Abstract
The aim of this study was to identify risk factors for early death (ED) in acute promyelocitic leukemia (APL) patients.
Clinical records of 49 APL patients who suffered ED were divided into 4 groups: death before treatment or within the first 3 days (immediate death; iED group), death during treatment at least 3 days after commencement (ED after treatment), low/intermediate risk, and high-risk groups.
White blood cell (WBC) count, high-risk cases, prothrombin time (PT) prolongation, international society on thrombosis and hemostasis (ISTH) scores (P < .05), bleeding (P = .05), and death due to severe hemorrhage (P = .010) were higher in iED group than ED after treatment. And the time from onset to initial hospitalization or death was significantly shorter (P < .05) in iED patients. LDH level (P = .002), PT prolongation (P = .014), and incidence of grades 3 or 4 bleeding (P = .049) were higher in high-risk group than in ED and low/intermediate-risk groups, while the times from onset to the initial hospitalization or death were lower for ED patients in high-risk group (P = .037).
We found that different types of EDs have different clinical features. A high WBC count contributes to the occurrence of more ED, which is usually not associated with delay of diagnosis and hospitalization. Current therapeutic strategies to reduce the incidence of ED in these cases are not adequate and will benefit from focused research attention.
Keywords: acute promyelocytic leukemia, early death, ISTH score and hospitalization, prothrombin time
1. Introduction
Acute promyelocytic leukemia (APL) is a distinct type of acute leukemia characterized by abnormal proliferation of promyelocytes, life-threatening coagulopathy, and the chromosome translocation t (15;17)(q22;q21). Introduction of all-trans retinoic acid (ATRA) and arsenic trioxide (ATO) have improved the prognosis of APL patients significantly.[1–6] However, epidemiology studies reveal that the incidence of early death (ED) in APL patients remains high. Indeed, 29% of APL patients die within 30 days of their diagnosis and 35% of the ED patient population never receives ATRA therapy.[7] In a recent study, Park et al[8] reported that the incidence of ED rate in APL patients from 1992 to 2007 was elevated at 17.3% despite the introduction of ATRA. Interestingly, Micol et al[9] reported that disease severity at diagnosis was a decisive factor to be included in clinical trials. Therefore, with the exclusion of the APL patients with a severe form of the disease, the ED rate of APL patients could be inaccurate and might be up to 29%. Consequently, ED might still be the primary cause of treatment failure in APL patients.
It is generally believed that delays in diagnosis and onset of therapy may contribute to the incidence of ED in APL patients. Interestingly, severe bleeding is often regarded as the main cause of ED in APL patients. Here, we review the demographic and clinical characteristics of the APL patients in our department who suffered from ED from 2002 to 2013 to determine the risk factors for ED in these patients.
2. Materials and methods
2.1. Patient data
Two hundred twelve patients with de novo APL were admitted to the Hematology department of our hospital between January 2003 and December 2013. In this group, 49 patients [34 males and 15 females, with a median age of 32 years (range 15–84)] who died at diagnosis or soon after the onset of therapy were retrospectively enrolled in this study. The remaining 163 patients [91 males and 72 females, with a median age of 31 years (range 15–54)] who achieved complete remission (CR) after induction treatment with ATRA, toxic agents, and/or ATO were included in the control group. Disease diagnosis was confirmed by bone marrow aspiration, chromosome karyotyping analysis, fluorescence in situ hybridization analysis, and PCR tests. Demographics, clinical parameters, and laboratory data at diagnosis were collected for analysis. We excluded 2 patients who died at diagnosis and 1 patient who died soon after admission after receiving treatment for 10 days because clinical data were missing. This study was approved by the ethical committee of our hospital.
2.2. Definitions
ED was defined as death during induction treatment from the first day of hospitalization. The ED group was divided into 2 groups: group 1 (death before treatment or within the first 3 days; immediate death, iED group; n = 24) and group 2 (death during treatment at least 3 days after commencement; n = 25).
Retinoic acid syndrome, also known as differentiation syndrome, was diagnosed on the basis of the incidence of at least 2 of the following clinical features: unexplained fever, acute respiratory distress with interstitial pulmonary infiltrates, acute renal failure, weight gain greater than 5 kg, unexplained hypotension, and pleuropericardial effusion.[10] Patients with this syndrome were categorized according to Sanz risk stratification score[11]: high risk [white blood cell (WBC) count ≥10 × 109/L at diagnosis], low risk (WBC count <10 × 109/L and PLT ≥40 × 109/L at diagnosis), and intermediate risk (WBC count <10 × 109/L and PLT <40 × 109/L). CR was defined as the presence of less than 5% of blast cells in bone marrow aspirates, PLT >100 × 109/L, and no juvenile cells in peripheral blood, according to the criteria set by the US National Cancer Institute.
2.3. Treatment strategy
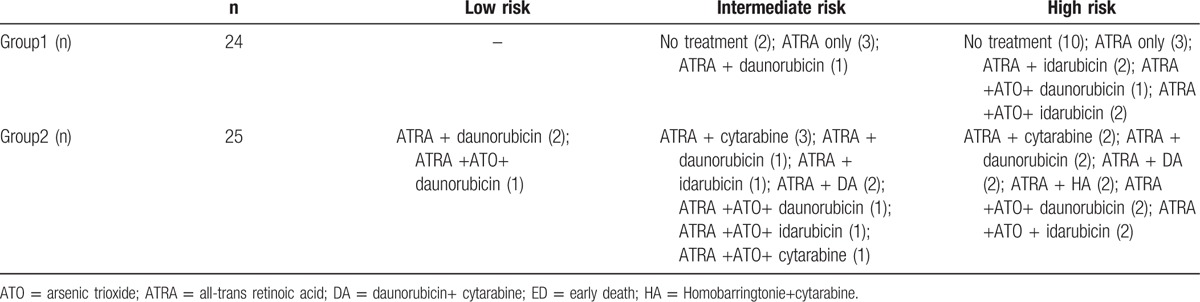
Following the diagnosis of APL, patients received ATRA (20 mg/m2/day) as induction treatment immediately until CR was achieved. Of the 24 patients included in group 1, 12 died before receiving an official diagnosis or at diagnosis and 12 received ATRA therapy for less than 3 days and had no chance of receiving other chemotherapy treatment. Two of the patients that received ATRA also received daunorubicin and four of the patients also received idarubicin. The patients in group 2 received daunorubicin alone (9 patients) or in combination with cytarabine (4 patients). Alternatively, the following chemotherapeutics were also administered in this group: idarubicin (4 patients), homoharringtonine and cytarabine (2 patients), and cytarabine alone (6 patients). The dosage protocol for all cytotoxic agents was as follows: idarubicin (8 mg/m2/day on days 1–3), daunorubicin (45 mg/m2/day on days 1–3), homoharringtonine (2 mg/m2/day on days 1−5), and/or cytarabine (100 mg/m2/day on days 1−7). Hydroxycarbamide was given before or after chemotherapy to reduce WBC count. Two patients in group 1 and 9 patients in group 2 received arsenic trioxide (details summarized in Table 1). Blood product support was applied to maintain platelet (PLT) level at ≥30 × 109/L, hemoglobin at ≥70 g/L, and plasma fibrinogen at ≥1.5 g/L.
Table 1.
Induction treatments in the 49 ED patients.
2.4. Statistical analysis
Statistical analysis was performed with the SPSS v.17.0 software (SPSS Inc., Chicago, IL). Clinical features are presented as percentages (%) for categorical variables and as mean values ± standard deviation (SD) for normally distributed continuous variables. The χ2 test was used to analyze the significance of differences in the distribution of categorical variables between the patient subsets. The t test was used to compare continuous parametric variables in 2 groups. When 3 groups were being compared, the analysis of variance (ANOVA) or Mann–Whitney test was used to analyze the significance of differences in the distribution of continuous parametric variables; if the data are normally distributed, ANOVA was chosen, and if not, the Mann–Whitney test was chosen.
3. Results
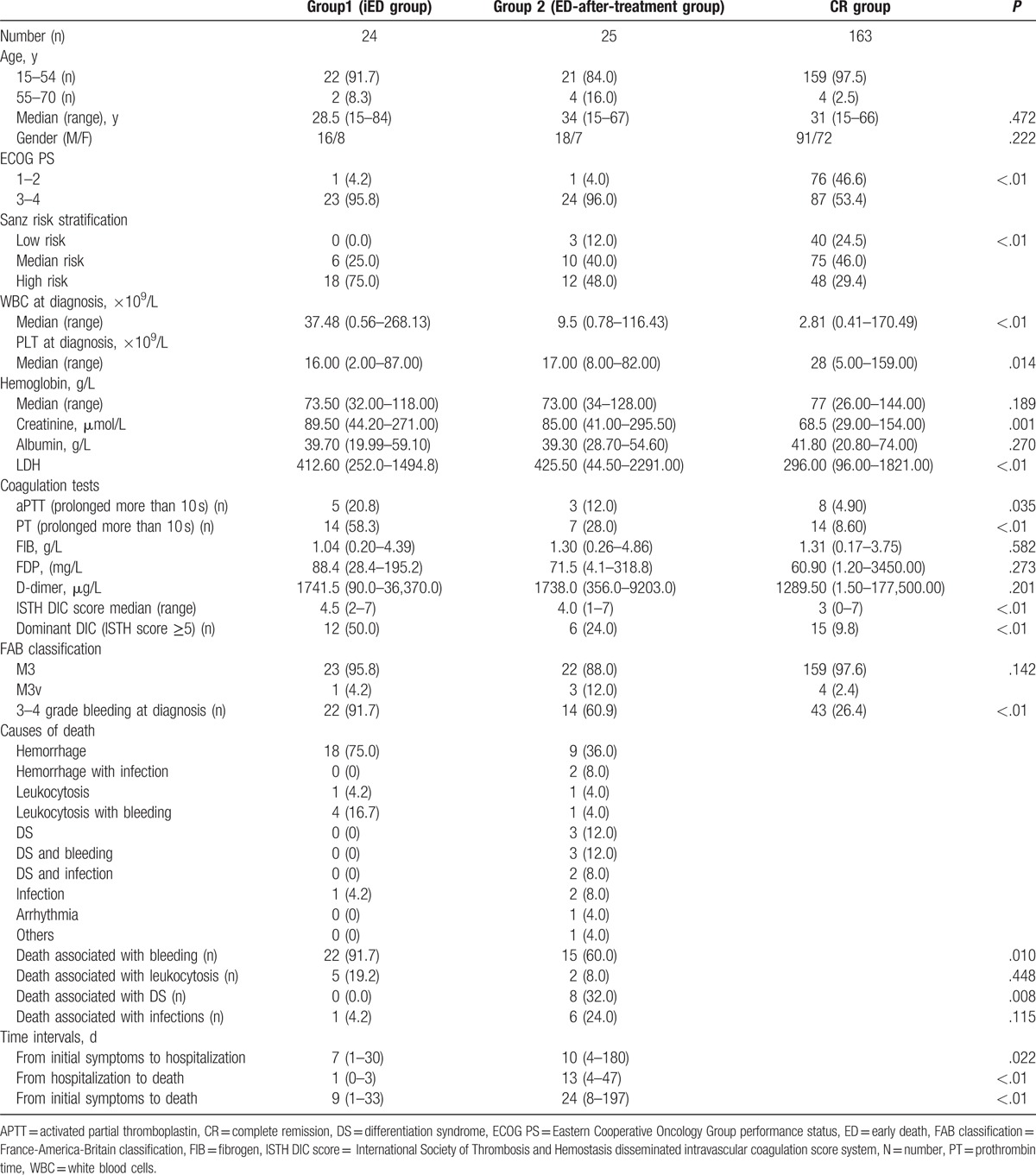
Table 2 summarizes a comparison of the clinical and laboratory parameters of the group1, group 2, and CR group. Forty-nine ED patients diagnosed with APL were included in the analysis (23.1% of the entire cohort of APL patients). Among the total patients, 12.2% were >55 years of age and 69.4% were male. An Eastern Cooperative Oncology Group (ECOG) score of 3 to 4 was observed in 95.9% of the total ED patients and 53.4% of CR patients (P < .01). In the ED group, there were 3 low-risk patients (6.1%), 16 intermediate-risk patients (32.7%), and 30 high-risk patients (61.2%). High-risk patients accounted for 29.4% of the CR group. ED patients in group 1 accounted for 11.3% of the total APL patients, similar to that of group 2 (11.79%). Almost half of the patients in group 1 did not receive treatment. We found the following differences among groups 1, 2, and CR. WBC counts at diagnosis were higher in group 1 (P < .01) than in group 2 and the CR group. Correspondingly, 75% patients of the APL in group 1 were high-risk compared with 48% in group 2 and 29.4% in the CR group (P < .01). PLT counts were significantly different among these 3 groups (P = .032), while multiple comparisons showed that the PLT count in group 1 was lower than that in the CR group (P = .041). There was no difference in the coagulation parameters as shown by the values for fibrinogen, fibrinogen degradation products (FDPs), and D-dimer. However, APTT prolongation and PT prolongation occurred most frequently in group 1. APTT prolongation was statistically different among these 3 groups (groups 1, 2, and CR; 20.8%, 12%, and 4.9%, respectively; P = .035), with a significantly higher incidence in group 1 than in the CR group (P = .015). The incidence of PT prolongation was 58.3%, 28%, and 8.59% in these 3 groups, respectively (P < .01) and was more frequent in groups 1 and group 2 than in the CR group. The ISTH score was significantly different among these 3 groups (P < .01) and it was consistently higher in groups 1 and 2 than in the CR group. Therefore, the incidence of hemorrhage was highest in group 1, with a rate of grade 3 to 4 bleeding of 91.7% compared with 60.9% in group 2 (P = .005) and 26.38% (P < .01) in the CR group. Creatinine was significantly higher in group 1 than in the other 2 groups (P = .001). The LDH level was higher in the ED groups than in the CR group (P < .01).
Table 2.
Baseline demographic, clinical and laboratory characteristics in mED, ED after treatment patients, and CR patients.
We found the following causes for ED in the patient population: hemorrhage [91.7% in group 1, 60% in group 2 (P = .010)] and differentiation syndrome [higher in group 2 (P = .008)]. For the patients in group 1, we found that the time from the onset of symptoms to initial hospitalization and from hospitalization to death was significantly shorter than that observed in the ED patients of group 2.
Table 3 summarizes a comparison of clinical and laboratory characteristics of low/intermediate, high-risk APL patients, and CR patients. We found that 19 of 134 (14.2%) low/intermediate-risk patients with APL suffered ED compared with 30 of the 78 high-risk patients (38.5%). No significant differences of sex, age, or ECOG score were found between the low/intermediate and high-risk ED groups. The LDH level was higher in the high-risk ED group (P = .002). Interestingly, coagulation parameters were similar between the low/intermediate and high-risk ED groups and there were no differences in the PLT, APTT, fibrinogen, FDP, D-dimer, and ISTH scores between the 2 groups. PT prolongation however was more frequent in the ED patients of the high-risk group (56.7%; P = .014). Similarly, the incidence of grade 3 to 4 bleeding was higher in the high-risk group (83.3%; P = .049).
Table 3.
Baseline demographic, clinical and laboratory characteristics of early death patients in the low/intermediate-risk and high-risk groups.
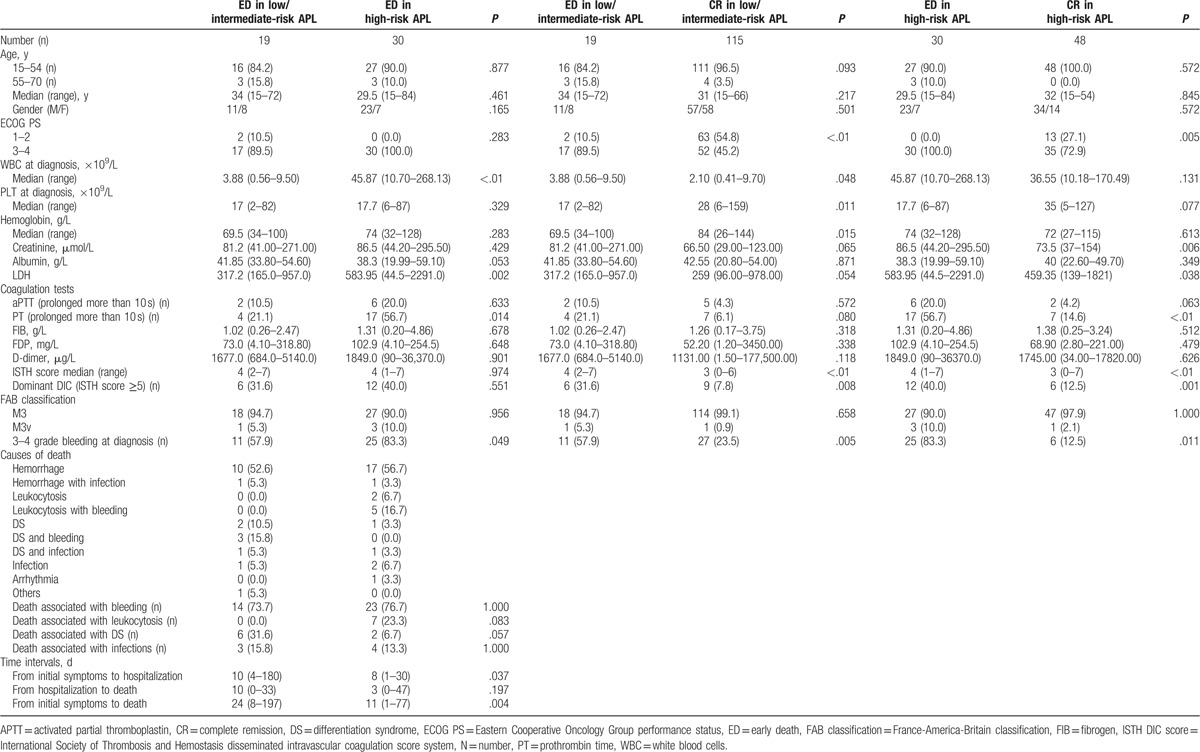
No differences were found in the causes of ED between the 2 ED groups and no delay in diagnosis was observed for the ED patients in the high-risk group. In contrast, both the time from the onset of symptoms to the initial hospitalization and from the onset of symptoms to death was shorter for the ED patients in the high-risk group than for the patients in the low/intermediate-risk group (P = .037, P = .004).
Compared with the CR patients of the low/intermediate-risk group, patients in the low/intermediate-risk ED group had a lower ECOG score (P < .01), higher WBC counts (P = .048), lower PLT counts (P = .011), lower hemoglobin levels (P = .015), and a higher ISTH score (P < .01). These patients were also at a higher risk for a grade 3 to 4 grade bleeding (P = .005). Patients in the high-risk ED group had higher WBC counts (P = .005), a higher frequency of PT prolongation (P < .01), higher ISTH (P < .01) and DIC scores (P = .001), and a higher rate of grade 3 to 4 bleeding (P = .011).
4. Discussion
There is still no exact definition of ED in APL, although it is usually defined as death during induction of treatment or within 30 days from initial treatment.[7,8] In this study, we defined ED as death occurring during induction treatment. All deaths occurred less than 30 days following treatment induction except for 1 patient who died on the 49th day from the onset of treatment. In general, the ED rate in APL patients remains high despite great advances in treatment options, suggesting that current therapeutic strategies are inadequate.[7,8]
The ED rate in our study was 23.1%. ED is traditionally defined as death at the onset of treatment, even though most patients may die before being diagnosed or starting treatment. In the present study, we found that 50% of EDs occurred before treatment was initiated or in the first 3 days after the initiation of ATRA therapy, while 24.5% of deaths occurred before treatment. Lehmann et al[7] reported that 35% of ED patients never receive ATRA treatment, and other studies have reported that 68% of deaths occur in the first 5 days after treatment is started.[12] However, only rare research studies distinguished the differences between the EDs before and after induction treatment. For patients who die before treatment was started or shortly thereafter, rapid disease progression and/or inadequate therapeutic choices may be to blame. However, for patients who die after treatment, differentiation syndrome, infections, or side effects of chemotherapy may be responsible. Therefore, the causes of ED need to be carefully delineated to improve the prognosis of APL patients.
The results of our study, presented herein, suggest that death before treatment or within the first 3 days of treatment is different from death occurring after the first 3 days of treatment. First, we found that hemorrhage was the leading cause of death in the iED population, while ED related to infection and differentiation syndrome was substantially higher in the ED after treatment group. Second, the iED group had more high-risk patients with higher WBC counts and higher levels of LDH and ED rates, suggesting that disease progression in APL patients with higher tumor load is faster and that a higher tumor load is a leading cause of ED. Third, for the 2 ED groups, PT prolongation was more frequent, the ISTH score was higher, and bleeding (grades 3–4) was observed more frequently than in the CR group. However, we found that APTT prolongation and detectable DIC was statistically different from the values of the CR group only for the iED group. These differences were not observed between the ED after treatment group and the CR group.
PT prolongation was found more frequently in the iED (56%) and high-risk ED groups (56.7%). However, there were no significant differences in the coagulation test results between the iED and ED after treatment groups or between the low/intermediate-risk and high-risk ED groups. In a recent study, Chang et al[13] reported that PT was a valuable parameter in the prediction of bleeding in de novo APL patients. As a prolonged PT is caused by abnormalities in the extrinsic coagulation pathway, studies have shown that overexpression of tissue factor (TF) and TF+ microparticles or exposure to phosphatidylserine—parameters associated with the extrinsic coagulation pathway—may be the reasons underlying the coagulopathy that occurs in APL patients.[14–16] To some extent, these parameters may explain the PT prolongation in de novo APL patients. Therefore, it is reasonable to speculate that patients in the iED and high-risk ED groups are more at risk for a life-threatening hemorrhage ISTH, itself another useful laboratory index to evaluate the status of bleeding in APL patients and a predictive value for hemorrhagic ED when it is equal to or greater than 6, as reported by Mitrovic et al.[17] Consistently, we found that the ISTH DIC score was higher in the iED group. Therefore, more aggressive support therapies are needed for the APL patients with high PT and ISTH scores to prevent ED.
WBC count is viewed as one of the most important indexes associated with prognosis in APL. In patients with low/intermediate risk, higher WBC counts were found in ED patients. Similar values were recorded for the high-risk ED patients and the high-risk CR patients. The results suggest that the WBC count is a useful index to predict ED.
It has been reported that delays in ATRA administration lead to a higher incidence of EDs from hemorrhage.[18] However, in the present study, we did not find a correlation between the number of deaths in the iED and high-risk ED groups and delays in diagnosis or treatment initiation. On the contrary, the times from the onset of symptoms to the initial hospitalization or death were shorter in the iED and high-risk groups than in the ED after treatment group. This finding is consistent with the results obtained by Rashidi and Fisher,[19] who purported that the group that receives ATRA promptly is somehow “sicker” than the delayed groups; here, the word “sicker” represents factors related to the patient, clinician, and hospital. Thus, the ED in the high-risk group is not a result of delay of hospitalization or diagnosis, and instead the nature of the disease or the rapid progression of high-risk APL should account for the ED.
Even if 49 ED patients with APL were included in this retrospective study, it is difficult to make the treatment before death homogeneous. However, we tried to balance the treatment factors between groups. In summary, in this study, we found that it was disease characteristics and not timing of treatment initiation that regulated the incidence of ED in APL patients. Specifically, a higher WBC, a prolonged PT, a higher ISTH score, and the speed of disease progression were found to be the distinctive risk factors among the patients in the iED group. Therefore, an optimization of treatment options and induction of therapy is necessary to reduce the incidence of ED after treatment. Specifically, more aggressive support therapy options are needed to reduce tumor load effectively, to improve the status of coagulopathy rapidly, to stop the progression of disease, and to eventually decrease the incidence of ED.
5. Conclusion
In all, different types of EDs have different clinical features. A high WBC count contributes to the occurrence of more ED, which is usually not associated with the delay of diagnosis and hospitalization. Current therapeutic strategies to reduce the incidence of ED in these cases are not adequate and will benefit from focused research attention.
Footnotes
Abbreviations: APL = acute promyelocitic leukemia, ATO = arsenic trioxide, ATRA = all-trans retinoic acid, CR = complete remission, DA = daunorubicin+ cytarabine, ECOG = Eastern Cooperative Oncology Group, ED = early death, FDP = fibrinogen degradation products, HA = homobarringtonie+cytarabine, iED group = immediate death, ISTH = International Society on Thrombosis and Hemostasis, PLT = platelet, PT = prothrombin time, SD = standard deviation, TF = tissue factor, WBC = death during treatment at least 3 days after commencement (ED after treatment) white blood cell.
All authors have declared no conflicts of interest.
References
- [1].Zhang Y, Zhang Z, Li J, et al. Long-term efficacy and safety of arsenic trioxide for first-line treatment of elderly patients with newly diagnosed acute promyelocytic leukemia. Cancer 2013;119:115–25. [DOI] [PubMed] [Google Scholar]
- [2].Avvisati G, Lo-Coco F, Paoloni FP, et al. AIDA 0493 protocol for newly diagnosed acute promyelocytic leukemia: very long-term results and role of maintenance. Blood 2011;117:4716–25. [DOI] [PubMed] [Google Scholar]
- [3].Shen Y, Fu YK, Zhu YM, et al. Mutations of epigenetic modifier genes as a poor prognostic factor in acute promyelocytic leukemia under treatment with all-trans retinoic acid and arsenic trioxide. EBioMedicine 2015;2:563–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hu J, Liu YF, Wu CF, et al. Long-term efficacy and safety of all-trans retinoic acid/arsenic trioxide-based therapy in newly diagnosed acute promyelocytic leukemia. Proc Natl Acad Sci U S A 2009;106:3342–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Iland HJ, Bradstock K, Supple SG, et al. All-trans-retinoic acid, idarubicin, and IV arsenic trioxide as initial therapy in acute promyelocytic leukemia (APML4). Blood 2012;120:1570–80. quiz 1752. [DOI] [PubMed] [Google Scholar]
- [6].Lo-Coco F, Avvisati G, Vignetti M, et al. Front-line treatment of acute promyelocytic leukemia with AIDA induction followed by risk-adapted consolidation for adults younger than 61 years: results of the AIDA-2000 trial of the GIMEMA Group. Blood 2010;116:3171–9. [DOI] [PubMed] [Google Scholar]
- [7].Lehmann S, Ravn A, Carlsson L, et al. Continuing high early death rate in acute promyelocytic leukemia: a population-based report from the Swedish Adult Acute Leukemia Registry. Leukemia 2011;25:1128–34.21502956 [Google Scholar]
- [8].Park JH, Qiao B, Panageas KS, et al. Early death rate in acute promyelocytic leukemia remains high despite all-trans retinoic acid. Blood 2011;118:1248–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Micol JB, Raffoux E, Boissel N, et al. Management and treatment results in patients with acute promyelocytic leukaemia (APL) not enrolled in clinical trials. Eur J Cancer 2014;50:1159–68. [DOI] [PubMed] [Google Scholar]
- [10].Frankel SR, Eardley A, Lauwers G, et al. The “retinoic acid syndrome” in acute promyelocytic leukemia. Ann Intern Med 1992;117:292–6. [DOI] [PubMed] [Google Scholar]
- [11].Sanz MA, Lo Coco F, Martin G, et al. Definition of relapse risk and role of nonanthracycline drugs for consolidation in patients with acute promyelocytic leukemia: a joint study of the PETHEMA and GIMEMA cooperative groups. Blood 2000;96:1247–53. [PubMed] [Google Scholar]
- [12].Visani G, Gugliotta L, Tosi P, et al. All-trans retinoic acid significantly reduces the incidence of early hemorrhagic death during induction therapy of acute promyelocytic leukemia. Eur J Haematol 2000;64:139–44. [DOI] [PubMed] [Google Scholar]
- [13].Chang H, Kuo MC, Shih LY, et al. Clinical bleeding events and laboratory coagulation profiles in acute promyelocytic leukemia. Eur J Haematol 2012;88:321–8. [DOI] [PubMed] [Google Scholar]
- [14].Ma G, Liu F, Lv L, et al. Increased promyelocytic-derived microparticles: a novel potential factor for coagulopathy in acute promyelocytic leukemia. Ann Hematol 2013;92:645–52. [DOI] [PubMed] [Google Scholar]
- [15].Yan J, Wang K, Dong L, et al. PML/RARalpha fusion protein transactivates the tissue factor promoter through a GAGC-containing element without direct DNA association. Proc Natl Acad Sci U S A 2010;107:3716–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhou J, Shi J, Hou J, et al. Phosphatidylserine exposure and procoagulant activity in acute promyelocytic leukemia. J Thromb Haemost 2010;8:773–82. [DOI] [PubMed] [Google Scholar]
- [17].Mitrovic M, Suvajdzic N, Bogdanovic A, et al. International Society of Thrombosis and Hemostasis Scoring System for disseminated intravascular coagulation >/ = 6: a new predictor of hemorrhagic early death in acute promyelocytic leukemia. Med Oncol 2013;30:478. [DOI] [PubMed] [Google Scholar]
- [18].Altman JK, Rademaker A, Cull E, et al. Administration of ATRA to newly diagnosed patients with acute promyelocytic leukemia is delayed contributing to early hemorrhagic death. Leuk Res 2013;37:1004–9. [DOI] [PubMed] [Google Scholar]
- [19].Rashidi A, Fisher SI. Prompt all-trans retinoic acid administration and early death in acute promyelocytic leukemia: what does the evidence say? Leukemia 2014;28:2425–6. [DOI] [PubMed] [Google Scholar]


