Abstract
Rationale:
Insulin autoimmune syndrome (IAS) is an uncommon disorder characterized by hyperinsulinemic hypoglycemia related to insulin-binding autoantibodies. To the best of our knowledge, we report the first case of a pregnant female with IAS.
Patient concerns:
The 26-year-old patient with Graves disease and 10 weeks pregnant developed IAS after approximately 6 months treatment with methimazole. The patient exhibited recurrent spontaneous hypoglycemia.
Diagnoses:
On evaluation, laboratory findings detected both high fasting insulin (>1000 mIU/L) and insulin autoantibodies. An oral glucose tolerance test showed elevated insulin concentrations with disproportionately elevated C-peptide levels. The imaging study showed nomasslesionsinthepancreas,and the patient was clinically diagnosed with IAS.
Interventions:
The patient had an abortion, discontinued methimazole and switched to oral prednisone (30 mg once daily) and propylth- iouracil (100 mg 3 times daily) for 3 months.
Outcomes:
At the 3-month follow-up visit, hypoglycemic episodes had disappeared and insulin antibody levels were no longer detectable.
Lessons:
We have described this case and reviewed the relevant literature concerning diagnosis and treatment of IAS. Importantly, this case indicates that clinicians should view pregnancy as another factor of hypoglycemia in IAS.
Keywords: hypoglycemia case report, insulin autoimmune syndrome, methimazole, pregnant
1. Introduction
Insulin autoimmune syndrome (IAS) was first reported in Tohoku Journal of Experimental Medicine in the 1970s by Hirata et al.[1] The first case report in China appeared in the Chinese Journal of Endocrinology and Metabolism in 1986 of a 49-year-old man with Graves disease who was treated with methimazole for 1 month and experienced severe spontaneous hypoglycemia.[2] IAS occurs when the titer of anti-insulin autoantibodies and immune reactive insulin increases causing transformation from benign autoimmunity to pathogenic autoimmunity.[3] This progression is determined by both genetic influences and environmental triggers such as medication, genetic instability, and other factors.[3] It is considered that IAS is associated with genetic immune deficiency, and that HLA-DR4 is the main susceptible gene (mainly with DRB1∗0406 and sometimes with DRB1∗0403 and DRB1∗0407).[4]
Patients with IAS are frequently aged >39 years with no difference between sexes. At present, there are no reports of IAS in pregnant women. IAS is the third leading cause of hypoglycemia, after insulinoma and pancreatic tumors. According to preliminary statistics,[5] between 1970 and 2013, there were up to 400 IAS case reports of which more than 200 occurred in Japan and only a few in Western countries. In addition, over 50% of patients who are diagnosed with IAS have previously received drugs containing a sulfhydryl group, such as methimazole, which has been proposed to be related to the production of insulin autoantibodies by chemical and immunological reactions with insulin molecules.[6] Unignored, pregnancy should be considered as another pathogenic factor of hypoglycemia in IAS. We report an uncommon case of a methimazole-associated IAS in a Chinese pregnant female patient.
2. Case report
A 26-year-old Chinese female patient was referred to our hospital for recurrent spontaneous hypoglycemia on January 14, 2016. Complaining of palpitations, sweating, tremors, and anxiety, she was diagnosed with Graves disease at the local hospital on August 24, 2015, and prescribed methimazole 10 mg 3 times daily. She became pregnant on October 31, 2015. On November 31, 2015, she frequently developed episodes of dizziness, fatigue, cold sweat, palpitations, tremor, and symptoms of drowsiness during the night and morning. In the evening of January 14, 2016, she suddenly lost consciousness and was immediately sent to a local hospital and diagnosed with hypoglycemia based on a plasma glucose concentration of 1.9 mmol/L (normal range, 3.90–6.10 mmol/L). Her symptoms were relieved following intravenous glucose infusion. Later, she repeatedly experienced hypoglycemia (blood glucose 2.1–2.8 mmol/L) at night and early morning. Laboratory measurements were fasting C-peptide 10.56 ng/mL, 2-h postprandial C peptide 12 ng/mL, and 2-h postprandial insulin 276.53 ng/L.
To identify the reasons for hypoglycemia and to exclude islet beta cell tumor disease, the patient was referred to our hospital for further evaluation and treatment. She had no history of diabetes mellitus, hypertension, or previous exposure to insulin or oral antidiabetic agents. There was no family history of diabetes mellitus. She had a 6-month history of Graves disease and pregnancy for 10 weeks.
On physical examination at admission, her weight was 52 kg, height 1.55 m, body mass index (BMI) 21.64 kg/m2, blood pressure 104/76 mm Hg, and pulse rate 106 beats per min. The swollen thyroid was II degrees and bump texture soft, no obvious palpable nodules or tenderness, and no extra vascular murmur.
Routine laboratory blood tests of hemoglobin concentration, erythrocyte sedimentation rate, renal function, adrenocorticotropic hormone (ACTH), growth hormone (GH), liver function, and adrenal function were found to be normal (Table 1). C-reactive protein, rheumatoid factor, antinuclear antibodies, and anti-dsDNA antibodies were negative. Human chorionic gonadotropin level was positive for pregnancy. The thyroid function test indicated hyperthyroidism (Table 1).
Table 1.
Laboratory investigations performed in patient.
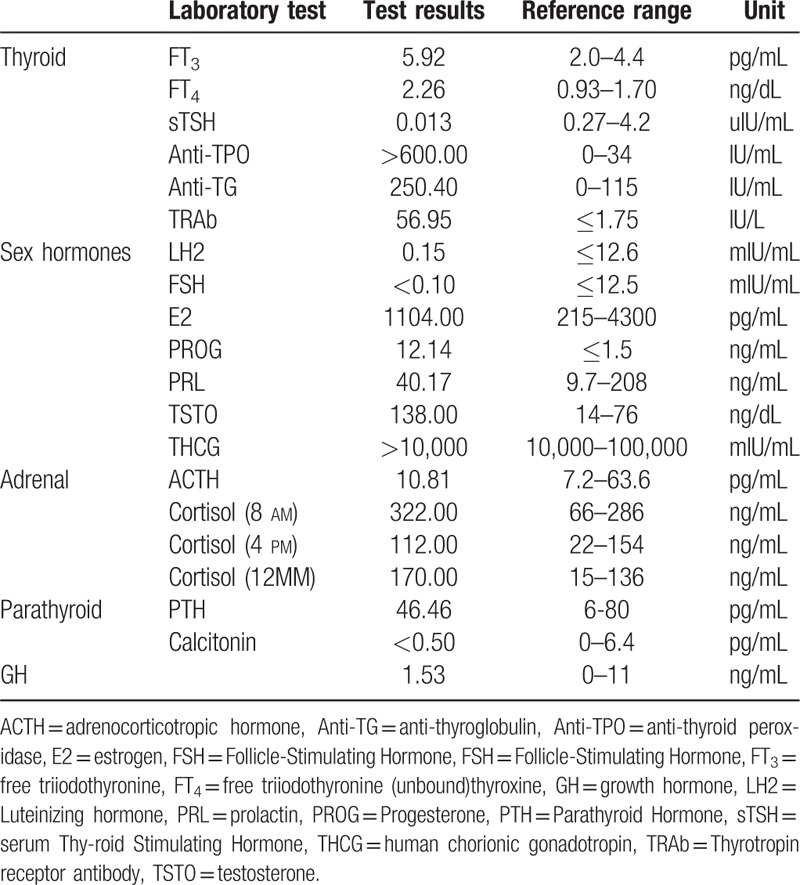
At 03:00 h on January 17, 2016, blood glucose was 2.26 mmol/L and glycosylated hemoglobin was 5.4% (normal range 4.5∼6.3%). She had no history of prolonged fasting. Spontaneous symptomatic hypoglycemic occurred again soon after admission and serum insulin, blood glucose, and serum C-peptide release were measured (Fig. 1). The serum levels of insulin and C-peptide were both inappropriately elevated in the presence of hypoglycemia, confirming the diagnosis of hyperinsulinemic hypoglycemia. Blood glucose monitor using the Dextrostix/Eyetone system (LifeScan Company, Johnson & Johnson, New Jersey, New Brunswick) results indicated hypoglycemia at 02:00 to 04:00 h, the lowest result was 1.65 mmol/L, but during the day glucose results were generally normal. In addition, unusually high levels of insulin autoantibodies (up to 33.69%; normal < 5%) were measured in the serum, and we assumed that these insulin-binding autoantibodies belonged to the IgG class. Anti-islet β-cell autoantibodies tests indicated that ICA, tyrosine phosphatase antibody (IAA-2), and glutamic acid decarboxylase antibody (GADA-65) were negative, but the insulin autoantibody (insulin resistance IgG antibody) level was positive (Table 3).
Figure 1.
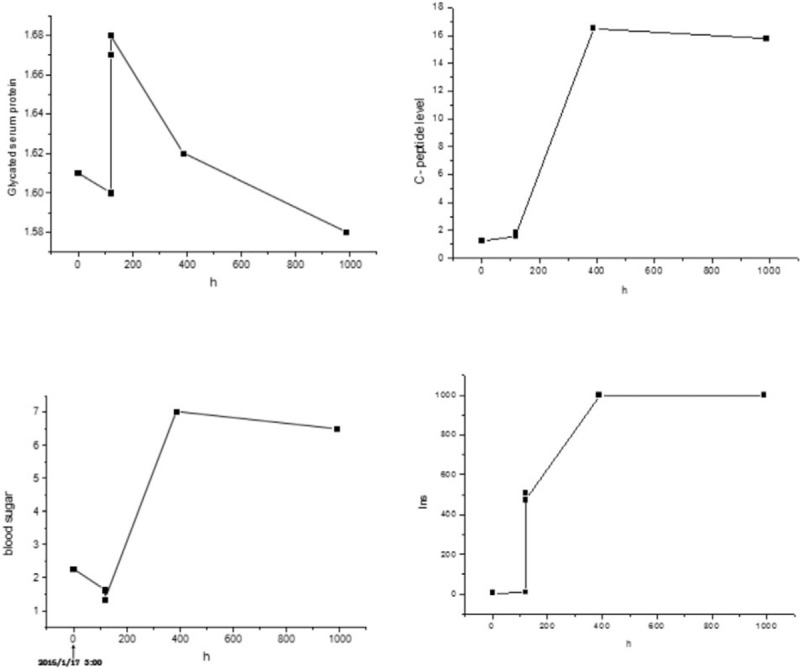
The figure illustrates the levels of serum insulin, blood glucose, glycated serum protein and C-peptide release during the episodes of spontaneous hypoglycemia in the patient. Results of serum insulin level, blood sugar level, glycated serum proteinserum, and C-peptide release during the patient are episodes of spontaneous hypoglycemia. The serum levels of insulin and C-peptide both were inappropriately elevated in the presence of hypoglycemia.
Table 3.
Anti-islet β-cell autoantibody tests results.
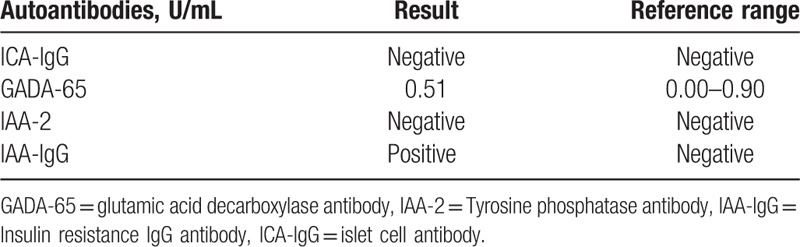
To further identity the relationship between the serum concentrations of insulin, glucose, and C-peptide, the patient had an oral glucose tolerance test (OGTT), insulin release test, and C-peptide release test (Table 2). The levels of insulin and C-peptide were markedly increased, and there was no history of exogenous insulin administration as the cause of hypoglycemia. Magnetic resonance of the abdomen and ultrasound results showed no space-occupying lesions in the pancreas. The outcomes indicated that IAS might have induced the hypoglycemia.
Table 2.
Insulin and C- peptide release test results.
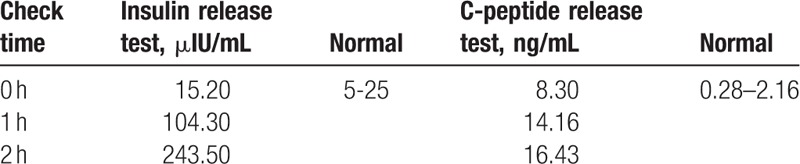
After inspection of the symptoms and laboratory, endocrinological, and imaging results together with no history of diabetes mellitus and hypertension, and no previous exposure to insulin or oral antidiabetic agents, we excluded insulinoma and adrenal hypofunction. Consequently, we made a diagnosis of IAS with possible methimazole association. However, pregnancy is another factor that can induce IAS.
The patient had an abortion, discontinued methimazole and switched to oral prednisone (30 mg once daily) and propylthiouracil (100 mg 3 times daily) for 3 months. During the follow-up visit after 3 months, the patient had not experienced any further hypoglycemic episodes, and prednisone was maintained at 5 mg once daily. The insulin and C-peptide levels had decreased to normal levels, and insulin autoantibodies levels were negative. The symptoms caused by Graves disease had also improved with drug treatment. The serum thyroid-stimulating hormone (sTSH) level was <0.005 mU/L, free triiodothyronine (FT3) level was 16.10 pg/mL, and free (unbound) thyroxine (FT4) was 5.58 ng/dL.
2.1. Ethical approval
Ethical approval was not necessary because our study is a case report.
2.2. Patient approval
This patient signed an informed consent before publication.
3. Discussion
The female patient took methimazole for nearly 6 months and had no history of exogenous insulin administration or insulin-stimulating drug therapy. This patient experienced recurrent hypoglycemia with symptoms that began with headache, anxiety, and hunger, which excited the parasympathetic nervous system, and progressed to a decrease in blood glucose. In addition, the patient had episodes of sympathetic nervous symptoms, such as sweating, heart palpitations, fatigue, and pale skin. The most severe symptom experienced was hypoglycemic coma.
The insulin release test and C-peptide release test showed that levels of insulin and C-peptide were inappropriately increased. The insulin autoantibody (insulin resistance IgG antibody) level was positive and the ICA and GADA tests were negative. Furthermore, there were no space-occupying lesions in the pancreas and no underlying autoimmune disorders. Therefore, we diagnosed that insulin autoantibody production caused the hypoglycemia.
The number of IAS case reports in the Chinese Biomedical Literature Database between 1994 and May 2017 is 142, including 62 males and 80 females with an age range of 9 to 82 years and a median of 46 years. Of the total cases, 18 are classified as idiopathic IAS. The outcome analyses of the other 124 cases show that methimazole for Graves disease is the most common primary inducement of IAS (64.5%). The other drugs listed include tiopronin (6.4%), captopril (1.6%), insulin (11.2%), D-penicillamine (2.4%), propranolol (0.8%), and ɑ-lipoic acid (1.6%) (Table 4).
Table 4.
Drug-induced IAS from 1994 and May of 2017 in china.
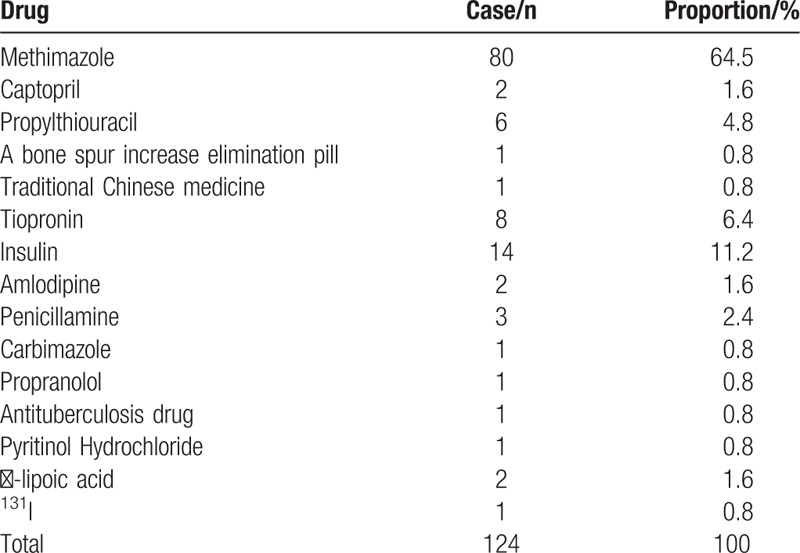
The Chinese database indicates that ∼86.7% (123/142) of the patients had taken drugs of the sulfhydryl group, such as methimazole (64.5%). In most cases, insulin autoantibodies appear a few weeks after treatment with a drug containing the sulfhydryl group.[7] The insulin-A-chain possesses a loop that is formed by the S-S bond, and the sulfhydryl group of drugs may cleave the disulfide bond of the insulin molecule in vivo to make the secreted endogenous insulin allosteric. This triggers an immune response, which produces insulin autoantibodies and activates the self-insulin-specific T-helper cells to enhance its immunogenicity.[8–10] When these autoantibodies combine with insulin antigen, the amount of insulin decreased induces hyperglycemia. Moreover, hyperglycemia stimulates β-cells to release insulin, which produces hyperinsulinemia and increases insulin antibodies. The increased activity of plasma insulin antibodies promotes the dissociation of insulin, which releases excessive free insulin.
Hypoglycemia is thought to be caused by the binding and release of insulin from the antibodies, which occurs in synchrony with the prevailing glucose concentration.[7,11,12] However, there is clinical and experimental evidence that maternal immune responses during pregnancy are biased toward antibody-mediated immunity.[13] The increased dominant hormones produced during pregnancy, such as corticosteroids, GH, and progesterone, are capable of polarizing the cytokine balance toward a Th2 dominance.[13,14] A Th2-biased immune response leads to increased antibody production.[15] These antibodies include insulin antibodies, ICA, and other disease-associated antibodies.[16–19] Therefore, hyperinsulinemic hypoglycemia is considered due to insulin autoantibodies induced by methimazole treatment and pregnancy.
We should consider a specialty differential diagnosis of insulinoma and IAS. Both conditions have similar episodes of recurrent hypoglycemia and hyperinsulinemia, but differ as follows[20,21]: In insulinoma, hypoglycemia occurs in the fasting state, while in IAS it is repeatedly experienced at night and early morning. A fasting test is a clinical diagnostic method. The IAA level of insulinoma is ≤1000 μU/mL, and the increase of insulin concentrations synchronize with the elevation of C-peptide levels. The oral glucose tolerance testing (OGTT) results of IAS indicates impaired glucose tolerance or postprandial blood glucose (2 h level ≧11.1 mmol/L), but blood glucose levels of IAS are a flat curve. The dynamic state of blood glucose monitoring is used to distinguish the conditions; levels fluctuate in IAS while insulinoma has low levels. The IAA level is the key for identifying the conditions. The IAA levels of IAS are mostly positive, but can turn negative. The IAA levels of insulinoma are negative. Imageological examination such as abdominal ultrasonography, pancreas magnetic resonance imaging, and endoscopic pancreas ultrasonography is an auxiliary method. Insulinoma frequently shows pancreas space-occupying lesions, while IAS is negative. In our case study, the patient had hyperinsulinemic hypoglycemia at night and early morning, the IAA level was positive, and imaging showed no mass lesion in the pancreas. Therefore, we excluded insulinoma.
Many patients with IAS get relief after drug discontinuation. Glucocorticoids are administered for IAS.[22] The mechanism of glucocorticoids for the treatment of IAS involves the elevation of blood glucose caused by insulin resistance, the suppression of insulin autoimmunity, and the repression of conversion of T4 (thyroxine) to T3 (Triiodothyronine).[23] In this study, we prescribed the patient with a small dose of prednisone, with an initial dosage of 30 mg daily and then maintained at 5 mg daily. We also suggested a change in eating habits to help reduce episodes of hypoglycemia. Furthermore, corticosteroids and immunosuppressant therapy could reduce levels of insulin autoantibodies.[22,24] Acarbose, diazoxide, octreotide, pancreatectomy, and plasmapheresis are other choices for the treatment of IAS.[25–27] The patient did not experience any further hypoglycemic episodes after the abortion and discontinuation of methimazole. Her insulin autoantibodies levels were negative after 3 months of prednisone treatment.
4. Conclusion
We report a rare case of IAS in a pregnant female. In cases involving hypoglycemia, clinicians should consider drug-induced IAS, but attention should also be given to pregnancy as another factor of the hypoglycemic symptoms.
Footnotes
Abbreviations: ACTH = adrenocorticotropic hormone, FT3 = free triiodothyronine, FT4 = free (unbound)thyroxine, GADA-65 = glutamic acid decarboxylase, GH = growth hormone, IAA-2 = Tyrosine phosphatase antibody, IAS = insulin autoimmune syndrome, ICA = islet cell antibody, OGTT = oral glucose tolerance testing, sTSH = serum thyroid stimulating hormone, T3 = Triiodothyronine, T4 = thyroxine.
Funding/support: The study was supported by grants from the National Natural Science Funds of China (nos. 81760168 and 81460018), Jiangxi Provincial Science Technology Foundation of China (No. 20151BBG70073), and Jiangxi Provincial Department of Education Scientific Research Funds of China (nos. GJJ13145 and GJJ13178).
The authors declare that no conflicts of interest exist.
References
- [1].Hirata Y, Ishizu H, Ouchi H, et al. Insulin autoimmunity in a case of spontaneous hypoglycemia. Jpn Diabetes Soc 1970;13:312–20. [Google Scholar]
- [2].Seino S, Fu ZZ, Marks W, et al. Characterization of circulating insulin in insulin autoimmune syndrome. J Clin Endocrinol Metab 1986;62:64–9. [DOI] [PubMed] [Google Scholar]
- [3].Ismail AA. The insulin autoimmune syndrome (IAS) as a cause of hypoglycaemia: an update on the pathophysiology, biochemical investigations and diagnosis. Clin Chem Lab Med 2016;54:1715–24. [DOI] [PubMed] [Google Scholar]
- [4].Rajpal A, Kassem LS, Moscoso-Cordero M, et al. Clopidogrel-induced insulin autoimmune syndrome: a newly recognized cause of hypoglycemia in a non-diabetic patient. J Endocr Soc 2017;1:1217–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wang YL, Yao PW, Zhang XT, et al. Insulin autoimmune syndrome: 73 cases of clinical analysis. Chin Med J (Engl) 2015;128:2408–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Uchigata Y, Hirata Y, Iwamoto Y. Insulin autoimmune syndrome (Hirata disease): epidemiology in Asia, including Japan. Diabetol Int 2010;1:21–5. [Google Scholar]
- [7].Virally ML, Timsit J, Chanson P, et al. Insulin autoimmune syndrome: a rare cause of hypoglycaemia not to be overlooked. Diabetes Metab 1999;25:429–36. [PubMed] [Google Scholar]
- [8].Takeuchi Y, Miyamoto TT, Shigematsu S, et al. Insulin autoimmune syndrome possibly caused by alpha lipoic acid. Intern Med 2007;46:237–9. [DOI] [PubMed] [Google Scholar]
- [9].Matsushita S, Takahashi K, Motoki M, et al. Allele specificity of structural requirement for peptides bound to HLA-DRB1∗0405 and -DRB1∗0406 complexes: implication for the HLA-associated susceptibility to methimazole-induced insulin autoimmune syndrome. J Exp Med 1994;180:873–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Uchigata Y, Hirata Y, Iwamoto Y. Drug-induced insulin autoimmune syndrome. Diabetes Res Clin Pract 2009;83:e19–20. [DOI] [PubMed] [Google Scholar]
- [11].Nasu T, Suzuki R, Okamoto Y, et al. Late postprandial hypoglycemia due to bioactive insulin dissociation from autoantibody leading to unconsciousness in a patient with insulin autoimmune syndrome. Intern Med 2011;50:339–43. [DOI] [PubMed] [Google Scholar]
- [12].Wong SL, Priestman A, Holmes DT. Recurrent hypoglycemia from insulin autoimmune syndrome. J Gen Intern Med 2014;29:250–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Hämäläinen AM, Savola K, Kulmala PK, et al. Disease-associated autoantibodies during pregnancy and at birth in families affected by type 1 diabetes. Clin Exp Immunol 2001;126:230–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wilder RI. Hormones, pregnancy, and autoimmune diseases. Ann N Y Acad Sci 1998;840:45–50. [DOI] [PubMed] [Google Scholar]
- [15].Wegmann TG, Hui L, Guilbert L, et al. Bidirectional cytokine interactions in the maternal-fetal relationship: is successful pregnancy a TH2 phenomenon? Immunol Today 1993;14:353–6. [DOI] [PubMed] [Google Scholar]
- [16].da Silva TN, Ferreira AG, Manita I, et al. Insulin autoimmune syndrome: the relationship between insulin, c-peptide and glucose in active and recovering disease states. Endocr Abstracts 2017;49:E486. [Google Scholar]
- [17].Mario UD, Fallucca F, Gargiulo P, et al. Insulin-anti-insulin complexes in diabetic women and their neonates. Diabetologia 1984;27:83–6. [DOI] [PubMed] [Google Scholar]
- [18].Martikainen A, Saukkonen T, Kulmala PK, et al. Disease-associated antibodies in offspring of mothers with IDDM. Diabetes 1996;45:1706–10. [DOI] [PubMed] [Google Scholar]
- [19].Ziegler AG, Hummel M, Schenker M, et al. Autoantibody appearance and risk for development of childhood diabetes in offspring of parents with type 1 diabetes: the 2-year analysis of the German BABYDIAB Study. Diabetes 1999;48:460–8. [DOI] [PubMed] [Google Scholar]
- [20].Basu A, Service FJ, Yu L, et al. Insulin autoimmunity and hypoglycemia in seven white patients. Endocr Pract 2005;11:97–103. [DOI] [PubMed] [Google Scholar]
- [21].Vaidakis D, Karoubalis J, Pappa T, et al. Pancreatic insulinoma: current issues and trends. Hepatobiliary Pancreat Dis Int 2010;9:234–41. [PubMed] [Google Scholar]
- [22].Saxon DR, Mcdermott MT, Michels AW. Novel management of insulin autoimmune syndrome with rituximab and continuous glucose monitoring. J Clin Endocrinol Metab 2016;101:1931–5. [DOI] [PubMed] [Google Scholar]
- [23].Gomez Cruz MJ, Jabbar M, Saini N, et al. Severe hypoglycemia secondary to methimazole-induced insulin autoimmune syndrome in a 16 year old African-American male. Pediatr Diabetes 2012;13:652–5. [DOI] [PubMed] [Google Scholar]
- [24].Ohtsuka Y, Kondo T, Shimada M, et al. Erythrocyte insulin receptor in insulin autoimmune syndrome: effects of corticosteroid therapy. Tohoku J Exp Med 1987;151:181–90. [DOI] [PubMed] [Google Scholar]
- [25].Lupsa BC, Chong AY, Cochran EK, et al. Autoimmune forms of hypoglycemia. Medicine 2009;88:141–53. [DOI] [PubMed] [Google Scholar]
- [26].Kim MR, Sheeler LR, Mansharamani N, et al. Insulin antibodies and hypoglycemia in diabetic patients. Can a quantitative analysis of antibody binding predict the risk of hypoglycemia? Endocrine 1997;6:285–91. [DOI] [PubMed] [Google Scholar]
- [27].Chu JP, Zheng XW, Lu J, et al. Insulin-induced autoimmune syndrome: a case report. Exp Ther Med 2016;12:3359–62. [DOI] [PMC free article] [PubMed] [Google Scholar]


