Abstract
Rationale:
Endometrial cancer patients with lung metastases are rare, and more rarely with long-term management of progesterone after recurrence.
Patient concerns:
Informed consent of the patients and their families.
Diagnoses:
Endometrial cancer (IVB) (Refer to 2009 FIGO stag of endometrial cancer).
Interventions:
the patient was treated with Megestrol Acetate Dispersible Tablets (trade name Yilizhi), 160 mg, orally, once daily, without interruption.
Outcomes:
The patient has been treated with progesterone therapy for stable conditions and her survival time is already roughly a decade (December 2006–October 2016).
Lessons:
Hormone therapy may as a long-term management for hormone receptor-positive patients with recurrent endometrial cancer.
Keywords: endometrial cancer, hormone therapy, lung metastasis, recurrence
1. Introduction
Endometrial cancer (EC) is one of the 3 malignant tumors of the female reproductive system whose most common histological subtype is endometriosis adenocarcinoma, and is also the most common gynecological cancer in developed countries.[1] Recently, with the lifestyle changes, the incidence of EC is increasing and, among gynecological cancers, its high morbidity is only second to cervical cancer. EC symptoms appear early and make early diagnosis possible, thus patients in stage I or II with 5-year survival rate can be more than 90%. However, there still remain 20–30% of patients who progress into stage III or IV and their 5-year survival rate is less than 30%, accounting for more than half of EC deaths.[2,3] A growing body of research indicates that the recurrence rate of early EC shows a tendency to increase, and 10 to 15% of patients with early stage EC have been reported to develop into recurrent EC.[4] Most patients with recurrent EC are inclined to select radiotherapy and chemotherapy as treatment, while those choosing hormone therapy are far fewer. Accordingly, how should choose hormone therapy after hysterectomy, and for the recurrent patients, especially for those patients with lung metastasis.
2. Case report
A 57-year-old woman, gestation 4, production 2, presented with irregular vaginal bleeding for 3 months, 7 months after the onset of menopause. The patient was examined in a gynecology clinic, in December 2006, she had no personal history of diabetes or cardiovascular disease and her body mass index (BMI) was within normal limits, despite having undergone subtotal thyroidectomy due to hyperthyroidism 13 months before. In addition, she had a family history of cancer and her brother died from esophageal cancer. Transvaginal B-ultrasound examination revealed irregular endometrial thickening, with a thickness of 1.1 cm, the echo was not uniform and showed abundant arteriovenous blood flow signal. Subsequently, curettage of the uterus was performed on the patient and a biopsy of the uterus revealed a moderately differentiated endometrioid adenocarcinoma. For the treatment of the EC, the patient, under general anesthesia, underwent an operation of epidural hysterectomy and bilateral salpingo-oophorectomy plus pelvic and para-aortic lymph nodes dissection. In addition the peritoneal cytology was negative. Postoperatively, the patient was diagnosed with endometrioid adenocarcinoma whose clinical FIGO stage and histological grade was IB and G2, respectively (referred to the 1988 FIGO stage of endometrial cancer at that time). The postoperative pathological report was consistent with the biopsy results, it showed that the carcinoma had a size of 2.5×0.8 cm and infiltrated the superficial muscle layer; immunohistochemical analysis was not performed during that period, however (Fig. 1A). Fortunately, the patient had no cervical or vaginal carcinoma invasion, no lymph node metastasis and no abnormality of double attachment. After being released from the hospital, the patient was followed-up regularly, exhibited no obvious change in tumor markers levels and on computed tomography (CT) analysis and maintained a good quality of life, without signs of tumor progression or recurrence.
Figure 1.
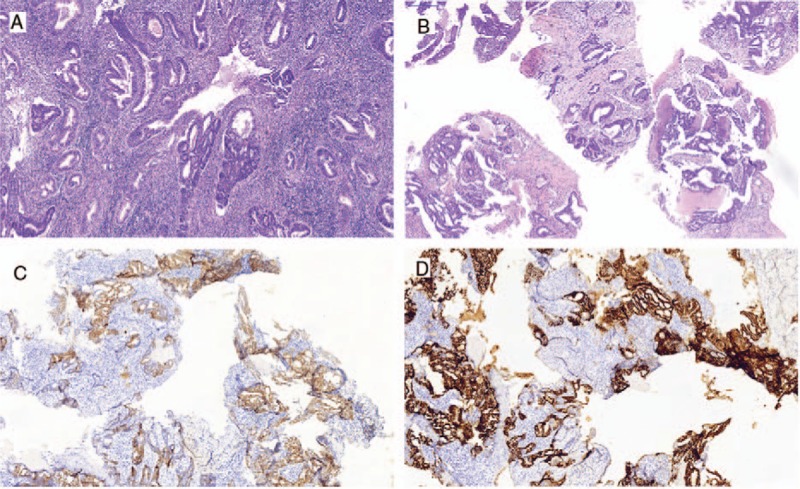
Endometrial biopsy after hysterectomy (A). Lung biopsy: After 54 months of remission (B), and immunohistochemical staining was found ER (++) (C), PR (++) (D). ER = estrogen receptor, PR = progesterone receptor.
The patient presented with irregular cough with no apparent cause after 54 months of remission, but appearance of pulmonary metastasis was suspected. Pulmonary CT showed multiple high-density pulmonary nodules indicating that it was likely a metastatic tumor (Fig. 2). Also, lung biopsy confirmed the moderately differentiated adenocarcinoma infiltration of the lung tissue, which according to our pathologist was from EC metastasis, combined with her cancer history (Fig. 1B). Additionally, immunohistochemical analysis revealed the staining status of ER (++), PR (++), C-erB-2(−) (Fig. 1C and D). In addition, the cancer antigen 125 (CA-125) level was 24.62 U/mL (normal range, 0–35 U/mL). After explaining the patient's condition to her daughter, the family made it clear that they refused the treatment by auxiliary chemotherapy, but agreed to hormone therapy. Accordingly, the patient was treated with Megestrol Acetate Dispersible Tablets (trade name Yilizhi), 160 mg, orally, once daily, without interruption. No significant progression of the tumor was observed in pulmonary CT scans 3, 9, 22, 24, 34 and 63 months after the lung metastasis was detected (Fig. 3) and corresponded with the CA-125 levels which remained steady within the normal range. To date, the patient has been treated with progesterone therapy for stable conditions and her survival time is already roughly a decade (December 2006–October 2016). This implies that rational hormone therapy is an effective means to treat EC patients postoperatively, and especially for the recurrent cases. During the treatment period with this drug, the monitoring of the patient's biochemical indices of the liver and kidney function revealed that they all were in the normal range.
Figure 2.
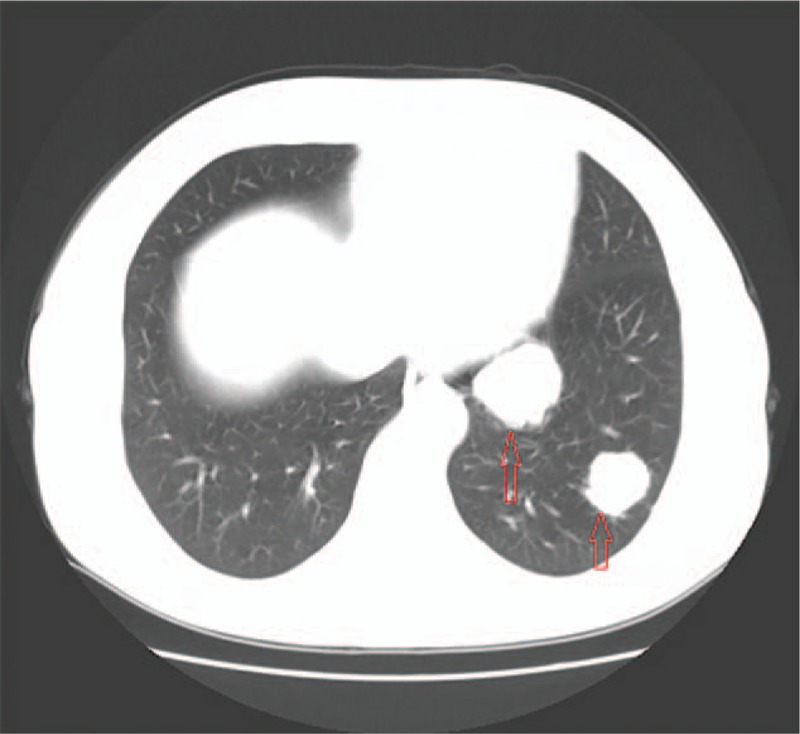
Pulmonary CT after 54 months of remission. It showed that multiple pulmonary nodular density increased shadow and it is likely metastatic tumor. CT = computed tomography.
Figure 3.
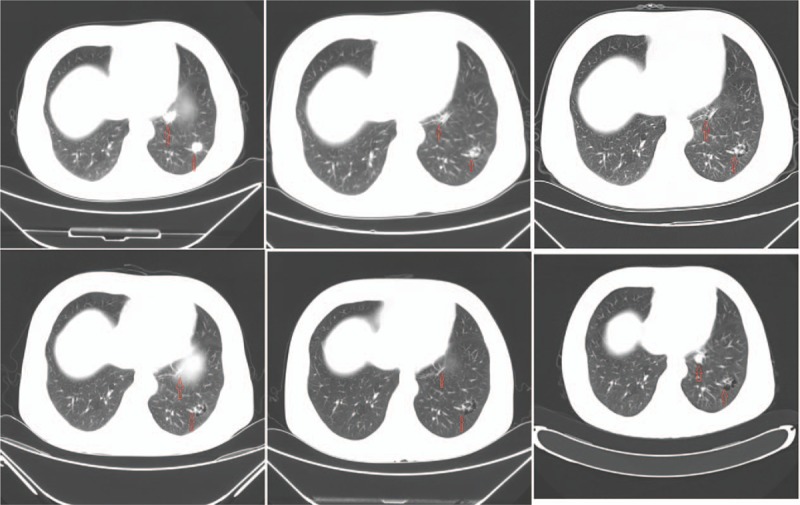
Pulmonary CT changes after treat with progesterone 3, 9, 22, 24, 34, and 46 months (from left to right and top to bottom.). There are no significant progress was seen in pulmonary CT. CT = computed tomography.
3. Discussion
3.1. Treatment of primary EC
Overall prognosis for these patients is excellent because cancer is detected in great majority of patients at the early stage of the disease when the lesion is confined to the uterus, which can be removed by hysterectomy, resulting in increase of the 5-year survival rates above 70%.[5] The main treatment alternative is surgery, and is performed on the primary endometrial cancer, which provides the basis for the removal of diseased tissue, identification of extra uterine metastasis, complementary treatment and scientific evaluation of the prognosis. However, how a treatment regimen is matched with a high-risk factor remains unclear. EC risk factors include the tumor type, FIGO stage, histological grade, and age.[6,7] They even include myometrial invasion depth, lymphatic vascular invasion, pelvic lymph node metastasis, positive peritoneal cytology, along with endogenous and exogenous estrogens associated with obesity, diabetes, early age at menarche, gravidity, late-onset menopause, older age (≥55 years), and use of tamoxifen.[8–10]
High-risk patients must be identified in order to give them the appropriate adjuvant therapy, which includes lymph node surgical strategy, vaginal short-range radiotherapy, and external beam pelvic radiotherapy (EBRT). Currently, adjuvant radiotherapy still remains as the routine postoperative treatment for early EC, such as for stage IIB, while chemotherapy is mainly used for advanced or recurrent EC, and the preferred first-line chemotherapy is the TC (paclitaxel and carboplatin) program. However, whether chemotherapy should be maintained after surgery, how to select antineoplastic agents, and whether to use combined hormones, are treatment options for which there is no consensus.
3.2. Recurrence of EC
There is evidence showing that low expression and even loss of expression of ER and PR are associated with invasion and metastasis of EC, as they can increase the risk of cancer cell invasion and metastasis. CA-125 is a common gynecologic tumor marker whose expression status is also related to the pathogenesis and progression of EC, and a study also found that CA-125 positive EC patients are more likely to have ectopic metastasis. Moreover, EC patients may also suffer from diabetes, hypertension, and obesity, which are also associated with ectopic metastasis of EC.[11] The history of malignant tumors in patients with solitary pulmonary nodules or masses indicates that in 25 to 46% of the cases is a metastatic tumor;[12] of course, this case has been confirmed by biopsy. In a Danish study, a population-based historical cohort of 2612 patients with early EC had a recurrence rate of 7% within 3 years after the initial treatment.[13]
Extra uterine metastasis often occurs in patients with late stage EC. An analysis of the pattern of recurrence and survival in an EC in Thailand revealed the local recurrence (LR) and distant/combined sites of recurrence (DCSR) at a median relapse time of 6.6 (95%CI = 4.6–8.6 months) and 16.9 months (95%CI = 5.6–28.2 months) (P = .36) after the primary treatment.[14] Kaewpangchan et al also noticed that the common site of relapse is the vaginal cuff, pelvis and lung. Jeppesen et al suggested that the recurrence site has a significant effect on the 5-year survival rate: vaginal recurrence and DCSR recurrence were 64.8% and 17.5%, respectively. In contrast, the recurrence rate was found to be relatively low in stage IB patients, even though most patients did not receive adjuvant therapy.[15]
The most common metastatic sites for EC are pelvic lymph nodes, followed by retroperitoneal lymph nodes, but distant metastases, such as lung, liver and brain metastasis are really rare.[16,17] Sohaib et al also reported that the pathological type of EC had no significant association with the prognosis of the patient's survival. However, an adverse histological type would reduce the specific survival of an individual and promote distant recurrence.
3.3. Treatment of recurrence EC
It is generally believed that the treatment after relapse, and whether or not it has been treated with radiotherapy, is associated with the location of the recurrence. In 2016 the National Comprehensive Cancer Network (NCCN) guidelines pointed out that for the isolated vaginal recurrence after radiotherapy the 5-year survival rate was 50 to 70%, whereas recurrence beyond the vagina or pelvic lymph node is a poor prognosis. The guidance for disseminated lesions is as follows: (1) patients with grade G1 or asymptomatic or ER/PR positive may be treated with hormone therapy, chemotherapy will be carried out if the condition progresses. (2) Patients with histological grading of G2 to G3 or those who have symptoms or massive focus underwent chemotherapy and/or palliative radiotherapy. Further, supportive treatment or clinical trial will be implemented, respectively.
Compared to adjuvant therapy, secondary surgery is also a choice for relapse patients. Borghesi et al[18] evaluated patients who underwent secondary surgery for stage IEC with intermediate and advanced recurrence risk, including the risk of lymph vascular space invasion (LVSI).Thus, LVSI is an independent factor for overall survival and progression-free survival, HR 7.2, 95% CI 3.1–17, P < .001and HR 3.7, 95% CI 1.6–8.5, P < .003, respectively. EC patients with recurrence after the initial treatment have aggressive cancer, and poor prognosis.
3.4. Hormone therapy for EC
It is not uncommon to find reports on the use of hormone therapy for fertility preservation in EC patients. A systematic review indicated that continuous combined hormone therapy is better than sequential combined therapy of unopposed estrogen and tibolone.[19] This case was a moderately differentiated adenocarcinoma, and the woman primary treatment was effective, but lung metastasis was detected 54 months after the operation. The family of the patient strongly required that the patient be treated by hormone therapy only and the condition has been well controlled for 63 months with the progesterone treatment.
Progestogens are known as inhibitors of endometrial carcinogenesis and cancer suppressive factors.[20,21] They can be divided into natural or synthetic depending on the source, and further subdivided, according to the structure, into progesterone (such as medroxy progesterone acetate, cyproterone acetate, and dydrogesterone) and testosterone (such as norethindrone, norgestimate, and gestodene).[20] Chlebowski et al[22] reported that the continuous combined estrogen plus progestin use reduced the incidence of EC by 35% in postmenopausal women, and the effects were observed in all histological types. Decruze and Green[23] reported that hormone therapy is mainly used for advanced or recurrent EC, if the EC has a high degree of differentiation, is hormone receptor positive, or shows no clinical symptoms, it will have higher response to the therapy. However, the literature on EC recurrence with lung metastasis and hormone treatment program is scarce. In the present case, the patient with pathological immunohistochemical staining showed ER (++), PR (++), which can allow the patient to also choose hormone therapy instead of radiotherapy or chemotherapy.
Most patients with EC have lesions which are confined to the uterus and the main pathological type is adenocarcinoma, with a good overall prognosis. In fact, patients with metastatic tumor are better guided by individualized treatment based on factors of comorbidities, tumor histological grade, performance status, and former treatments. Although the overall prognosis is still poor, several therapy plans are available for the recurrent EC, whose management traditionally includes hormone therapy and cytotoxic chemotherapy and molecular biology targeted therapy that involves cell growth and proliferation pathways to inhibit angiogenesis and cell signaling.[5,24] The cases of EC complicated with lung metastasis are rare in the clinic, we reported the case that treatment with progesterone achieved survival for about a decade, and the lung metastasis is well controlled with no apparent progression. Maybe it was also an occasional opportunistic event, what is needed now is to assess the efficacy of progesterone in a number of similar cases, and to comprehensively evaluate the treatment of patients with lung metastasis from EC.
Footnotes
Abbreviations: CA-125 = cancer antigen 125, EBRT = external beam pelvic radiotherapy, EC = endometrial cancer, ER = estrogen receptor, LR = local recurrence, DCSR = distant/combined sites of recurrence, LVSI = lymph vascular space invasion, NCCN = National Comprehensive Cancer Network, PR = progesterone receptor, TC = paclitaxel and carboplatin.
Ethics: The project was approved by the Ethics Committee of the Affiliated Hospital of Qingdao University.
Patient consent: The informed consent of the patient has been explicitly obtained prior to this study.
The authors have no conflicts of interest to disclose.
References
- [1].Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11–30. [DOI] [PubMed] [Google Scholar]
- [2].Straughn JM, Jr, Huh WK, Kelly FJ, et al. Conservative management of stage I endometrial carcinoma after surgical staging. Gynecol Oncol 2002;84:194–200. [DOI] [PubMed] [Google Scholar]
- [3].Hicks ML, Phillips JL, Parham G, et al. The National Cancer Data Base report on endometrial carcinoma in African–American women. Cancer 1998;83:2629–37. [DOI] [PubMed] [Google Scholar]
- [4].Kong A, Johnson N, Kitchener HC, Lawrie TA. Adjuvant radiotherapy for stage I endometrial cancer. The Cochrane database of systematic reviews. Apr 18 2012(4):Cd003916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Temkin SM, Fleming G. Current treatment of metastatic endometrial cancer. Cancer Control 2009;16:38–45. [DOI] [PubMed] [Google Scholar]
- [6].Eggink FA, Mom CH, Boll D, et al. Compliance with adjuvant treatment guidelines in endometrial cancer: room for improvement in high risk patients. Gynecol Oncol 2017;146:380–5. [DOI] [PubMed] [Google Scholar]
- [7].Morice P, Leary A, Creutzberg C, et al. Endometrial cancer. Lancet 2016;387:1094–108. [DOI] [PubMed] [Google Scholar]
- [8].Renehan AG, Tyson M, Heller RF, et al. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet (London, England) 2008;371:569–78. [DOI] [PubMed] [Google Scholar]
- [9].Purdie DM, Green AC. Epidemiology of endometrial cancer. Best practice & research. Clin Obstetr Gynaecol 2001;15:341–54. [DOI] [PubMed] [Google Scholar]
- [10].Kaaks R, Lukanova A, Kurzer MS, et al. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev 2002;11:1531–43. [PubMed] [Google Scholar]
- [11].Jung IK, Kim SS, Suh DS, et al. Tumor-infiltration of T-lymphocytes is inversely correlated with clinicopathologic factors in endometrial adenocarcinoma. Obstetr Gynecol Sci 2014;57:266–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Seo JB, Im JG, Goo JM, et al. Atypical pulmonary metastases: spectrum of radiologic findings. Radiographics 2001;21:403–17. [DOI] [PubMed] [Google Scholar]
- [13].Jeppesen MM, Jensen PT, Gilsa Hansen D, et al. The nature of early-stage endometrial cancer recurrence—a national cohort study. Eur J Cancer 2016;69:51–60. [DOI] [PubMed] [Google Scholar]
- [14].Kaewpangchan P, Cheewakriangkrai C. Relapse patterns and outcomes following recurrence of endometrial cancer in northern Thai women. Asian Pac J Cancer Prev 2015;16:3861–6. [DOI] [PubMed] [Google Scholar]
- [15].Rahatli S, Dizdar O, Kucukoztas N, et al. Good outcomes of patients with stage IB endometrial cancer with surgery alone. Asian Pac J Cancer Prev 2014;15:3891–3. [DOI] [PubMed] [Google Scholar]
- [16].Sohaib SA, Houghton SL, Meroni R, et al. Recurrent endometrial cancer: patterns of recurrent disease and assessment of prognosis. Clin Radiol 2007;62:28–34. discussion 35-26. [DOI] [PubMed] [Google Scholar]
- [17].Park JW, Hwang SO, Choi SJ, et al. Liver recurrence in early endometrial cancer with focal myometrial invasion. Obstetr Gynecol Sci 2013;56:338–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Borghesi Y, Narducci F, Bresson L, et al. Managing endometrial cancer: the role of pelvic lymphadenectomy and secondary surgery. Ann Surg Oncol 2015;22(suppl 3):S936–943. [DOI] [PubMed] [Google Scholar]
- [19].Sjogren LL, Morch LS, Lokkegaard E. Hormone replacement therapy and the risk of endometrial cancer. A systematic review. Maturitas 2016;91:25–35. [DOI] [PubMed] [Google Scholar]
- [20].Stanczyk FZ, Hapgood JP, Winer S, et al. Progestogens used in postmenopausal hormone therapy: differences in their pharmacological properties, intracellular actions, and clinical effects. Endocr Rev 2013;34:171–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yang S, Thiel KW, Leslie KK. Progesterone: the ultimate endometrial tumor suppressor. Trends Endocrinol Metab 2011;22:145–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chlebowski RT, Anderson GL, Sarto GE, et al. Continuous combined estrogen plus progestin and endometrial cancer: the women's health initiative randomized trial. J Natl Cancer Inst 2016;108:pii: djv350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Decruze SB, Green JA. Hormone therapy in advanced and recurrent endometrial cancer: a systematic review. Int J Gynecol Cancer 2007;17:964–78. [DOI] [PubMed] [Google Scholar]
- [24].Del Carmen MG, Boruta DM, 2nd, Schorge JO. Recurrent endometrial cancer. Clin Obstetr Gynecol 2011;54:266–77. [DOI] [PubMed] [Google Scholar]


