Abstract
Background
: This study aimed to assess the efficacy and safety of drotaverine hydrochloride (DHC) in Chinese patients with irritable bowel syndrome (IBS).
Methods
: Totally, 144 patients with IBS were included and randomly divided into treatment group and placebo group in a 1:1 ratio. Patients received either DHC or placebo 80-mg tablet, 3 times daily for a total of 4 weeks. The primary outcome included abdominal pain, measured by the visual analog scale (VAS), and weekly stool frequency. The secondary outcomes were measured by the Bristol scale, and the 36-item short form health survey (SF-36), as well as the adverse events recorded during the treatment period. All those outcomes were measured at the end of 4-week treatment.
Results
: The total and different types of IBS in VAS, stool frequency, and Bristol score were significantly better in the treatment group than those in the placebo group at the end of 4-week treatment. However, no significant difference was found in quality of life, measured by SF-36 scale between 2 groups. Additionally, no serious and significant differences in adverse events were found in and between both groups.
Conclusion
: The findings suggest that DHC has promising efficacy to enhance symptoms of IBS in Chinese population.
Keywords: clinical trial, drotaverine hydrochloride, efficacy, irritable bowel syndrome
1. Introduction
Irritable bowel syndrome (IBS) is a common functional gastrointestinal disorder.[1–3] It often manifests with the abdominal pain, discomfort such as cramping, bloating, gas, diarrhea, and/or constipation.[4–7] It has been reported the prevalence ranged from 3.7% to 22% in Asian population.[8,9] The other study reported that the prevalence was at 24.0%,[10] with a 3:1 female predominance.[11]
It has been found that IBS consists of 4 different bowel patterns, including diarrhea predominant of IBS (IBS-D), constipation predominant of IBS (IBS-C), mixed diarrhea and constipation of IBS, and alternating diarrhea and constipation of IBS. However, most patients with IBS seek medical care because of the abdominal pain.[12] In addition, this kind of condition often significantly affect their quality of life.[13]
The goal of the treatment for this condition is to relieve its symptoms. The therapies for patients with IBS in clinics mainly include the suggestions from doctor about the diet and lifestyle change, and medications. The diet and lifestyle change often consists of avoiding foods that trigger the symptoms, eating high-fiber foods, drinking plenty of fluids, exercising regularly, and getting enough sleep. Additionally, several specific medications are often used to treat such condition, such as alosetron, eluxadoline, rifaximin, lubiprostone, and linaclotide.
Current treatment for IBS include medication, stress relief, and eating habits changes for patients with IBS.[14–16] Although those therapies can relieve some symptoms of IBS, more effective and safe therapies are still looked for treating and relieving this kind of condition. Previous studies also reported to use antispasmodics to treat IBS.[17,18] However, they often accompanied with lots of anticholenergic side effects, and restricted their use.
Drotaverine is an another kind of antispasmodic.[19–22] It is reported that it has a good relaxing effect on intestinal smooth muscle.[20,22] It has showed promising effect of abdominal pain relief without side effects like anticholinergics. Although several studies have explored the effect and safety of drotaverine hydrochloride (DHC) for treating IBS, no study specifically focused on Chinese patients with IBS treated by DHC only.
This study aimed to conduct a randomized controlled trial to assess the efficacy and safety of DHC for treating Chinese patients with IBS specifically. We hypothesized that DHC for the treatment of IBS in Chinese population would be superior to the efficacy of placebo.
2. Methods/design
2.1. Study design
This study was approved by the ethics committee of The People's Hospital of Yan’an. It was conducted at the People's Hospital of Yan’an from April 2014 to August 2016. A total of 144 patients were randomly allocated to the treatment group and the placebo group in a 1:1 allocation ratio. The patients received either DHC or placebo 80-mg tablet, 3 times daily for a total of 4 weeks. Outcome evaluation and data analysis were performed at the end of 4-week treatment.
2.2. Patients
Patients with following criteria were included: aged between 18 and 70 years old; confirmed diagnosis of IBS according to the Rome II diagnostic criteria for IBS; the tests of stool for ova and/or parasites, blood test for full count, and liver function are all normal; and the provision of written informed consent prior to enrollment into the study. However, patients were excluded if they were pregnancy or breast-feeding, history of fever, blood in stool, weight loss during the recent months, or other gastrointestinal tract diseases, and any other medication for the treatment of abdominal pain, and bowel disorders.
2.3. Randomization and blinding
All eligible patients were randomly allocated to the treatment group or placebo group in a 1:1 ratio. Randomization was conducted using a computerized number generator with SAS package (Version 9.3; SAS Institute Inc., Cary, NC) by a professional statistician. The allocation and assignments information were to the patients, investigators, outcome assessors, and data analysts.
2.4. Intervention schedule
Patients in the treatment group received DHC 80 mg tablet, 3 times daily for a total of 4 weeks. Participants in the placebo group received placebo, the same administration schedule as the DHC. Additionally, the placebo also has the similar appearance, taste, and color as the DHC.
2.5. Outcomes
The primary outcome was abdominal pain, measured by the visual analog scale (VAS),[23] and average weekly stool frequency. The secondary outcome measurements were Bristol scale,[24] and the 36-item short form health survey (SF-36).[25] In addition, adverse events (AEs) were also recorded during the treatment period. All outcome data were measured and analyzed at the end of 4-week treatment.
2.6. Statistical analysis
All outcome data were analyzed by the SAS package (Version 9.3; SAS Institute Inc.) with intention-to-treat approach. t-test or Mann–Whitney rank test was used to analyze the continuous data. Pearson's Chi-square test or Fisher's exact test was used to analyze the categorical data. P < .05 was set as the statistical significance level.
3. Results
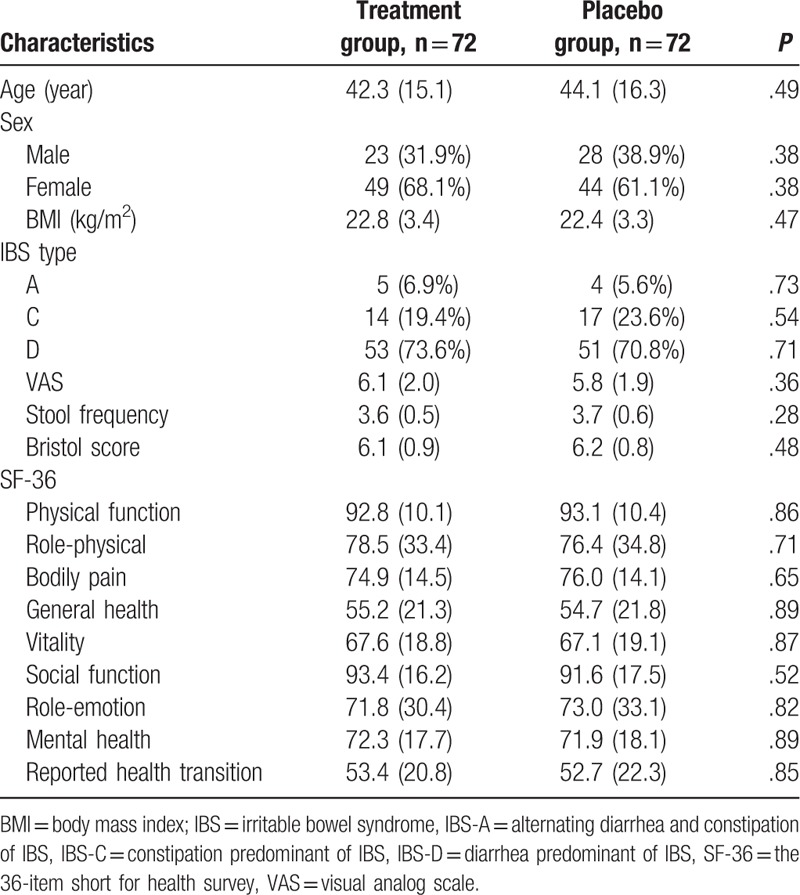
In this study, 225 participants were initially screened and entered the study (Fig. 1). Eighty-one participants were excluded after the selection. Thus, 144 patients were included and were randomly allocated into a treatment group and placebo group in a ratio of 1:1. At the end of 4-week treatment, 5 patients withdrew from the treatment group, and 4 subjects withdrew from the placebo group (Fig. 1). The patient characteristics at baseline are summarized in Table 1. The 2 groups did not differ significantly in all the characteristics at the baseline visit (Fig. 1).
Figure 1.
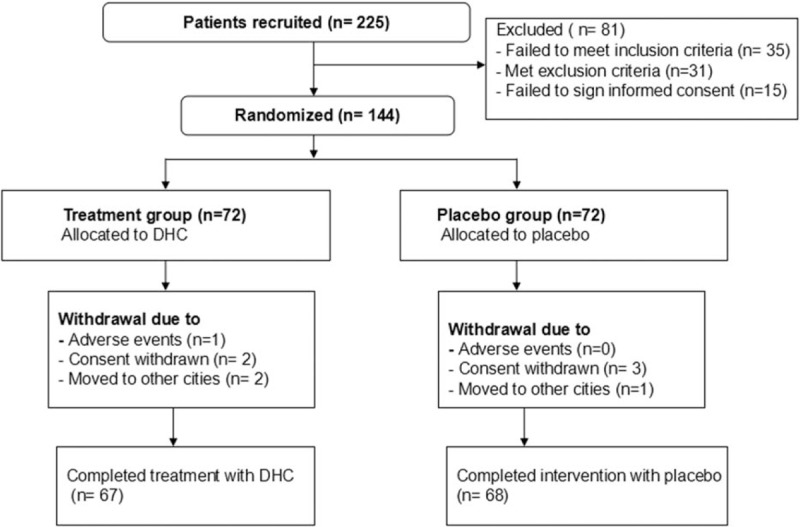
Flow of participants through the trial. DHC = drotaverine hydrochloride.
Table 1.
Patients characteristics at baseline.
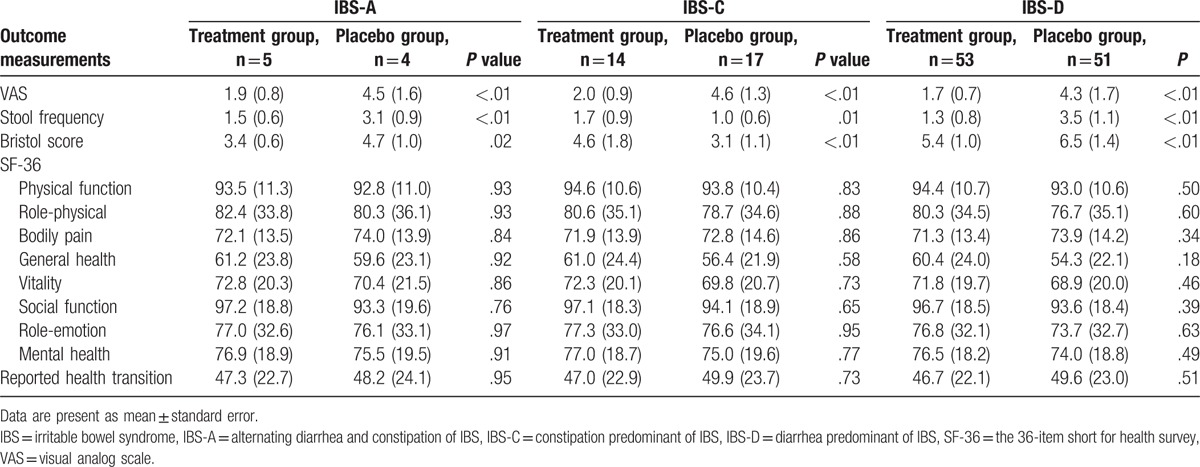
The measurements of the primary and secondary outcomes are summarized in Tables 2 and 3. The total symptoms of IBS showed that DHC can reduce the severity of pain evaluated by the VAS scale (P < .01), and also decreased the stool frequency (P < .01), as well as the Bristol score (P < .01) compared with the placebo at the end of the 4-week treatment (Table 2). The different types of IBS also found that DHC can decrease the pain intensity, evaluated by the VAS scale for all types (P < .01); enhance stool frequency for all symptoms (P < .01), except IBS-C (P = .01); and reduce Bristol scores for all types (P < .01), except ISB-A (P = .02; Table 3). However, DHC did not exhibit significant improvements in health-related life quality, measured by SF-36 scale compared with the placebo (Tables 2 and 3).
Table 2.
Outcome measurements of total symptoms at the end of the 4 weeks treatment.
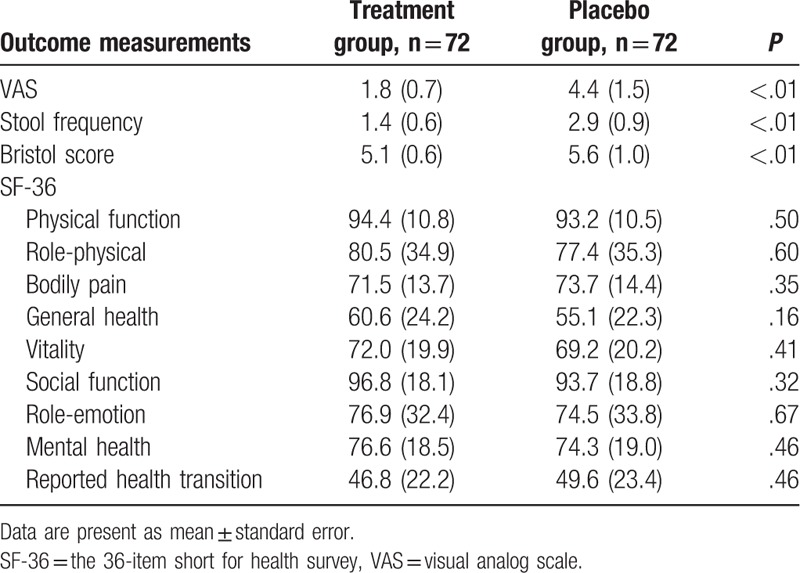
Table 3.
Outcome measurements of different types of IBS at the end of the 4 weeks treatment.
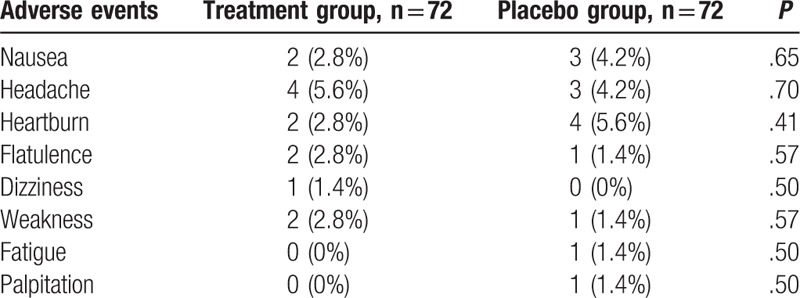
AEs of both groups are listed in Table 4. No significant differences in AEs were found between both groups (Table 4). No serious AEs occurred in the either group.
Table 4.
Adverse events between 2 groups.
4. Discussion
IBS is a very common digestive condition among the population, especially toward the youth and women.[26,27] It not only generates substantial workload for health care, but also considerably reduces the quality of life for patients with IBS.[28] It often manifests as the abdominal pain, headache, backache, and dizziness. Of those symptoms, abdominal pain is 1 of the most common reasons for patients to visit doctors.[29]
Traditional therapies for IBS treatment include bulking agents, prokinetics, antispasmodics, alosetron, tegaserod, 5-hydroxytryptamine agonists and antagonists, smooth muscle relaxants, and antidepressants. However, most clinical trials of those drugs suffered from poor methodology and inconclusive findings.[30] The lacking of randomized placebo-controlled trials for those therapies provides limited evidence for clinical practice.[31] In this study, we provide the strongest clinical evidence by utilizing the randomized double-blinded placebo-controlled trial on evaluating the efficacy and safety of DHC for treating Chinese population with IBS specifically. This study recommends that DHC can effectively enhance symptoms of IBS in Chinese population.
Previous studies have evaluated the efficacy of DHC for the treatment of IBS. They found that DHC plays an important role in enhancing symptoms of patients with IBS. Of them, 1 study evaluated the efficacy and safety of DHC in Indian patients with IBS.[19] It included 180 patients with IBS and was allocated to DHC or placebo intervention. It found that DHC significantly improved abdominal symptoms in patients with IBS. The other study explored the efficacy of DHC in the Indian children population with recurrent abdominal pain.[32] Its results also found that DHC is an effective and safe pharmaceutical agent in the management of recurrent abdominal pain in children. However, no study specifically focused on Chinese patients with IBS treated by DHC only.
The results of this study confirmed our hypothesis that DHC has promising efficacy for the treatment of IBS in Chinese population, compared with the placebo. To our best knowledge, this study is the first randomized, double-blinded, placebo-controlled trial to explore the efficacy of DHC for treating Chinese population with IBS specifically. Our findings showed the efficacy of DHC for Chinese patients with IBS.
In this study, the abdominal pain intensity, measured by the VAS scale, and stool frequency, and Bristol score reduced significantly in the treatment group, compared with those in the placebo group. However, no significant improvements in quality of life were found in the treatment group, when compared with the placebo group. Thus, DHC treatment appears to be promising for improving the symptoms of Chinese patients with IBS.
This study had several limitations. First, this study was only conducted at a single hospital with Chinese patients with IBS. Thus, it may be limited to the generalization to other hospitals and other population. Second, this study only conducted 4-week without follow-up evaluation because of its short duration. Therefore, the longer period of treatment and follow-up effect assessment of DHC on Chinese patients with IBS are still needed in the future.
5. Conclusion
The results of this study found that DHC can improve the symptoms of Chinese patients with IBS. Future studies are still needed to warrant the results of this study.
Footnotes
Abbreviations: AEs = adverse events, DHC = drotaverine hydrochloride, IBS = irritable bowel syndrome, IBS-A = alternating diarrhea and constipation of IBS, IBS-C = constipation predominant of IBS, IBS-D = diarrhea predominant of IBS, IBS-M = mixed diarrhea and constipation of IBS, SF-36 = 36-item short form health survey, VAS = visual analog scale.
The authors have no conflicts of interest to disclose.
References
- [1].Yang TY, Chen CS, Lin CL, et al. Risk for irritable bowel syndrome in fibromyalgia patients: a National Database Study. Medicine (Baltimore) 2017;96:e6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Hsu TY, He GY, Wang YC, et al. Alcohol use disorder increases the risk of irritable bowel disease: a Nationwide Retrospective Cohort Study. Medicine (Baltimore) 2015;94:e2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wang H, Zhou G, Luo L, et al. Serological screening for Celiac disease in adult Chinese patients with diarrhea predominant irritable bowel syndrome. Medicine (Baltimore) 2015;94:e1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Qin Z, Li B, Wu J, et al. Acupuncture for chronic diarrhea in adults: protocol for a systematic review. Medicine (Baltimore) 2017;96:e5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tseng PT, Zeng BS, Chen YW, et al. A meta-analysis and systematic review of the comorbidity between irritable bowel syndrome and bipolar disorder. Medicine (Baltimore) 2016;95:e4617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zheng H, Li Y, Zhang W, et al. Electroacupuncture for patients with diarrhea-predominant irritable bowel syndrome or functional diarrhea: a randomized controlled trial. Medicine (Baltimore) 2016;95:e3884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Li J, Zhu W, Liu W, et al. Rifaximin for irritable bowel syndrome: a meta-analysis of randomized placebo-controlled trials. Medicine (Baltimore) 2016;95:e2534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rey E, Talley NJ. Irritable bowel syndrome: novel views on the epidemiology and potential risk factors. Dig Liver Dis 2009;41:772–80. [DOI] [PubMed] [Google Scholar]
- [9].Gwee KA, Wee S, Wong ML, et al. The prevalence, symptom characteristics, and impact of irritable bowel syndrome in an Asian urban community. Am J Gastroenterol 2004;99:924–31. [DOI] [PubMed] [Google Scholar]
- [10].Cañón M, Ruiz AJ, Rondón M, et al. Prevalence of irritable bowel syndrome and health-related quality of life in adults aged 18 to 30 years in a Colombian University: an electronic survey. Ann Gastroenterol 2017;30:67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Cremonini F, Talley NJ. Irritable bowel syndrome: epidemiology, natural history, health care seeking and emerging risk factors. Gastroenterol Clin North Am 2005;34:189–204. [DOI] [PubMed] [Google Scholar]
- [12].Mangel AW, Northcutt AR. Review article: the safety and efficacy of alosetron, a 5-HT3 receptor antagonist, in female irritable bowel syndrome patients. Aliment Pharmacol Ther 1999;13(suppl 2):77–82. [DOI] [PubMed] [Google Scholar]
- [13].Ghoshal UC, Abraham P, Bhatt C, et al. Epidemiological and clinical profile of irritable bowel syndrome in India: report of the Indian society of gastroenterology task force. Indian J Gastroenterol 2008;27:22–8. [PubMed] [Google Scholar]
- [14].Leventer SM, Raudibaugh K, Frissora CL, et al. Clinical trial: dextofisopam in the treatment of patients with diarrhoea-predominant or alternating irritable bowel syndrome. Aliment Pharmacol Ther 2008;27:197–206. [DOI] [PubMed] [Google Scholar]
- [15].Zijdenbos IL, de Wit NJ, van der Heijden GJ, et al. Psychological treatments for the management of irritable bowel syndrome. Cochrane Database Syst Rev 2009;1:CD006442. [DOI] [PubMed] [Google Scholar]
- [16].Reed-Knight B, Squires M, Chitkara DK, et al. Adolescents with irritable bowel syndrome report increased eating-associated symptoms, changes in dietary composition, and altered eating behaviors: a pilot comparison study to healthy adolescents. Neurogastroenterol Motil 2016;28:1915–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Fisher M, Walker A, Falqués M, et al. Cost-effectiveness of linaclotide compared to antidepressants in the treatment of irritable bowel syndrome with constipation in Scotland. Eur J Health Econ 2016;17:1091–100. [DOI] [PubMed] [Google Scholar]
- [18].Barker WR, Glass GG. Which is better for IBS pain in women: antispasmodics or antidepressants? J Fam Pract 2015;64:734–5. [PubMed] [Google Scholar]
- [19].Rai RR, Dwivedi M, Kumar N. Efficacy and safety of drotaverine hydrochloride in irritable bowel syndrome: a randomized double-blind placebo-controlled study. Saudi J Gastroenterol 2014;20:378–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ruchkina IN, Fadeeva NA, Parfenov AI, et al. The role of small bowel microflora in the development of secondary lactase deficiency and the possibilities of its treatment with probiotics. Ter Arkh 2013;85:21–6. [PubMed] [Google Scholar]
- [21].Khalif IL, Quigley EM, Makarchuk PA, et al. Interactions between symptoms and motor and visceral sensory responses of irritable bowel syndrome patients to spasmolytics (antispasmodics). J Gastrointestin Liver Dis 2009;18:17–22. [PubMed] [Google Scholar]
- [22].Makarchuk PA. Dynamics of indices of visceral sensitivity in patients with irritable bowel syndrome treated with spasmolytics. Eksp Klin Gastroenterol 2007;6:126–9. [PubMed] [Google Scholar]
- [23].Crichton N. Visual analogue scale (VAS). J Clin Nurs 2001;10:706–16. [Google Scholar]
- [24].Riegler G, Esposito I. Bristol scale stool form. A still valid help in medical practice and clinical research. Tech Coloproctol 2001;53:163–4. [DOI] [PubMed] [Google Scholar]
- [25].Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care 1992;306:473–83. [PubMed] [Google Scholar]
- [26].Hungin AP, Whorwell PJ, Tack J, et al. The prevalence, patterns, and impact of irritable bowel syndrome: an international survey of 40,000 subjects. Aliment Pharmacol Ther 2003;17:643–50. [DOI] [PubMed] [Google Scholar]
- [27].Ruigomez A, Wallander MA, Johansson S, et al. One-year follow-up of newly diagnosed irritable bowel syndrome patients. Aliment Pharmacol Ther 1999;13:1097–102. [DOI] [PubMed] [Google Scholar]
- [28].Drossman DA, Camilleri M, Mayer EA, et al. AGA technical review on irritable bowel syndrome. Gastroenterology 2002;123:2108–31. [DOI] [PubMed] [Google Scholar]
- [29].Samuels LA. Pharmacotherapy update: Hyoscine butylbromide in the treatment of abdominal spasms. Clin Med Ther 2009;1:647–55. [Google Scholar]
- [30].Lesbros-Pantoflickova D, Michetti P, Fried M, et al. Meta-analysis: the treatment of irritable bowel syndrome. Aliment Pharmacol Ther 2004;20:1253–69. [DOI] [PubMed] [Google Scholar]
- [31].Hussain Z, Quigley EM. Systematic review: complementary and alternative medicine in the irritable bowel syndrome. Aliment Pharmacol Ther 2006;23:465–71. [DOI] [PubMed] [Google Scholar]
- [32].Narang M, Shah D, Akhtar H. Efficacy and safety of drotaverine hydrochloride in children with recurrent abdominal pain: a randomized placebo controlled trial. Indian Pediatr 2015;52:847–51. [DOI] [PubMed] [Google Scholar]


