Abstract
Because of improving treatments and survival, 40% to 58% of patients with bone metastases from nonsmall cell lung cancer (NSCLC) will suffer from at least one skeletal-related event (SRE), affecting their quality of life, but the natural history of SRE is poorly understood. The study aimed to examine the factors involved in SRE-free survival (SRS) and overall survival (OS) of patients with NSCLC and bone metastases.
This was a retrospective study of 211 patients with bone metastasis from NSCLC and treated at the Tumor Hospital Affiliated to Harbin Medical University between January 2007 and January 2012. OS and SRS were evaluated by the Kaplan–Meier method. The factors associated with SRS and OS were examined using multivariate Cox analyses.
The 1 year OS was 55.9% and the median OS was 30 months (range, 1–98 months). Multivariate analyses showed that clinical staging at initial diagnosis (P < .001) and SRE (P = .033) were independently associated with OS, and clinical staging at initial diagnosis (P = .009), bone pain (P = .008), primary tumor radiotherapy (P < .001), and chemotherapy (P = .031) were independently associated with SRS. Stage I, II, and III patients under biphosphonate therapy fared better than those without biphosphonate treatment, but there was no difference for stage IV patients.
The identification of factors associated with OS and SRS of patients with NSCLC and bone metastases should provide new clues for a better management of these patients.
Keywords: bone metastasis, nonsmall cell lung cancer, skeletal-related events, survival
1. Introduction
Lung cancer has a high incidence and mortality.[1] Nonsmall cell lung cancer (NSCLC) represents about 85% of all cases of lung cancer.[2] NSCLC mainly affects men aged 55 to 65 years.[3] In China, the incidence of NSCLC is 61.9/100,000 men and 29.5/100,000 women, with mortality rates of 50.0/100,000 men and 23.3/100,000 women.[4] The incidence of NSCLC has increased over the last decade in China, particularly among men living in urban areas.[4] Survival to NSCLC is poor, with 5-year overall survival (OS) of 58%, 35%, 10%, and 2% for stage I, II, III, and IV disease, respectively.[5] Because of this poor survival, the morbidity of lung cancer is often overlooked among survivors. Nevertheless, because of the improving OS of patients with advanced NSCLC due to better therapeutic approaches,[6] managing survivorship is becoming an important clinical issue. Bone metastasis is one of the most common complications among lung cancer survivors.[7] These patients often have a dramatic decline of quality of life (QOL) due to pain and physical limitations and a shortened OS.[7,8]
Some patients with bone metastases may suffer from skeletal-related events (SREs), that is, pathological fracture, spinal cord compression, and malignant hypercalcemia.[7,9] Previous studies showed that about 40% to 58% of patients with bone metastases from NSCLC will have at least 1 SRE during their lifespan.[7,10] SRE usually appears within the first 5 to 6 months after the diagnosis of bone metastasis.[7,10] Risk factors for SREs include bone metastases at diagnosis, number of bone metastases, male gender, poor performance status, and baseline hypercalcemia.[9,11] SREs are associated with pain that requires advanced management and with declines in all components of QOL.[12,13] In addition, OS is shorter among patients with SRE compared with patients with bone metastases but without SRE.[9] Severe bone symptoms may require surgery or radiotherapy to improve the QOL of the patients.[7,9]
Nevertheless, the natural history of SREs in patients with NSCLC and bone metastases is poorly understood, especially in Chinese patients. In addition, there is a need to improve the SRE-free survival (SRS) among these patients in order to improve their management and QOL. Therefore, the aim of the present study was to examine the factors involved in SRS and OS among patients with NSCLC and bone metastases.
2. Materials and methods
2.1. Study design and patients
This was a retrospective study of 211 patients with bone metastasis from NSCLC admitted to the Tumor Hospital Affiliated to Harbin Medical University between January 2007 and January 2012. The medical records were searched for cases of NSCLC with bone metastasis. All patients have been confirmed to be with NSCLC according to symptoms and signs (cough, bloody sputum or hemoptysis, shortness of breath or stridor, fever, weight loss, chest pain, hoarseness, dysphagia, hydrothorax, symptoms and signs caused by metastasis, and paraneoplastic syndrome) and histological examination based on the 2004 World Health Organization criteria.[14] Diagnostic criteria for bone metastasis were: bone abnormal radioactive concentration by ECT bone scan; and confirmed by X-ray, computed tomography, magnetic resonance imaging, and/or positron emission tomography. Exclusion criteria were: incomplete clinical and pathological data; or the presence of a primary tumor other than NSCLC.
This study was approved by the ethics committee of the Tumor Hospital Affiliated to Harbin Medical University. The need for individual consent was waived by the committee because of the retrospective nature of the study.
2.2. Data collection
The clinical and pathological data included gender, age (≥60 vs <60 years old), histological type (adenocarcinoma vs squamous cell carcinoma), ECOG performance score at first bone metastasis (≤2 vs >2),[15] number of bone metastases (<3 vs ≥3), TNM stage according to the 7th edition of AJCC TNM staging, lactate dehydrogenase (normal: 100–300 U/L), alkaline phosphatase (normal: 40–150 U/L), and albumin (normal: 35–50 g/L).
2.3. Follow-up and outcomes
After diagnosis of lung cancer, patients were followed up every 3 months for 2 years and then yearly thereafter. Death or last follow-up was considered as the end of follow-up. For surviving patients, follow-up was censored on June, 2016. The follow-ups were conducted by phone, text message, outpatient service, or medical records.
SRE diagnostic criteria were: pathological fracture; spinal cord compression; malignant hypercalcemia; and/or severe bone-related symptoms that require surgery or radiotherapy for management, including (but not limited to) pain and symptom of nerve compression.[7,9]
SRS was calculated as the time from the diagnosis of primary lung cancer to diagnosis of SRE. Survival without SRE was calculated as the time from the diagnosis of primary lung cancer to death or last follow-up. OS was calculated as the time from the diagnosis of primary lung cancer to death or last follow-up.
2.4. Statistical analysis
Continuous data were presented as mean ± standard deviation and analyzed using the Student t-test. Categorical data were presented as frequencies and analyzed using the Fisher exact test. The Kaplan–Meier method was used for survival analysis and the log-rank test was used to compare the curves. Multivariate analysis was conducted using the Cox regression model to screen for factors independently associated with OS and SRS. Subgroup analysis was performed according to clinical staging at first diagnosis, using the Kaplan–Meier method and the log-rank test for comparison between the biphosphonate and nonbiphosphonate groups. SPSS 22.0 (IBM, Armonk, NY) was used for statistical analysis. Two-sided P-values <0.05 were considered statistically significant.
3. Results
3.1. Characteristics of the patients
Table 1 presents the characteristics of the patients. There were 125 males and 86 females (ratio of 1.45:1). The median age was 57 years old (range, 22–80). Most patients were stage IV at diagnosis (77.7%). The ECOG performance status was >2 in 172 patients (81.5%). Most patients had ≥3 bone metastases (67.8%) and were suffering from bone pain (82.9%). Bone metastases were observed in the spine in 146 patients (69.2%), ribs in 120 (56.9%), pelvis in 70 (33.2%), limb bone in 31 (14.7%), and skull in 14 (6.6%).
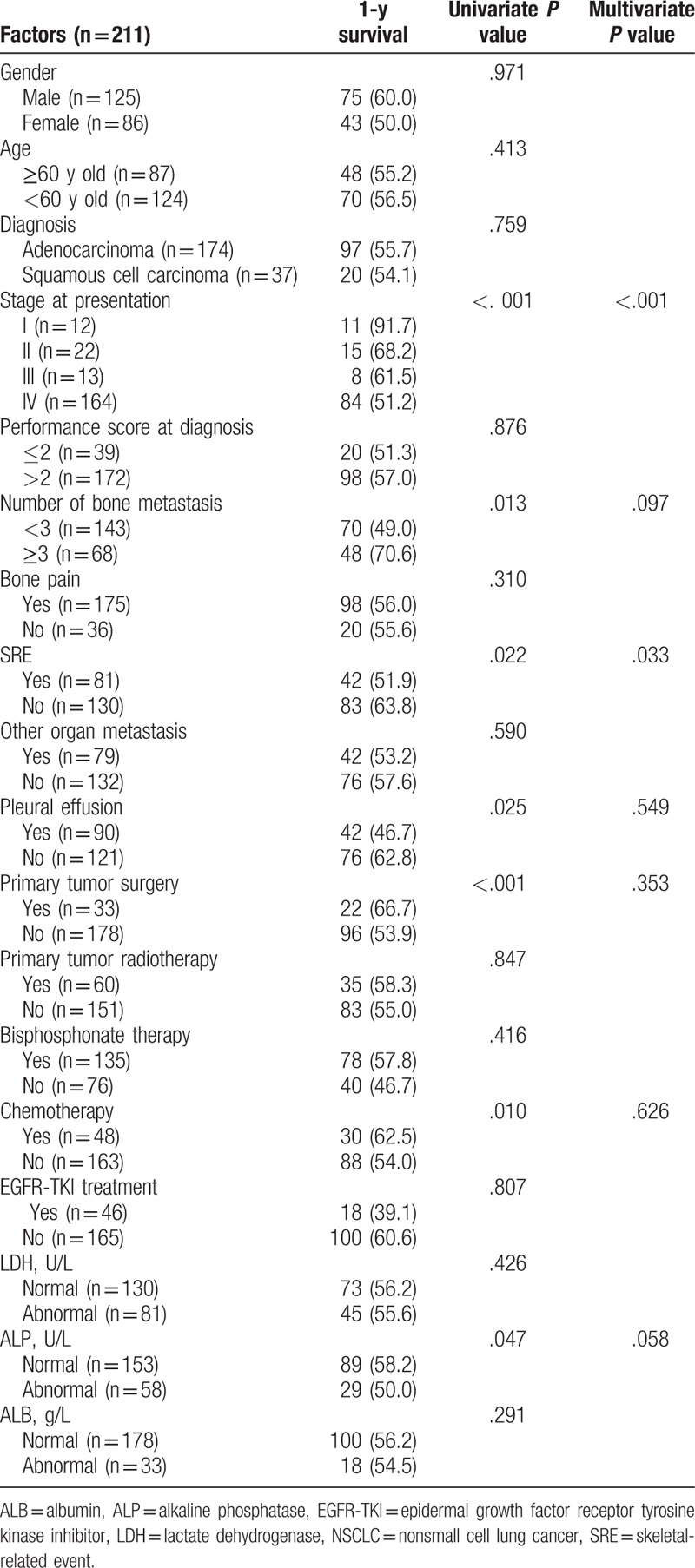
Table 1.
Univariate and multivariate‘ analyses of factors affecting overall survival of patients with bone metastasis from NSCLC.
3.2. Skeletal-related events
Eighty-one patients had a reported SRE (38.4%). Eleven patients had 2 or more types of SRE (5.2%), for a total of 92 SRE events. Among these events, 75 were serious SREs that needed surgery or radiotherapy, 7 were pathological fractures, 7 were spinal cord compressions, and 3 were malignant hypercalcemia.
3.3. Overall survival
Six patients were lost to follow-up, for an overall follow-up rate of 97.2%. The median follow-up was 18 months and the longest was 98 months. The 1 year OS rate of the 211 patients was 55.9% and the median OS was 30 months (range, 1–98 months) (Fig. 1).
Figure 1.
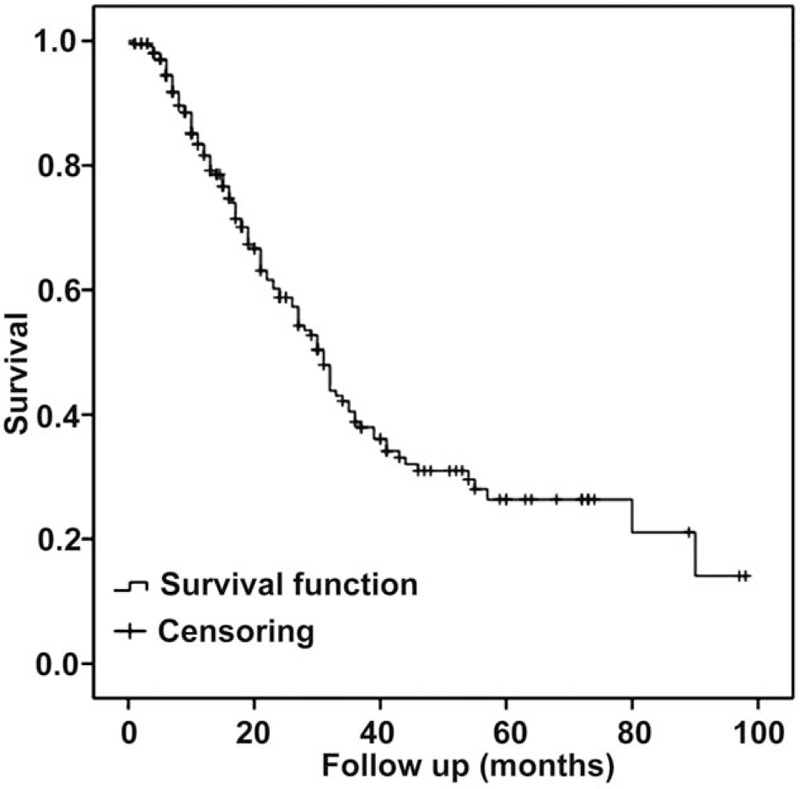
Overall survival.
3.4. Univariate and multivariate analyses of OS
Univariate analyses showed that the clinical staging at initial diagnosis (P < .001), number of bone metastases (P = .013), SRE (P = .022), pleural effusion (P = .025), primary tumor surgery (P < .001), chemotherapy (P = .010), and alkaline phosphatase levels (P = .047) were significantly associated with OS of patients with bone metastasis from NSCLC (Table 1). These factors were included in a Cox regression model and the results showed that the clinical staging at initial diagnosis (P < .001) and SRE (P = .033) were independently associated with OS (Table 1).
3.5. Univariate and multivariate analyses of SRS
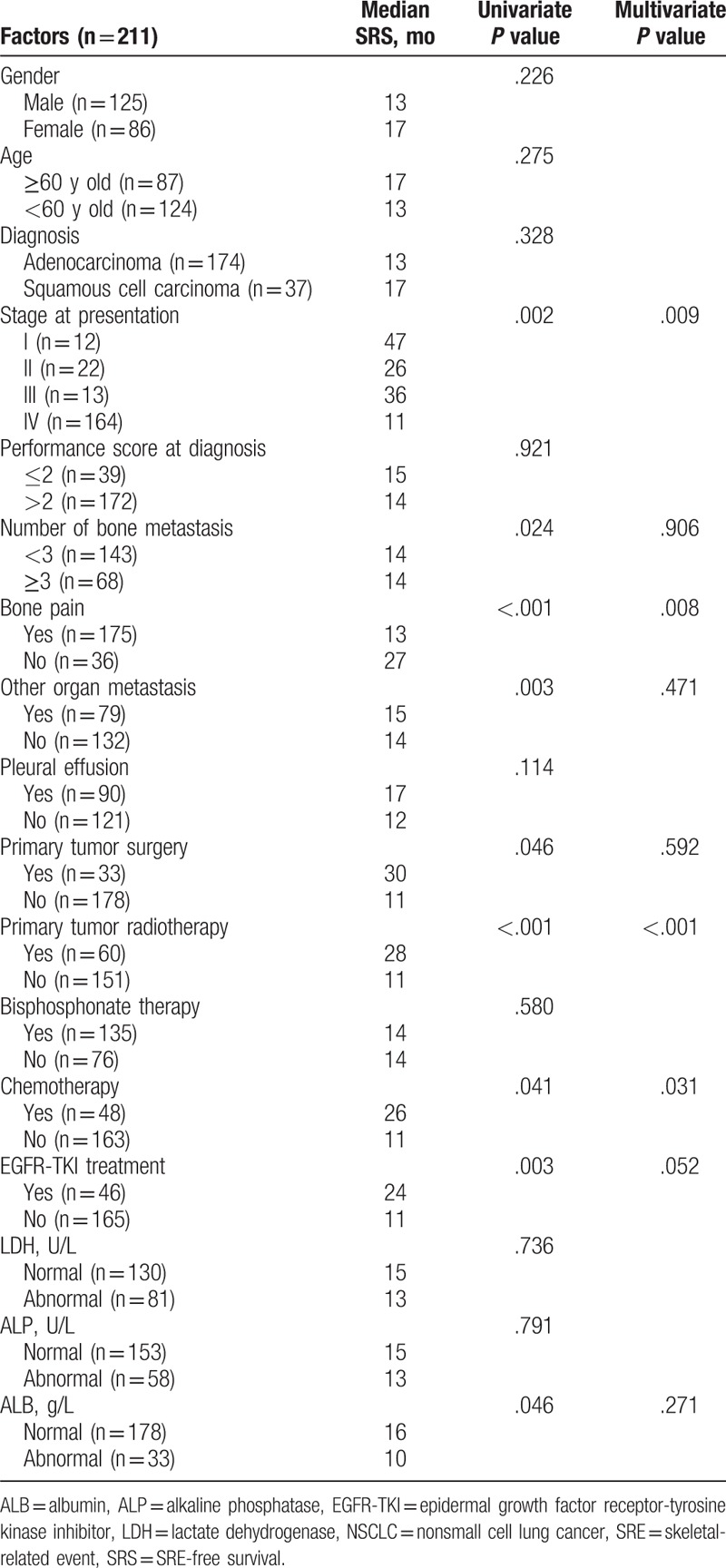
Univariate analyses showed that the clinical staging at initial diagnosis (P = .002), number of bone metastases (P = .024), bone pain (P < .001), metastases to other organs (P = .003), primary tumor radiotherapy (P < .001), chemotherapy (P = .041), endothelial growth factor receptor-tyrosine kinase inhibitor (EGFR-TKI) treatment (P = .003), and albumin levels (P = .046) were associated with SRS (Table 2). These factors were included in a Cox regression model and the results showed that clinical staging at initial diagnosis (P = .009), bone pain (P = .008), primary tumor radiotherapy (P < .001), and chemotherapy (P = .031) were independently associated with SRS of patients with bone metastasis from NSCLC (Table 2).
Table 2.
Univariate and multivariate analyses of factors affecting SRS of patients with bone metastasis from NSCLC.
3.6. Subgroup analysis based on biphosphonate therapy
The Kaplan–Meier SRS analyses and the log-rank test showed that stage I, II, and III patients under biphosphonate therapy fared better than those without biphosphonate treatment (P = .023, P = .009, and P = .042, respectively (Fig. 2A–C). There was no difference among stage IV patients (P = .923) (Fig. 2D). Based on the available data, there were no differences in OS and SRS for chemotherapy versus no chemotherapy and for EGFR-TKI versus no EGFR-TKI (data not shown).
Figure 2.
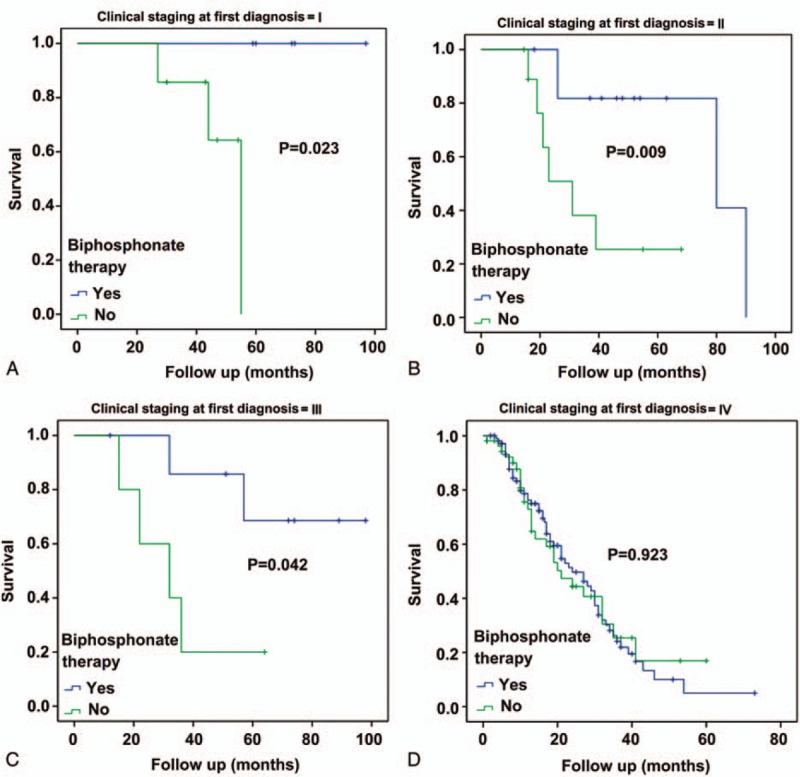
Subgroup analysis of skeletal-related event-free survival according to cancer stage and biphosphonate therapy. (A) Stage I, (B) stage II, (C) stage III, and (D) stage IV.
4. Discussion
Because of improving treatments and survival, 40% to 58% of patients with bone metastases from NSCLC have at least 1 SRE during their lifespan, affecting their QOL,[7,10] but the natural history of SRE is poorly understood. Therefore, this study aimed to examine the factors involved in SRS and OS of patients with NSCLC and bone metastases. Multivariate analyses showed that the clinical staging at initial diagnosis and SRE were independently associated with OS, and that clinical staging at initial diagnosis, bone pain, primary tumor radiotherapy, and chemotherapy were independently associated with SRS. The identification of factors associated with OS and SRS of patients with NSCLC and bone metastases should provide new clues for a better management of these patients.
Previous studies showed that the spinal column is the most common site of bone metastasis,[16] but in the present study, we found that the most common sites of bone metastasis were the spinal column (69.2%) and the rib cage (56.9%), which is supported by Tsuya et al.[17]
SRE not only reduces the QOL of patients with NSCLC, but is also associated with higher treatment costs and poorer prognosis. Cetin et al[18] showed that the incidence of bone metastasis and SRE was higher in patients with NSCLC than in patients with small cell lung cancer and that SRE led to higher mortality. Another study showed that the OS of patients without SRE was longer than in patients with SRE and the survival rates of bone metastasis patients with and without SRE were 36% and 45%, respectively.[9] In the present study, a similar tendency was observed, but with higher survival rates (51.9% and 63.8%, respectively). The present study showed that SRE was an independent factor affecting the OS of patients with bone metastasis from NSCLC, which is supported by Ulas et al.[9]
Previous studies revealed a variety of factors associated with SRS. Ulas et al[9] showed that the presence of bone metastases at initial diagnosis, the number of bone metastases, baseline hypercalcemia, and palliative radiotherapy were independently associated with SRS. Sekine et al[11] showed that male gender and multiple bone metastases were associated with SRS. Sun et al[19] showed that smoking, histological subtype, performance status, and no EGFR-TKI therapy were associated with SRS. Finally, da Silva et al[20] showed that smoking, performance status, and multiple bone metastases were associated with SRS. In the present study, clinical staging at initial diagnosis, bone pain, primary tumor radiotherapy, and chemotherapy were independently associated with SRS of patients with bone metastasis from NSCLC. Discrepancies among studies could be due to a number of factors such as study population, available data, and genetic variations around the globe.
Radiotherapy combined with chemotherapy is the main treatment approach for lung cancer.[21] This approach can kill tumor cells, both local and disseminated, reducing the novel and/or progression of bone metastases and delay SRE occurrence. The present study showed that radiotherapy of the primary tumor did not prolong OS, but extended SRS, which has not been shown in previous studies. It is generally considered that tumors of the skeletal system have a low sensitivity to cytotoxic drugs,[22] despite anecdotal case reports suggesting a possible efficacy of chemotherapy combined to biphosphonate.[23] The results of this study showed that chemotherapy was independently associated with SRS. In addition, the results suggest that the use of biphosphonate improved survival in patients with stage I, II, and III cancer at diagnosis. Biphosphonate is usually used as prevention to reduce the occurrence of SRE in patients with bone metastasis from breast cancer and multiple myeloma,[24] but Ulas et al[9] showed that SRS was significantly longer with biphosphonate therapy than without biphosphonate among patients with NSCLC. In addition, Henk et al[25] found that the fracture risk of patients with bone metastases was lower when using zoledronic acid. Biphosphonate can inhibit the activity of osteoclasts and decrease the dissolution and destruction of trabecular bone, thereby preventing a variety of SRE caused by tumor metastasis.
The present study is not without limitations. The sample size was small and from a single center. Because of the retrospective nature of the study, some variables that were not included in the medical charts could not be studied. Systematic screening for all metastases in other organs was not performed in all patients, and many had suspicions of metastases based on imaging, but without histopathological confirmation. Because of the retrospective nature of the study and because some patients were managed at more than one hospital, we could not obtain the exact treatment data for the whole follow-up of all patients, especially for EGFR-TKI drugs that are given orally. Finally, because the patients were from before 2015, the 2004 WHO criteria were used for diagnosis[14] instead of the 2015 criteria.[26] Additional studies are still necessary to assess the factors associated with SRE in patients with NSCLC.
5. Conclusions
In conclusion, clinical staging at initial diagnosis and SRE were independently associated with OS of patients with NSCLC and bone metastases. Clinical staging at initial diagnosis, bone pain, radiotherapy of primary tumor, and chemotherapy were independently associated with the SRS of patients with NSCLC and bone metastases. Biphosphonate could improve the prognosis of patients with stage I, II, and III disease. The identification of factors associated with OS and SRS of patients with NSCLC and bone metastases should provide new clues for a better management of these patients.
Acknowledgments
The authors thank the National Natural Science Foundation of China (No. 81201437), the Science Foundation of Educational Commission of Heilongjiang Province of China (No. 12531328), and the Science Foundation of Health and Family Planning Commission of Heilongjiang Province of China (No. 2012-550) for the support.
Footnotes
Abbreviations: EGFR-TKI = endothelial growth factor receptor-tyrosine kinase inhibitor, NSCLC = nonsmall cell lung cancer, OS = overall survival, QOL = quality of life, SRE = skeletal-related event, SRS = SRE-free survival.
DL, YJ, YW, JZ, JW, XH, and XY contributed equally and share the second authorship.
Ethics approval and consent to participate: This study was approved by the ethics committee of the Tumor Hospital Affiliated to Harbin Medical University. The need for individual consent was waived by the committee because of the retrospective nature of the study.
Funding/support: This work was supported by the National Natural Science Foundation of China (No. 81201437), the Science Foundation of Educational Commission of Heilongjiang Province of China (No. 12531328), and the Science Foundation of Health and Family Planning Commission of Heilongjiang Province of China (No. 2012-550).
The authors have no conflicts of interest to disclose.
References
- [1].Kim ST, Uhm JE, Lee J, et al. Randomized phase II study of gefitinib versus erlotinib in patients with advanced non-small cell lung cancer who failed previous chemotherapy. Lung Cancer 2012;75:82–8. [DOI] [PubMed] [Google Scholar]
- [2].Molina JR, Yang P, Cassivi SD, et al. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc 2008;83:584–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Thun MJ, Hannan LM, Adams-Campbell LL, et al. Lung cancer occurrence in never-smokers: an analysis of 13 cohorts and 22 cancer registry studies. PLoS Med 2008;5:e185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Chen W, Zheng R, Zeng H, et al. Epidemiology of lung cancer in China. Thorac Cancer 2015;6:209–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ries LAG, Young JL, Keel GE, et al. SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988-2001, Patient and Tumor Characteristics (NIH Pub. No. 07-6215). Bethesda: National Cancer Institute, SEER Program; 2007. [Google Scholar]
- [6].Sandler A, Gray R, Perry MC, et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006;355:2542–50. [DOI] [PubMed] [Google Scholar]
- [7].Daniele S, Sandro B, Salvatore I, et al. Natural history of non-small-cell lung cancer with bone metastases. Sci Rep 2015;5:18670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Shimada Y, Saji H, Yoshida K, et al. Prognostic factors and the significance of treatment after recurrence in completely resected stage I non-small cell lung cancer. Chest 2013;143:1626–34. [DOI] [PubMed] [Google Scholar]
- [9].Ulas A, Bilici A, Durnali A, et al. Risk factors for skeletal-related events (SREs) and factors affecting SRE-free survival for nonsmall cell lung cancer patients with bone metastases. Tumour Biol 2016;37:1131–40. [DOI] [PubMed] [Google Scholar]
- [10].Rosen LS, Gordon D, Tchekmedyian NS, et al. Long-term efficacy and safety of zoledronic acid in the treatment of skeletal metastases in patients with nonsmall cell lung carcinoma and other solid tumors: a randomized, phase III, double-blind, placebo-controlled trial. Cancer 2004;100:2613–21. [DOI] [PubMed] [Google Scholar]
- [11].Sekine I, Nokihara H, Yamamoto N, et al. Risk factors for skeletal-related events in patients with non-small cell lung cancer treated by chemotherapy. Lung Cancer 2009;65:219–22. [DOI] [PubMed] [Google Scholar]
- [12].Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev 2001;27:165–76. [DOI] [PubMed] [Google Scholar]
- [13].Coleman RE. Bisphosphonates: clinical experience. Oncologist 2004;9(Suppl 4):14–27. [DOI] [PubMed] [Google Scholar]
- [14].Travis WD. Pathology and Genetics of Tumours of the Lung, Pleura, Thymus, and Heart. Lyon: IARC Press; 2004. [Google Scholar]
- [15].Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 1982;5:649–55. [PubMed] [Google Scholar]
- [16].Yoh K, Kubota K, Ohmatsu H, et al. Feasibility study of zoledronic acid plus cisplatin-docetaxel as first-line treatment for advanced non-small cell lung cancer with bone metastases. Anticancer Res 2012;32:4131–5. [PubMed] [Google Scholar]
- [17].Tsuya A, Kurata T, Tamura K, et al. Skeletal metastases in non-small cell lung cancer: a retrospective study. Lung Cancer 2007;57:229–32. [DOI] [PubMed] [Google Scholar]
- [18].Cetin K, Christiansen CF, Jacobsen JB, et al. Bone metastasis, skeletal-related events, and mortality in lung cancer patients: a Danish population-based cohort study. Lung Cancer 2014;86:247–54. [DOI] [PubMed] [Google Scholar]
- [19].Sun JM, Ahn JS, Lee S, et al. Predictors of skeletal-related events in non-small cell lung cancer patients with bone metastases. Lung Cancer 2011;71:89–93. [DOI] [PubMed] [Google Scholar]
- [20].da Silva GT, Bergmann A, Thuler LC. Skeletal related events in patients with bone metastasis arising from non-small cell lung cancer. Support Care Cancer 2016;24:731–6. [DOI] [PubMed] [Google Scholar]
- [21].NCCN. Clinical Practice Guidelines in Oncology, (NCCN, Guidelines), Non-Small Cell Lung, Cancer. 2016;Fort Washington: National Comprehensive Cancer Network, Version, 4. 2016. [Google Scholar]
- [22].Shiono S, Harada M, Abiko M, et al. A case of recurrent lung cancer with bone metastases treated with tegafur-uracil and zoledronic acid for long-term survival. Gan To Kagaku Ryoho 2014;41:757–9. [PubMed] [Google Scholar]
- [23].Sugiura H, Yamada K, Sugiura T, et al. Predictors of survival in patients with bone metastasis of lung cancer. Clin Orthop Relat Res 2008;466:729–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Rosen LS, Gordon D, Kaminski M, et al. Long-term efficacy and safety of zoledronic acid compared with pamidronate disodium in the treatment of skeletal complications in patients with advanced multiple myeloma or breast carcinoma: a randomized, double-blind, multicenter, comparative trial. Cancer 2003;98:1735–44. [DOI] [PubMed] [Google Scholar]
- [25].Henk HJ, Kaura S, Teitelbaum A. Retrospective evaluation of the clinical benefit of long-term continuous use of zoledronic acid in patients with lung cancer and bone metastases. J Med Econ 2012;15:195–204. [DOI] [PubMed] [Google Scholar]
- [26].Travis WD, Brambilla E, Nicholson AG, et al. The 2015 World Health Organization Classification of Lung Tumors: Impact of Genetic, Clinical and Radiologic Advances Since the 2004 Classification. J Thorac Oncol 2015;10:1243–60. [DOI] [PubMed] [Google Scholar]


