Abstract
Background:
Homocysteine (Hcy) plays an important role in vascular function and Hcy level contributes to pathogenesis of ischemic stroke (IS). MTHFR gene polymorphism may have effects on IS risks by influencing the Hcy metabolic pathway. In the present study, a case–control study was designed to evaluate the relationship among MTHFR C677Tpolymorphism, plasma Hcy level, and susceptibility of IS in Chinese population.
Methods:
A total of 300 patients with IS and 261 matched control subjects were recruited. Plasma Hcy concentration was determined using enzymatic cycling assay. MTHFR C677T polymorphisms were genotyped by PCR-RFLP.
Results:
Compared with controls, the plasma Hcy level was significantly higher in the IS patients (P < .05). After adjusting for conventional risk factors, the T allele frequency of MTHFR C677T in IS group (54%) was significantly higher than that in the controls (38.3%) (P < .05; OR = 1.890, 95% CI: 1.489–2.399). Additionally, the plasma Hcy level of the TT genotype is significantly higher than that of the CC and CT genotypes (P < .05).
Conclusion:
Our study provided evidence that hyperhomocysteinemia (HHcy) and MTHFR C677T polymorphism were associated with IS. More importantly, suggesting that a possible synergistic effect of MTHFR C677T polymorphism on Hcy level variations increased risk for IS in Chinese population.
Keywords: homocysteine level, ischemic stroke, MTHFR C677T polymorphism
1. Introduction
Stroke is a leading cause of death or disability in the world and is an emergent public health problem.[1,2] Ischemic stroke (IS) accounts for 87% of all strokes.[3] Several risk factors, such as hypertension, diabetes, hyperlipidemia, and smoke, have been well studied, but explain only a small part of IS risk.[4] Homocysteine (Hcy) plays an important role in vascular function and there are growing evidences that Hcy contributes to pathogenesis of IS. Hcy leads to atherogenesis and thrombogenesis via endothelial damage, vascular smooth muscle proliferation, and coagulation abnormalities. Hcy levels are determined by the interaction of genetic and environmental factors. Mutations in the genes which are involved in the Hcy metabolic pathway are related to elevate plasma of the Hcy levels and may confer an increased risk for IS.
5,10-Methylenetetrahydrofolate reductase (MTHFR), encoded by the MTHFR gene in humans, is a key controlling enzyme related to Hcy metabolism.[5] The most common polymorphism of MTHFR is the cytosine (C) to thymine (T) substitution at position 677 which results in the conversion of alanine to valine at amino acid 222.[6] The C677T polymorphism is associated with decreased enzyme activity and eventually leads to elevation of Hcy concentrations.[7–8] However, most previous studies have simply focused on MTHFR C677T polymorphisms and IS risk. The role of Hcy variation in individual susceptibility to IS and the association between Hcy and MTHFR gene polymorphisms have not been extensively explored. Therefore, the aim of the present study was to investigate the role of serum total Hcy level, MTHFR C677T polymorphisms, and their association in IS risk in a central Chinese Han population.
2. Materials and methods
2.1. Study population
In the present study, 300 patients with IS (130 males and 170 females, mean age of 64.2 ± 13.2 years) were recruited from the Department of Neurology at the First People's Hospital of Zhengzhou from December 2015 to May 2016. IS was diagnosed by the criteria based on a loss of global or focal cerebral function persisting for >24 hours with corresponding infarction on brain imaging with a probable vascular cause.[9] We classified the IS subtypes according to the Trial of Org10172 in Acute Stroke Treatment (TOAST).[10] Brain imaging with CT and/or MRI as well as ancillary diagnostic investigations and standardized blood tests were performed. Patients with cerebral hemorrhage, atrial fibrillation, hyperthyroidism, cardioembolic stroke, venous thrombosis, peripheral vascular diseases, liver disorders, or kidney diseases were excluded from the study. The comorbidities of IS in our study were hypertension and diabetes. High blood pressure subjects were defined by blood pressure ≥140/90 mm Hg, and had been diagnosed and drug control. Diabetic subjects were defined by a fasting plasma glucose ≥7.0 mmol/L, or by the use of antidiabetic drugs. Patients were considered diabetic if diabetes was previously known. All the patients were first ever stroke. And the treatments of IS patients were antiplatelet aggregation, reducing lipid and stable plaque and scavenging free radicals. All the high Hcy patients were given folic acid tablets, vitamin B12, and vitamin B6.
The control group comprised 261 individuals (127 males and 134 females, mean age of 63.8 ± 13.7) selected from the same demographic area. And they were well matched with IS patients by age and gender. The individuals who had cerebrovascular disease, cardiovascular disease, and cancer, hepatic, or renal diseases were excluded. The study protocols were approved by the Ethics Committee on Human Research of Zhengzhou University and signed consent form was obtained from each participant.
2.2. Plasma Hcy assay
Fasting blood samples from IS patients were collected within 24 hours after stroke symptoms onset. Two milliliters of whole blood were collected into a tube containing EDTA. Blood samples were fractionated by centrifugation at 3000 g for 5 minutes. Hcy reagent (Beijing Strong Biotechnologies, Inc, Beijing, China) was used to detect the levels of Hcy with enzymatic cycling assay according to the manufacturer's instructions. The normal range is 5 to 15 μmol/L, and a plasma Hcy level higher than 15 μmol/L is an indication of hyperhomocysteinemia (HHcy).
2.3. Genotyping of MTHFR
Genomic DNA was extracted from 2 mL of peripheral venous blood anticoagulated by EDTA with the phenol-chloroform method. Polymerase chain reaction-restriction fragment length polymorphism (PCR-RFLP) method was used to genotype MTHFR gene polymorphisms. DNA was amplified using the following primer pairs: 5′-TGAAGGAGAAGGTGTCTGCGGGA-3′ (sense) and 5′-AGGACGGTGCGGTGAGAGTG-3′ (antisense). The PCR mixture (25 μL) contained 2.5 μL of STR buffer (MgCl2, dNTPs and 10 × buffer), 2.5 μL of each sense and antisense primer, 0.4 μL Taq DNA polymerase (Shanghai Bao Sheng Co Ltd., China), and 1.5 μL of genomic DNA, and the total volume was adjusted to 25 μL using dd H2O. Amplification was performed as follows: predenaturation at 94 °C for 2 minutes; followed by 40 cycles of denaturation at 94 °C for 30 seconds, 62 °C for 30 seconds, and 72 °C for 30 seconds; and extension at 72 °C for 5 minutes.
The PCR products were digested with the restriction enzyme Hinf I (Promega Corp, Madison). A reaction mixture of 20 μL was prepared by mixing 10 μL of PCR products, 0.5 μL of Hinf I, 2.0 μL of buffer R, and 7.5 μL of ddH2O. The reaction mixture was incubated at 37 °C for 3 hours. Then, PCR fragments were separated by electrophoresis on a 2% agarose gel for 30 minutes at 100 V. The wild-type (CC) genotype without restriction enzyme sites produced a 198 bp fragment. The heterozygous (CT) genotype produced 2 fragments: 198 and 175 bp, and the homozygous mutant (TT) produced a 175 bp fragment.
2.4. DNA sequencing analysis
For verifying the genotyping results of PCR-RFLP, 20 subjects with different genotypes were randomly selected for DNA sequencing analysis, which was conducted by the Shang Hai Bao Sheng Company. All genotypes were identical with those obtained from the first round of PCR-RFLP genotyping.
2.5. Statistical analysis
All the analyses related to the case–control study were performed using the Statistical Software Package for the Social Sciences v.17.0 (IBM [formerly SPSS Inc], Armonk, NY). Quantitative data are expressed as mean ± SD. The difference between the cases and controls was evaluated using the independent 2-sample t test for quantitative variables. Allele and genotype frequencies in both groups were calculated and compared using the chi-square test. Logistic regression model was done to study the independent association of MTHFR genotype by adjusting the confounding variables such as age, gender. The results were expressed as odds ratios (ORs) and 95% confidence intervals (CIs). P values less than .05 were considered statistical significant.
3. Results
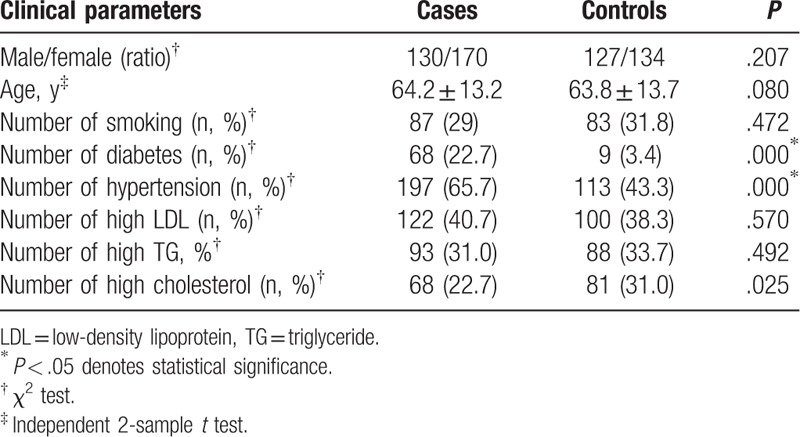
3.1. Clinical characteristics of subjects
The clinical characteristics of the study population are given in Table 1. There was no significant difference in age and sex between the 2 groups (P > .05). The proportion of smokers, low-density lipoprotein, and triglyceride (TG) showed no statistically significant differences (P > .05). While the incidences of diabetes, hypertension, and hypercholesteremia in the IS patients were significantly higher than that in control (P < .05).
Table 1.
Clinical parameters of patients and controls.
3.2. Plasma Hcy levels
The data obtained from the experiments of plasma Hcy level examination indicated that the patients had significantly higher plasma Hcy level than control subjects (P < .05). More than half of stroke patients had HHcy, whereas only some of controls had abnormally elevated Hcy levels (see Fig. 1).
Figure 1.
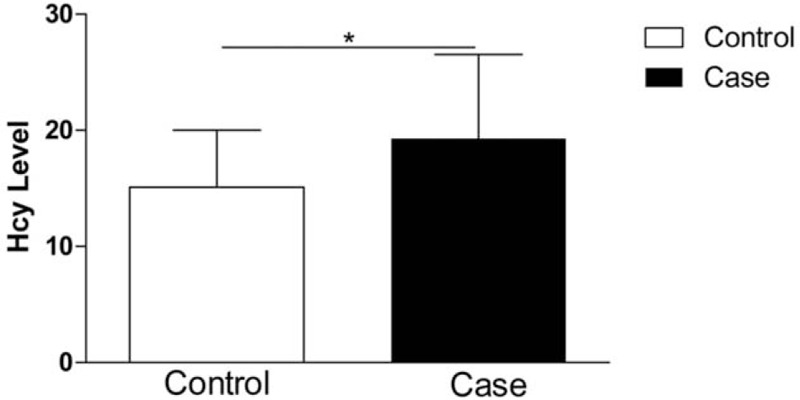
Plasma Hcy levels in IS patients and controls, ∗P < .05. Hcy = homocysteine, IS = ischemic stroke
3.3. Association analysis and inherited model test
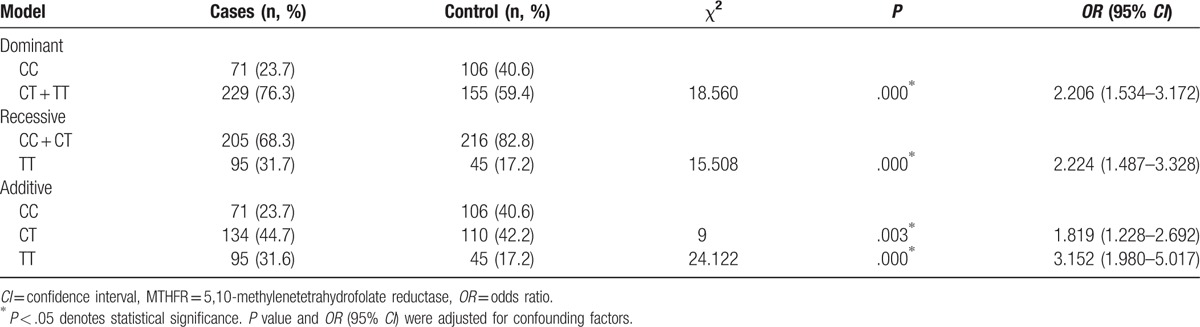
The genotype distributions and allele frequencies of the MTHFR C677T(C/T) polymorphism in the patients and controls were shown in Table 2. There was no significant deviation from the Hardy–Weinberg equilibrium test at MTHFR C677T in the IS group and controls (P > .05). The T allele frequency of MTHFR C677T was significantly higher in IS group (54%) than in control group (38.3%) (P = .000; OR = 1.890, 95% CI: 1.489–2.399). Both the homozygous TT genotype frequency (31.6%) and heterozygote CT genotype frequency (44.7%) in the IS group were significantly higher than those (42.2% and 17.2%, respectively) in the control group (P = .000, OR = 3.152, 95% CI = 1.980–5.017; P = .003, OR = 1.819, 95% CI = 1.228–2.692, respectively). To assess the effect of MTHFR C677T on the risk of IS, we compared dominant, recessive, and additive models. The effect of MTHFR C677T was best described with dominant and additive models (Table 3).
Table 2.
Frequencies of MTHFR C677T genotype and alleles in controls and patients.
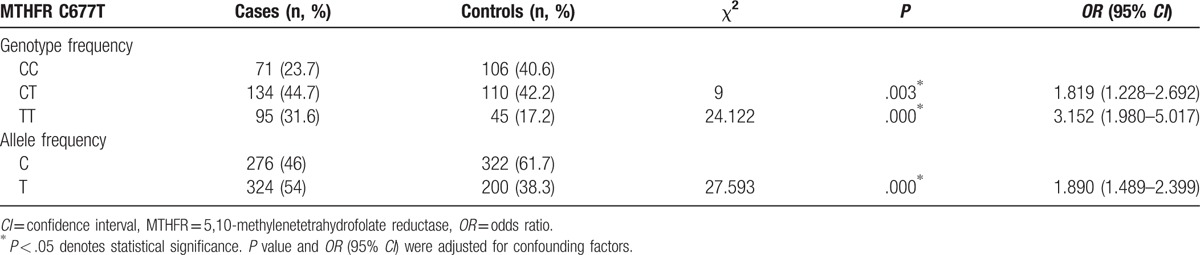
Table 3.
Detailed association of MTHFR C677T between patients and controls under different genetic models.
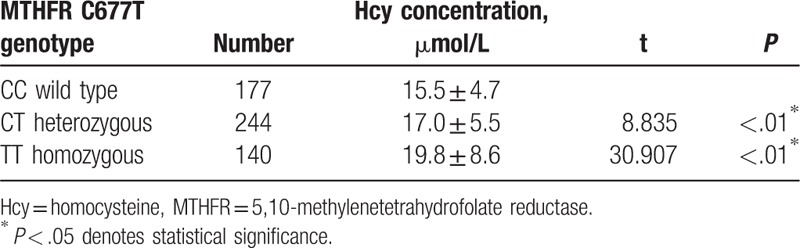
3.4. Influence of MTHFR 677T polymorphism on plasma Hcy level
Genetic polymorphisms were analyzed with respect to plasma Hcy levels. Hcy levels for carriers of the polymorphism (heterozygotes and homozygotes) were considered together versus wild type for the analysis. The association of MTHFR C677T polymorphism genotypes with the plasma Hcy level was shown in Table 4. The result demonstrated that the plasma Hcy level of the TT homozygous genotype and CT heterozygous genotype were significantly higher than that of the CC wild-type genotypes (P < .05).
Table 4.
Comparison of plasma Hcy levels in different genotypes of MTHFR C677T polymorphism.
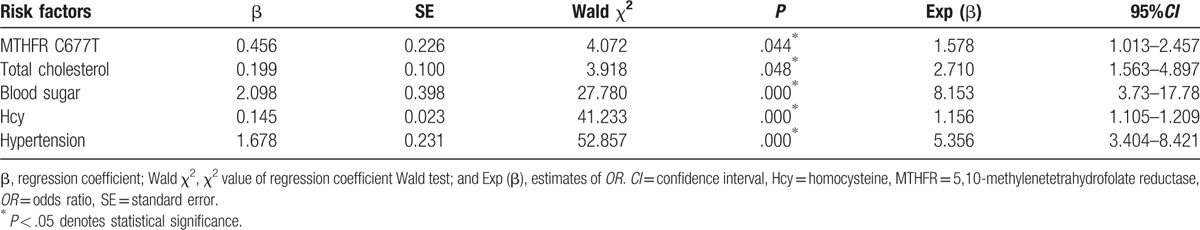
3.5. Correlation of risk factors with ischemic stroke
As shown in Table 5, the conventional risk factors such as diabetes, hypertension, and the plasma Hcy level significantly increased IS risks via a stepwise logistic regression analysis (P < .05). Although MTHFR C677T polymorphism could increase IS risks by logistic regression analysis in this study (P < .05).
Table 5.
Results of stepwise logistic regression analysis for ischemic stroke.
4. Discussion
We designed a case–control study to explore the association of serum total Hcy level, MTHFR C677T polymorphism, and IS risk in a central Chinese Han population. Our findings showed that an elevated plasma Hcy level and MTHFR C677T polymorphism were significantly associated with an increased risk of IS (P = .000, OR = 1.890, 95% CI = 1.489–2.399). More importantly, we investigated the influence of MTHFR 677T polymorphism on plasma Hcy level, suggesting a possible synergistic effect of MTHFR C677T polymorphism on Hcy level variations increased risk for IS.
HHcy has been proved to be one of the risk factors for thromboembolism including IS.[11] First, we examined the relationship between the serum total Hcy level and IS. The mean plasma Hcy level of IS patients was significantly higher than that of control subjects (P < .05). More than half of IS patients had HHcy, whereas only some of controls had abnormally elevated Hcy levels. Our result supported the opinion that HHcy is associated with IS risk.[12] Hcy is a kind of sulfur-containing amino acid, the important intermediate product of methionine metabolism. A high level of Hcy makes a person more prone to injury to the endothelial and vascular smooth muscle cells, which leads to endothelial proliferation, activation of coagulation factors, and expression of plasminogen activator inhibitor. This in turn inhibits synthesis of sulfated heparin, thrombomodulin expression, and synthesis of tissue-type plasminogen activator, which can result in platelet aggregation and subsequently IS.[13] HHcy is strongly determined by dietary intake of B vitamins, which has been assessed by several studies among different ethnic populations.[14] Moreover, it was reported that folic acid supplementation could be effective in stroke prevention.[15]
The strongest association with increased Hcy level is the cytosine (C) to thymine (T) substitution at position 677 of the MTHFR gene. Second, we made an association study between the MTHFR C677T polymorphisms and IS. We investigated 300 patients with IS and 261 controls by PCR-RFLP. And we found that MTHFR C677T CT genotype frequencies (44.7%), TT genotype frequencies (31.6%), and T allele frequencies (54%) of the patients were significantly higher than those (42.2%, 17.2%, and 38.3%, respectively) of the controls (P < .05). Stepwise logistic regression analysis showed that MTHFR C677T polymorphism was associated with an increased risk of IS (95% CI, 1.013–2.457; P = .044). Next we performed genotype association test with dominant, recessive, and additive models. And the MTHFR C677T genotype was associated with IS in dominant and additive genetic models (P < .05). This association was consistent with the results reported previously. Song et al[16] who showed that TT and CT genotypes as well as the T allele of MTHFR C677T genetic polymorphism were associated with IS risk and that there were associations between MTHFR C677T genetic polymorphism and susceptibility to IS under all genetic models. Li and Qin[17] showed MTHFR C677T polymorphism was a risk factor in IS. Kelly et al[18] came to the conclusion that MTHFR 677C>T polymorphism might play a proper role in IS via analysing 6044 IS patients and 13916 controls.
Third, we investigated the influence of MTHFR 677T polymorphism on plasma Hcy level. We found that the plasma Hcy level of the TT genotype was significantly higher than that of the CC and CT genotypes (P < .05). The exact mechanism of synergism between MTHFR C677T polymorphisms and HHcy remains to be determined. We proposed a possible molecular mechanism as follows: the cytosine (C) to thymine (T) substitution at position 677 results in the conversion of alanine to valine at amino acid 222, and then reduces the MTHFR enzyme activity, which is a key controlling enzyme involved in Hcy metabolism. The decreased MTHFR enzyme activity eventually leads to elevating Hcy concentrations, which contributes to IS risk.
Several limitations of our study need to be addressed. The sample size of the study may not be sufficiently large to evaluate gene–environment interactions. Besides, because the cases and controls were recruited from hospital, there was potential selection bias.
In conclusion, we demonstrated that HHcy is associated with IS and MTHFR C677T polymorphism might be a genetic risk factor for IS in Chinese Han population. More importantly, we investigated the influence of MTHFR 677T polymorphism on plasma Hcy level, suggesting that the synergistic effect between elevated Hcy levels and MTHFR C677T variant was found to be associated with IS. Further large-scale studies are necessary to investigate other genetic risk factors of IS and their possible synergistic effects on Hcy level variations.
Acknowledgments
The authors thank the National Natural Science Foundation of China (No. 81671163), the grant from the Zhengzhou City general Science and Technology Research Projects (project number 20150001), and the grant from the Key projects of Henan Provincial Education Department (project number 16A310016) for the support. The authors also thank the technical assistance of staff members of the Department of Neurology, The First People's Hospital of Zhengzhou and all patients and controls for providing blood samples.
Footnotes
Abbreviations: Hcy = homocysteine, HHcy = hyperhomocysteinemia, IS = ischemic stroke, MTHFR = 5,10-methylenetetrahydrofolate reductase.
AL and YS contributed equally.
Authorship: YCZ and XJZ performed experiments. HLZ made the statistical analysis. QML and XHC was involved in local study implementation and participator recruitment. AFL, YSS, and LYX wrote the manuscript. HZ and YH conceived of the study and participated in its design and coordination. All authors read and approved the final manuscript.
Funding/support: This study was supported by the National Natural Science Foundation of China (No. 81671163), the grant from the Zhengzhou City general Science and Technology Research Projects (project number 20150001), and the grant from the Key projects of Henan Provincial Education Department (project number 16A310016).
The authors have no conflicts of interest to disclose.
References
- [1].Johnston SC, Mendis S, Mathers CD. Global variation in stroke burden and mortality: estimates from monitoring, surveillance, and modelling. Lancet Neurol 2009;8:345–54. [DOI] [PubMed] [Google Scholar]
- [2].Somarajan BI, Kalita J, Mittal B, et al. Evaluation of MTHFR C677T polymorphism in ischemic and hemorrhagic stroke patients. A case-control study in a Northern Indian population. J Neurol Sci 2011;304:67–70. [DOI] [PubMed] [Google Scholar]
- [3].Slomka A, Switonska M, Sinkiewicz W, et al. Haemostatic factors do not account for worse outcomes from ischaemic stroke in patients with higher C-reactive protein concentrations. Ann Clin Biochem 2017;54:378–85. [DOI] [PubMed] [Google Scholar]
- [4].Fekih-Mrissa N, Mrad M, Klai S, et al. Methylenetetrahydrofolate reductase (C677T and A1298C) polymorphisms, hyperhomocysteinemia, and ischemic stroke in Tunisian patients. JStroke Cerebrovasc Dis 2013;22:465–9. [DOI] [PubMed] [Google Scholar]
- [5].Vijayan M, Chinniah R, Ravi PM, et al. MTHFR (C677T) CT genotype and CT-apoE3/3 genotypic combination predisposes the risk of ischemic stroke. Gene 2016;591:465–70. [DOI] [PubMed] [Google Scholar]
- [6].Jadavji NM, Wieske F, Dirnagl U, et al. Methylenetetrahydrofolate reductase deficiency alters levels of glutamate and gamma-aminobutyric acid in brain tissue. Mol Genet Metab Rep 2015;3:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Atadzhanov M, Mwaba MH, Mukomena PN, et al. Association of the APOE, MTHFR and ACE genes polymorphisms and stroke in Zambian patients. Neurol Int 2013;5:e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ou W, Liu X, Shen Y, et al. Association of CVD candidate gene polymorphisms with ischemic stroke and cerebral hemorrhage in Chinese individuals. PloS One 2014;9:e105516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Saleheen D, Bukhari S, haider SR, et al. Association of phosphodiesterase 4D gene with ischemic stroke in a Pakistani population. Stroke 2005;36:2275–7. [DOI] [PubMed] [Google Scholar]
- [10].Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in acute stroke treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- [11].Wardlaw JM, Murray V, Berge E, et al. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet 2012;379:2364–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kalita J, Kumar G, Bansal V, et al. Relationship of homocysteine with other risk factors and outcome of ischemic stroke. Clin Neurol Neurosurg 2009;111:364–7. [DOI] [PubMed] [Google Scholar]
- [13].Hainsworth AH, Yeo NE, Weekman EM, et al. Homocysteine, hyperhomocysteinemia and vascular contributions to cognitive impairment and dementia (VCID). Biochim Biophys Acta 2016;1862:1008–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Banecka-Majkutewicz Z, Sawu?a W, Kadzi?ski L, et al. Homocysteine, heat shock proteins, genistein and vitamins in ischemic stroke – pathogenic and therapeutic implications. Acta Biochim Pol 2012;59:495. [PubMed] [Google Scholar]
- [15].Huo Y, Qin X, Wang J, et al. Efficacy of folic acid supplementation in stroke prevention: new insight from a meta-analysis. Int J Clin Pract 2012;66:544–51. [DOI] [PubMed] [Google Scholar]
- [16].Song Y, Li B, Wang C, et al. Association between 5, 10-methylenetetrahydrofolate reductase C677T gene polymorphism and risk of ischemic stroke: a meta-analysis. J Stroke Cerebrovasc Dis 2016;25:679–87. [DOI] [PubMed] [Google Scholar]
- [17].Li P, Qin C. Methylenetetrahydrofolate reductase (MTHFR) gene polymorphisms and susceptibility to ischemic stroke: a meta-analysis. Gene 2014;535:359–64. [DOI] [PubMed] [Google Scholar]
- [18].Fallon UB, Ben-Shlomo Y. Homocysteine, MTHFR 677C–>T polymorphism, and risk of ischemic stroke: results of a meta-analysis. Neurology 2002;59:529–36. [DOI] [PubMed] [Google Scholar]


