provide proof of the concept of PDMSCs hydrogel and its efficacy in tissue repair and engineering.
MSCs are multipotent stromal cells that were first found in bone marrow by Friedenstein et al[20] in 1966. They were later found in various other tissue types, including umbilical cord blood, placenta,[10,21,22] adipose tissue, and amniotic membrane. It is important to establish the best cell source. Currently, bone marrow is the main cell source of MSCs, whereas the human placenta is a better choice. First, PDMSCs are readily acquired and raise no ethical conflicts. Second, and most importantly, larger amounts of cells can be isolated from human placenta compared with bone marrow. Third, human placenta displays lower immunogenicity.[9–11] The main component of PDMSCs hydrogel is placenta-derived MSCs, combined with sodium alginate powder. Specifically, the sodium alginate powder can be adopted to maintain the activity and cellular compatibility of PDMSCs. PDMSCs have multidirectional differentiation capacities and secretory capabilities that could improve wound healing.[9–11,14] To the best of our knowledge, this is the first time that PDMSCs hydrogel has been applied to treat DFU. Our current observation demonstrated that PDMSCs hydrogel applied in a diabetic foot wound, and monitored, could markedly decrease the wound size, shorten the duration of wound healing, and induce thick granulation-tissue formation to promote wound healing. Several mechanisms might explain the efficient therapy of PDMSCs hydrogel application in the diabetic-foot patient, the most obvious being paracrine and immunomodulation. In the present study, PDMSCs stimulated wound tissue to generate granulation tissues (Fig. 2). Consistent with our study, Du et al[23] demonstrated that PDMSCs could secrete a variety of cytokines, including stromal cell-derived factor 1, VEGF, bFGF, and hepatocyte growth factor (HGF), and act in a paracrine manner to stimulate the formation of new blood vessels and granulation tissue and the migration of epithelial keratinocytes and fibroblast cells to improve ulcer healing. Several studies have demonstrated that PDMSCs contributed to wound repair by secreting various cell factors.[15,23] Another mechanism by which PDMSCs hydrogel promotes wound healing is inflammation. PDMSCs could inhibit the production of TNF-α, interferon-γ, IL-6, and IL-4, and promote the expression of the anti-inflammatory factor IL-10.[14] Recent in vitro studies described that PDMSCs highly expressed IL-10 while suppressing the expression of TNF-α, IL-6, IL-8, IL-1, and ICAM-1, that they induced high immunomodulation activity, and that they reduced inflammation during wound healing.[12,14,24]
The present case study trialed the safety and clinical efficacy of PDMSCs hydrogel in the treatment of DFU. To the best of our knowledge, this is the first time globally that PDMSCs hydrogel has been applied to treat DFU. We observed that PDMSCs hydrogel markedly decreased the wound size, shortened the duration of wound healing, increased granulation-tissue formation, and succeeded in avoiding lower extremity amputation in the DFU patient. The healing process was supported by cultured PDMSCs hydrogel complex that is capable of promoting neovascularization, differentiating into a variety of cell types, and releasing growth factors. Kong et al[12] elucidated the possible mechanism of PDMSCs in the treatment of DFU. On the one hand, PMSCs through increasing angiogenesis and collagen accumulation in the ulcer tissue promote wound healing, while on the other hand, PMSCs could secrete numerous proangiogenic molecules, including VEGF, HGF, bFGF, TGF-β, and IGF-1 at levels that are bioactive. Above all, the efficacy of PMSCs may be attributed to vascular regeneration and paracrine mechanisms.
4. Conclusion
This case study demonstrated that the PDMSCs hydrogel may provide a new method for DFU treatment, but further studies with larger patient numbers are still required.
Footnotes
Abbreviations: bFGF = basic fibroblast growth factor, DFU = diabetic foot ulcer, DM = diabetes mellitus, FBS = fetal bovine serum, GMP = good manufacturing practice, HGF = hepatocyte growth factor, ICAM-1 = intercellular adhesion molecule 1, IL = interleukin, MSC = mesenchymal stem cell, PDMSC = placenta-derived mesenchymal stem cell, TNF = tumor necrosis factor, VEGF = vascular endothelial growth factor.
The study was supported by grants from the National Natural Science Funds of China (No. 81760168 and 81460018), the Jiangxi Provincial Science Technology Foundation of China (No. 20151BBG70073), and the Jiangxi Provincial Department of Education Scientific Research Funds of China (No. GJJ13145 and GJJ13178).
The authors have no conflicts of interest to disclose.
References
- [1].Da RF, Ogurtsova K, Linnenkamp U, et al. IDF Diabetes Atlas estimates of 2014 global health expenditures on diabetes. Diabetes Res Clin Pract 2016;117:48–54. [DOI] [PubMed] [Google Scholar]
- [2].Whiting DR, Guariguata L, Weil C, et al. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract 2011;94:311–21. [DOI] [PubMed] [Google Scholar]
- [3].Sandu MM, Protasiewicz DC, Firănescu AG, et al. Data regarding the prevalence and incidence of diabetes mellitus and prediabetes. Rom J Diabetes Nutr Metab Dis 2016;23:95–103. [Google Scholar]
- [4].Reiber GE, Vileikyte L, Boyko EJ, et al. Causal pathways for incident lower-extremity ulcers in patients with diabetes from two settings. Diabetes Care 1999;22:157–62. [DOI] [PubMed] [Google Scholar]
- [5].Pedras S, Carvalho R, Pereira MG. Sociodemographic and clinical characteristics of patients with diabetic foot ulcer. Rev Assoc Med Bras 2016;62:171–8. [DOI] [PubMed] [Google Scholar]
- [6].Anichini R, Zecchini F, Cerretini I, et al. Improvement of diabetic foot care after the Implementation of the International Consensus on the Diabetic Foot (ICDF): results of a 5-year prospective study. Diabetes Res Clin Pract 2007;75:153–8. [DOI] [PubMed] [Google Scholar]
- [7].Cavanagh PR, Lipsky BA, Bradbury AW, et al. Treatment for diabetic foot ulcers. Digest World Core Med J 2005;366:1725–35. [DOI] [PubMed] [Google Scholar]
- [8].Jiang Y, Ran X, Jia L, et al. Epidemiology of type 2 diabetic foot problems and predictive factors for amputation in China. Int J Low Extrem Wounds 2015;14:19–27. [DOI] [PubMed] [Google Scholar]
- [9].Chang CJ, Yen ML, Chen YC, et al. Placenta-derived multipotent cells exhibit immunosuppressive properties that are enhanced in the presence of interferon-gamma. Stem Cells 2006;24:2466–77. [DOI] [PubMed] [Google Scholar]
- [10].Fukuchi Y, Nakajima H, Sugiyama D, et al. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells 2004;22:649–58. [DOI] [PubMed] [Google Scholar]
- [11].Mihu CM, Mihu D, Costin N, et al. Isolation and characterization of stem cells from the placenta and the umbilical cord. Rom J Morphol Embryol 2008;49:803–8. [PubMed] [Google Scholar]
- [12].Kong P, Xie X, Li F, et al. Placenta mesenchymal stem cell accelerates wound healing by enhancing angiogenesis in diabetic Goto-Kakizaki (GK) rats. Biochem Biophys Res Commun 2013;438:410–9. [DOI] [PubMed] [Google Scholar]
- [13].Kinzer M, Hingerl K, König J, et al. Mesenchymal stromal cells from the human placenta promote neovascularization in a mouse model in vivo. Placenta 2014;35:517–9. [DOI] [PubMed] [Google Scholar]
- [14].Wang H, Chen L, Liu Y, et al. Implantation of placenta-derived mesenchymal stem cells accelerates murine dermal wound closure through immunomodulation. Am J Transl Res 2016;8:4912–21. [PMC free article] [PubMed] [Google Scholar]
- [15].Kranz A, Wagner DC, Kamprad M, et al. Transplantation of placenta-derived mesenchymal stromal cells upon experimental stroke in rats. Brain Res 2010;1315:128–36. [DOI] [PubMed] [Google Scholar]
- [16].Han Z. Safety and effect of placenta mesenchymal stem cells transplantation in the treatment of diabetic foot. China Med News 2012;27:19–19. [Google Scholar]
- [17].Jiang R, Han Z, Zhuo G, et al. Transplantation of placenta-derived mesenchymal stem cells in type 2 diabetes: a pilot study. Front Med 2011;5:94–100. [DOI] [PubMed] [Google Scholar]
- [18].Lu LL, Liu YJ, Yang SG, et al. Isolation and characterization of human umbilical cord mesenchymal stem cells with hematopoiesis-supportive function and other potentials. Haematologica 2006;91:1017–26. [PubMed] [Google Scholar]
- [19].Brooke G, Rossetti T, Pelekanos R, et al. Manufacturing of human placenta-derived mesenchymal stem cells for clinical trials. Br J Haematol 2009;144:571–9. [DOI] [PubMed] [Google Scholar]
- [20].Friedenstein AJ, Piatetzkyshapiro II, Petrakova KV. Osteogenesis in transplants of bone marrow cells. J Embryol Exp Morphol 1966;16:381–90. [PubMed] [Google Scholar]
- [21].Barlow S, Brooke G, Chatterjee K, et al. Comparison of human placenta- and bone marrow-derived multipotent mesenchymal stem cells. Stem Cells Dev 2008;17:1095–107. [DOI] [PubMed] [Google Scholar]
- [22].Li X, Wen L, Pennisi A, et al. Human placenta-derived adherent cells prevent bone loss, stimulate bone formation, and suppress growth of multiple myeloma in bone. Stem Cells 2015;29:263–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Du WJ, Chi Y, Yang ZX, et al. Heterogeneity of proangiogenic features in mesenchymal stem cells derived from bone marrow, adipose tissue, umbilical cord, and placenta. Stem Cell Res Ther 2016;7:163–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yang ZX, Han ZB, Ji YR, et al. CD106 identifies a subpopulation of mesenchymal stem cells with unique immunomodulatory properties. PLoS ONE 2013;8:e59354. [DOI] [PMC free article] [PubMed] [Google Scholar]


