Abstract
Background:
5% to 40% of infants cry excessively, usually accompanied by fussiness and excessive of gas. There are no uniform criteria for treatment of infantile colic. Lactobacillus reuteri DSM 17938 has been used with promising results. The objective of this network-meta-analysis (NMA) is to compare the efficacy of L reuteri DSM 17938 with other interventions for infantile colic.
Methods:
RCTs, published between 1960 and 2015 for the treatment of infantile colic were included. Primary outcome was duration of crying after 21 to 28 days of treatment. Different databases were searched. Information was analyzed using control group as central axis. A random effect model was used. Hedges standard mean difference (SMD) and odds ratio (OR) were calculated. A SUCRA analysis was performed to evaluate superiority for each intervention.
Results:
32 RCTs were analyzed, including 2242 patients. Studies with L reuteri DSM 17938 versus Ctrl., Diet versus Ctrl. and Acupuncture versus Ctrl. were the most influential studies in the NMA. L reuteri DSM 17938 [WMD −51.3 h (CI95% −72.2 to −30.5 h), P .0001] and dietetic approaches [WMD −37.4 h (CI95% −56.1 to −18.7 h), P .0001] were superior compared to the other treatments.
Conclusions:
L reuteri DSM 17938 and some dietetic approaches are better to other interventions for treatment of infantile colic.
Keywords: infantile colic, L reuteri DSM 17938, network meta-analysis
1. Introduction
Crying is generally thought to be a normal behavior during infancy, serving as an infant's means of survival. Through crying, infants can alert to and elicit help for problems, such as hunger, soiled diapers, harsh temperature, and discomfort or pain.[1,2] However, 5% to 40% of infants cry inconsolably and excessively, and this can be accompanied by bouts of fussiness and passing of gas.[3–5] Wessel et al[6] coined the term “infantile colic” to describe a fussy infant with colic as one who is otherwise healthy and well-fed, but with paroxysms of irritability, fussing or crying, lasting for a total of at least 3 hours a day, occurring on more than 3 days a week for a period of 3 weeks.[7] In 2006, Rome III criteria was published modifying these criteria to consider the diagnosis of “infantile colic” applicable to infants with paroxysms of irritability, fussing, or crying that start and stop without obvious cause, lasting 3 or more hours per day and occurring at least 3 days per week, but for at least 1 week and no failure to thrive.[8] Infantile colic can manifest as early as 1 to 2 weeks of age, with peak crying duration and fussiness typically between 6 and 8 weeks of age, and diminishing gradually until disappearing between 3 and 4 months of age.[1,6,9,10] The exact etiology of infantile colic remains elusive; however, various theories have been proposed, some of which include overproduction of intestinal gas, forceful intestinal contraction, miscommunication between brain and intestine, hypersensitivity to cow's milk protein, transient lactase deficiency, negative or inadequate maternal–infant bonding or parental overstimulation, difficult infant temperament, insecure parental attachment, or changes in intestinal microbiota.[2,6,7,10–12] Diverse studies have identified different microbiota patterns between infants with/without colic, which seems to affect intestinal fatty acid profiles.[12–16] In 2004, Savino et al[15] evaluated intestinal microflora in breastfed colicky and noncolicky infants. Seventy-one breastfed infants, aged 3.2 ± 0.6 weeks old, free from episodes of gastroenteritis and without previous use of antibiotics and probiotics, were enrolled in the study. They were divided into 2 groups: colicky (42 cases) and noncolicky (29 cases). Colicky infants were less frequently colonized by Lactobacillus spp., and more frequently by anaerobic gram-negative bacteria. Additionally, it seems that colicky babies are more frequently colonized with the gas-forming Clostridium difficile, Escherichia spp, and/or Klebsiella spp.[17,18] From a therapeutic point of view, there are no uniform criteria for a specific therapeutic regimen for infantile colic. The first recommended step is to look for potential “red flags.” In 2013 Vandenplas et al[19] published different algorithms for practical approach of gastrointestinal functional disorders. In this paper, the authors pointed out the importance of identifying signs/symptoms such as arching (Sandifer), GI bleeding or failure to thrive which could be associated to organic disease. If no red flags are apparent, it is recommendable to evaluate the feeding technique; then, reassure the caregivers and offer general advice, emphasizing the self-limiting nature of the condition. For breast-fed infants, clinicians should advise mothers to continue breast-feeding, with some authors recommending that nursing mothers should omit cow's milk protein (CMP) intake. The elimination diet should be continued for a minimum of 2 weeks and should continue if the infant responds well. For formula-fed infants, other authors have recommended the use of hydrolyzed and low protein-content infant formula.[20] Considering the evidence about the microbiota pattern in these infants, diverse authors have published different randomized clinical controlled trials (RCTs) where the ability of Lactobacillus reuteri to reduce crying time in these infants has been evaluated.[21–26] In 2010, Savino et al conducted an RCT to test the efficacy of this strain on infantile colic and to evaluate its relationship to the gut microbiota. Fifty exclusively breastfed colicky infants, diagnosed according to modified Wessel's criteria, were randomly assigned to receive either L reuteri DSM 17938 (108 colony-forming units) or placebo daily for 21 days. Parental questionnaires monitored daily crying time and adverse effects. Forty-six infants (L reuteri group: 25; placebo group: 21) completed the trial. Daily crying times in minutes/day (median [interquartile range]) were 370 (120) vs 300 (150) (P = .127) on day 0 and 35.0 (85) vs 90.0 (148) (P = .022) on day 21 with no differences in weight gain, stooling frequency, or incidence of constipation or regurgitation between groups, and no adverse events related to the supplementation were observed.[22] Three years later, Sajewska et al published with a similar design a second RCT in 80 infants aged < 5 months, identifying that the rate of responders to treatment was significantly higher in the probiotic group compared with the placebo group at day 7 (P = .026), at day 14 (relative risk [RR] 4.3, 95% CI 2.3–8.7), at day 21 (RR 2.7, 95% CI 1.85−4.1), and at day 28 (RR 2.5, 95% CI 1.8−3.75).[23] After these RCTs, 3 additional RCTs were published: 2 with similar results in support of L reuteri DSM 17938[25,26] and 1, a very controversial RCT with similar effects between L reuteri and placebo.[24] Additionally, other therapeutic strategies have been used, including the use of dicyclomine, cimetropium or simethicone;[27–32] infant formulas with the addition of hydrolyzed protein, soy protein, low-protein and/or prebiotics;[33–39] some herbal products;[40–43] acupuncture, chiropractic techniques, spinal massages, support to family/caregivers, counseling therapies, car rides during colic episodes, and/or decrease of stimulating actions.[44–56] Considering there are some conflicting results related to the use of some of these strategies, the aim of this paper is to compare the efficacy of L reuteri DSM 17938 with other plausible therapeutic approaches for infantile colic, through a systematic review with network meta-analysis (NMA) approach attempting to identify on an evidence-based analysis which could be the best therapeutic choice.
2. Methods
2.1. Study protocol register and search strategy
This systematic review was assembled considering The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions[57] and approved by the Internal Review Board (IRB) of the Hospital General Dr Manuel Gea González, México. We included in this review only double-blind, randomized, controlled clinical trials (RTCs), published between January, 1960 and August, 2015 in English or Spanish language. A systematic and exhaustive search was conducted in Medline, Embase, Cumulative Index to Nursing and Allied Health (CINAHL), PsycINFO, the Cochrane Central Register of Controlled Trials, Lilacs, Artemisa and in the databases of the principal international regulatory agencies in order to identify relevant studies published between 1960 and August 2015. PubMed searching algorithms was ((“infant” [MeSH Terms] OR “infant” [All Fields]) OR infantile [All Fields]) AND (“colic” [MeSH Terms] OR “colic” [All Fields]) AND ((“probiotics” [MeSH Terms] OR “probiotics” [All Fields]) OR ((“infant” [MeSH Terms] OR “infant” [All Fields]) AND formula [All Fields]) OR (“diet” [MeSH Terms] OR “diet” [All Fields]) OR (“pharmaceutical preparations” [MeSH Terms] OR (“pharmaceutical” [All Fields] AND “preparations” [All Fields]) OR “pharmaceutical preparations” [All Fields] OR “drugs” [All Fields]) OR (((“protons” [MeSH Terms] OR “protons” [All Fields] OR “proton” [All Fields]) AND pump [All Fields] AND (“antagonists and inhibitors” [Subheading] OR (“antagonists” [All Fields] AND “inhibitors” [All Fields]) OR “antagonists and inhibitors” [All Fields] OR “inhibitors” [All Fields])) OR (“dicyclomine” [MeSH Terms] OR “dicyclomine” [All Fields]) OR (“dicyclomine” [MeSH Terms] OR “dicyclomine” [All Fields] OR “dicycloverine” [All Fields]) OR (“cimetropium” [Supplementary Concept] OR “cimetropium” [All Fields]) OR (“simethicone” [MeSH Terms] OR “simethicone” [All Fields])) OR ((familiar [All Fields] OR (“caregivers” [MeSH Terms] OR “caregivers” [All Fields])) AND support[All Fields]) OR ((“counselling” [All Fields] OR “counseling” [MeSH Terms] OR “counseling” [All Fields]) AND (“therapeutics” [MeSH Terms] OR “therapeutics” [All Fields] OR “therapies” [All Fields])) OR car-rides [All Fields] OR (stimulating [All Fields] AND actions [All Fields]) OR (“chiropractic” [MeSH Terms] OR “chiropractic” [All Fields]) OR (“massage” [MeSH Terms] OR “massage” [All Fields] OR “massages” [All Fields]) OR (“acupuncture” [MeSH Terms] OR “acupuncture” [All Fields] OR “acupuncture therapy” [MeSH Terms] OR (“acupuncture” [All Fields] AND “therapy” [All Fields]) OR “acupuncture therapy” [All Fields]) OR herbal [All Fields]) AND (Clinical Trial[ptyp] AND (“1960/01/01” [PDAT]: “2015/08/31” [PDAT]) AND “humans” [MeSH Terms] AND (English[lang] OR Spanish[lang])).
2.2. Study selection and outcome measures
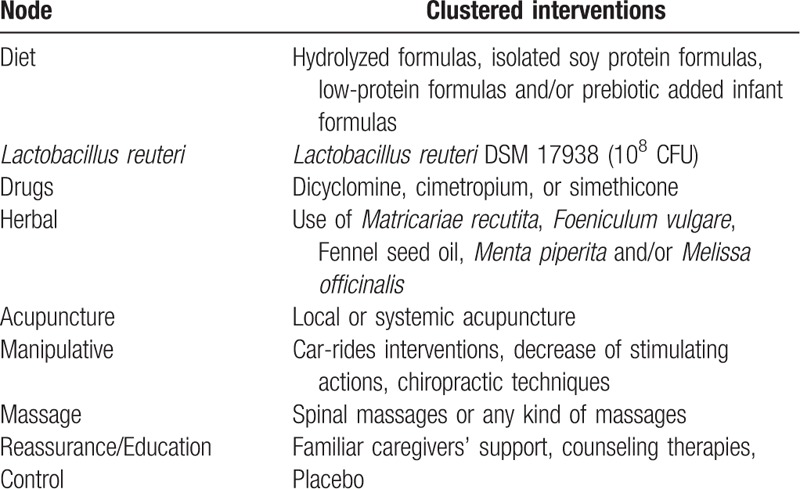
RCTs that compared the use of L reuteri; use of extensively or partially hydrolyzed formulas, isolated soy protein formulas, low-protein or lactose-free formulas or prebiotic added infant formulas; dicyclomine, cimetropium, or simethicone; familiar caregivers’ support, counseling therapies; car-rides interventions during colic episodes, decrease of stimulating actions, chiropractic techniques, spinal massages; acupuncture or use of herbal options versus placebo or active treatment in outpatient infantile colic infants less than 6 months old were selected for this network meta-analysis. All different treatments were included in 9 nodes (Table 1). Primary outcome analyzed was the duration of crying (in hours) observed 7 to 28 days after the beginning of treatment.
Table 1.
Intervention included in the node for analysis.
2.3. Data extraction and quality analysis
Quality evaluation of studies was performed in pairs, in a blinded and independent fashion using CONSORT statement for RCTs.[58] Any discrepancy in the evaluation of the articles was resolved using Delphi methodology, which was coordinated by the principal investigator. Analyzed data included the research setting, the source of funding, characteristics of participants (age, gender, baseline pathologies, duration, and intensity of colic prior to study entry), type of therapeutics (dose, duration, frequency) and reported outcomes.
2.4. Data synthesis and analysis
From a statistic point of view, the information was analyzed with the strategy of multiple treatment meta-analysis. Considering that the common denominator of the majority of the studies was the use of placebo as comparator, we decided to use this intervention as the central axis for direct comparisons. Dichotomous outcomes were analyzed with the total number of randomly assigned participants as the denominator. For the secondary analysis of efficacy, measured as a binary outcome, the outcomes for missing information were generated, assuming that all participants with missing data did not respond to treatment. When reported, information on participants that abandoned the studies was included in the analysis. For each potentially eligible study, descriptive statistics of the population characteristics and their results were reported, describing the type of comparison as well as the most important clinical and methodological variables. For each pairwise comparison (direct or indirect), the Hedges standard mean difference (SMD) was calculated for continuous numeric variables, whereas the respective odds ratio (OR) was calculated for dichotomous outcomes. Both were calculated with their respective 95% confidence interval (CI95%). The first meta-analysis was a paired comparison of all published studies. We used a random effect model, considering that different studies estimated different treatment effects. Concomitantly, we calculated I2 for heterogeneity and its corresponding P value. Thereafter we assembled a NMA, using a random effect model with a Bayesian approach[59,60] and summarized the results using effect sizes and CI95%. We used the adjusted model as described by Salanti et al.[61] Additionally, we calculated the probability of superiority for each “anti-colic” intervention through a SUCRA analysis and presented the results in a ranked graph.[62] To estimate the inconsistency (discordance between direct and indirect evidence with a CI95% that did not include zero), we calculated the difference between the direct and indirect estimates, taking as reference only the constructed indicators that had included a placebo.[63] Finally, we adjusted the model with and without assumptions of consistency and compared the 2 models in terms of fit and parsimony.[64] In the case of a significant inconsistency we investigated the distribution of clinical and methodological variables that might have been a potential source of heterogeneity or inconsistency in each group of specific comparisons. All analysis and graphic depictions were performed on the version 13 of STATA for Mac.
3. Results
About 32 RCTs were analyzed[22–52] (Fig. 1), including 2242 patients randomized to 9 nodes of intervention (L reuteri DSM 17938, n = 175; dietetic and nutritional, n = 324; pharmacologic, n = 150; herbal, n = 133; acupuncture, n = 81; manipulative, n = 136; massage, n = 48; reassurance/education, n = 84 and placebo, n = 1,111) (Table 1 and Fig. 2). The RCTs were published between 1977 and 2015. sample sizes ranged from 10 to 111 patients per trial, with a median of 30. Fifty-six percent of total participants were females. The mean age of participants was 35 days (8 days–3 months). The number of visits during the study was 4 to 5 (Basal, day 7, 14, 21, and 28) (Table 2). The risk of bias was rated as low concerning randomized generation of the allocation sequence, allocation concealment and outcome evaluation for L reuteri DSM 17938 RCTs and moderate for the rest of RCTs. Through the contributive plot analysis, we were able to identify that studies with L reuteri DSM 17938 versus Ctrl., Diet versus Ctrl. and Acupuncture versus Ctrl. were the most influential studies in the NMA. The most informative direct evidence in the network was for these 3 comparisons, contributing with around 14% to 15% each one (Fig. 3). Regarding efficacy, considering the weighted mean differences (WMD) effect and the heterogeneity of studies we identify a superiority of L reuteri DSM 17938 [WMD −51.3 h (CI95% −72.2 to −30.5 h), P 0.0001] and dietetic approaches [WMD −37.4 h (CI95% −56.1 to −18.7 h), P 0.0001] (Table 3, Fig. 4). Through Forest plots of the network meta-analysis, we identify a low risk of bias (Fig. 5). Finally, we created hierarchies of effect size on the basis of SUCRA rankings for efficacy outcomes (Fig. 6). The best treatment, according to the curves, was L reuteri DSM 17938 and the least effective treatment was reassurance/education probably due to the high risk of bias identified on the 2 studies that we include in this analysis.
Figure 1.
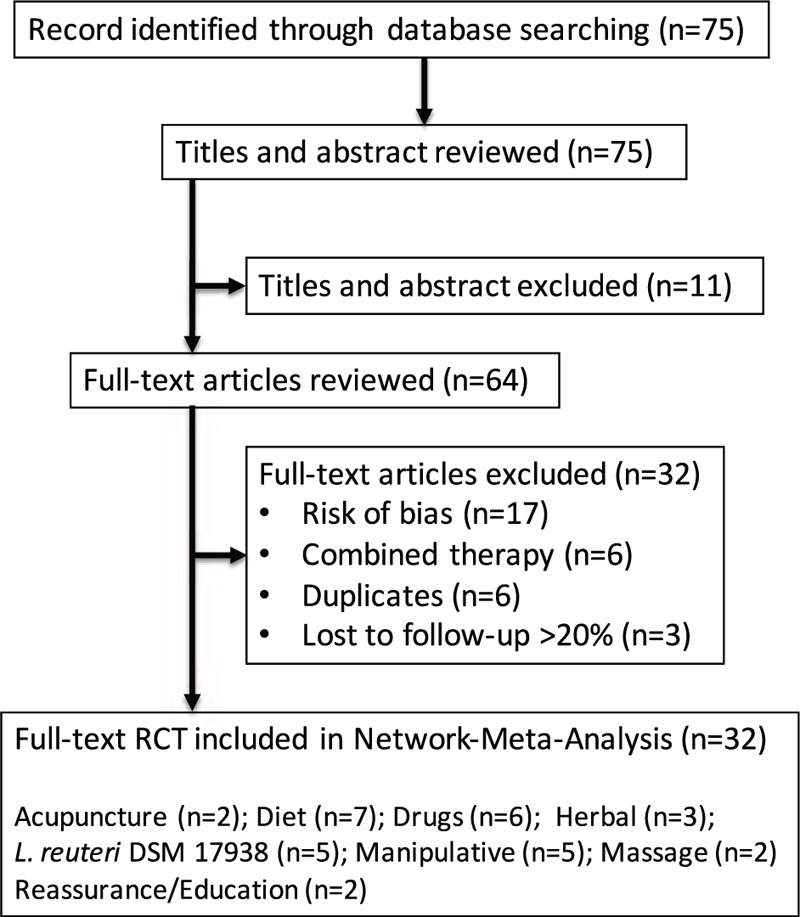
Flow chart of analyzed studies.
Figure 2.
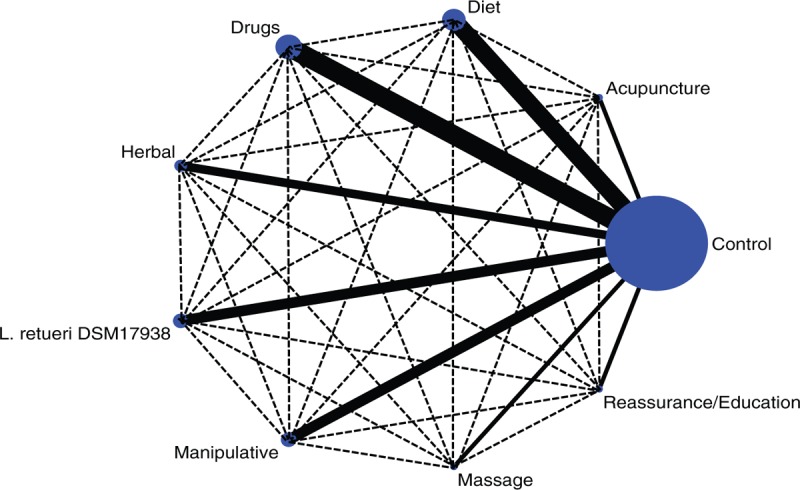
Network meta-analysis of multiple treatments for infantile colic.
Table 2.
Characteristics of includes studies.
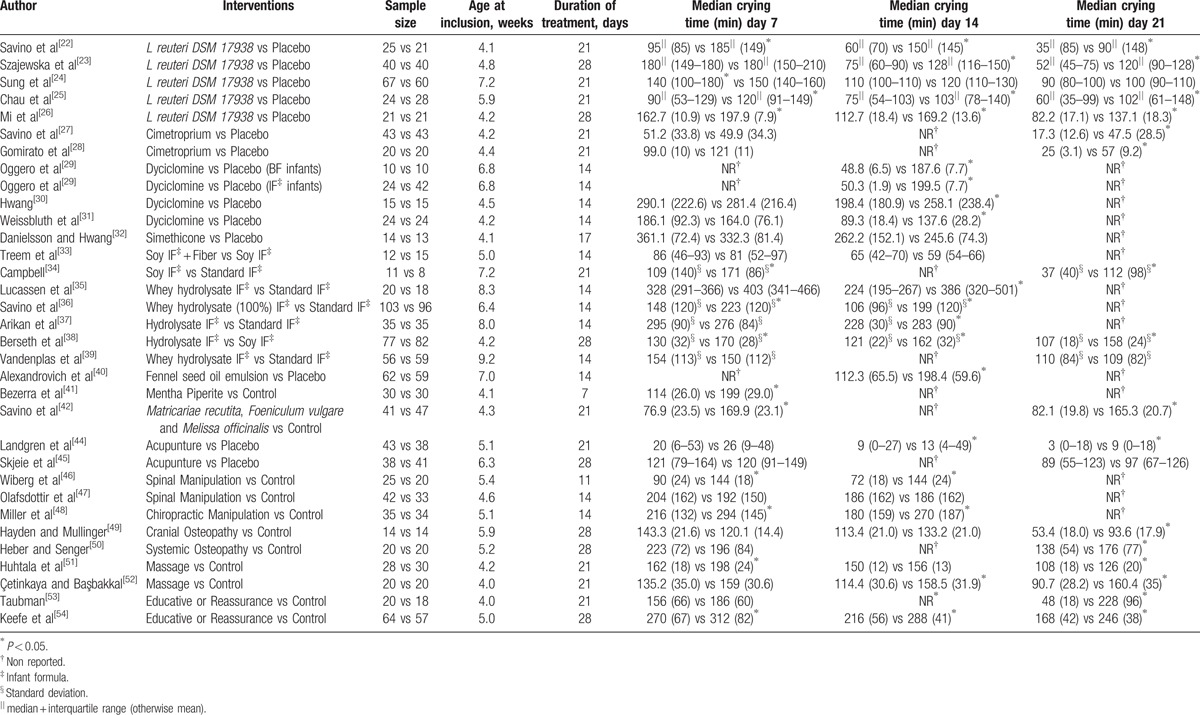
Figure 3.
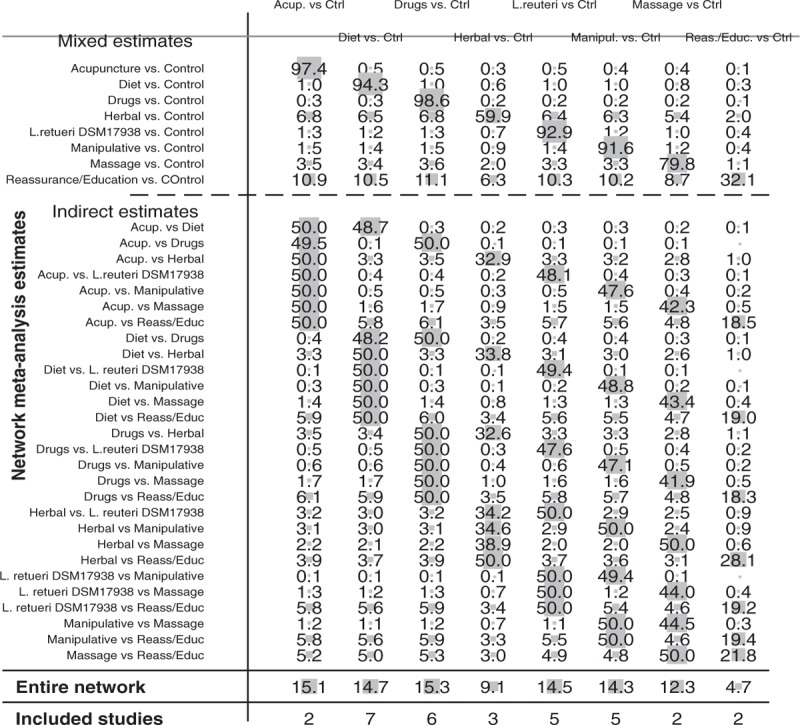
Contribution plot for the network meta-analysis.
Table 3.
Comparative efficacy of treatments for infantile colic.
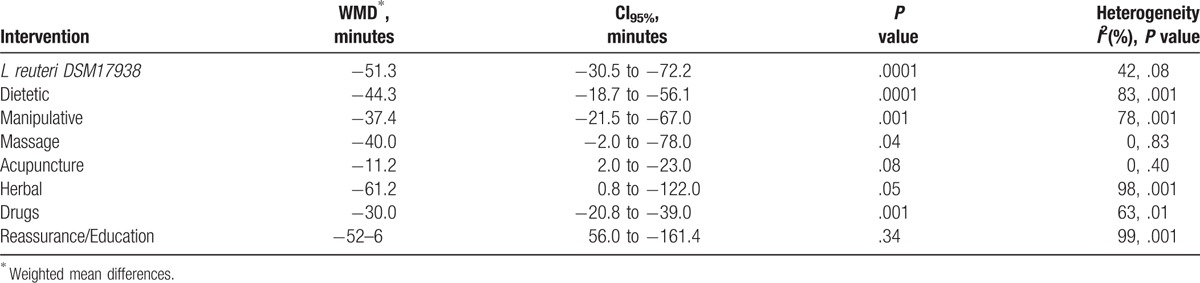
Figure 4.
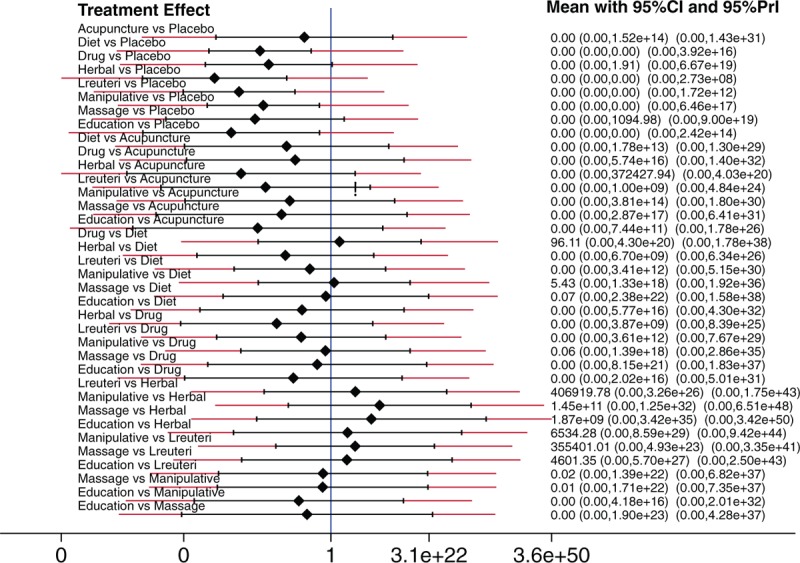
Forrest plot of multiple treatments for infantile colic.
Figure 5.
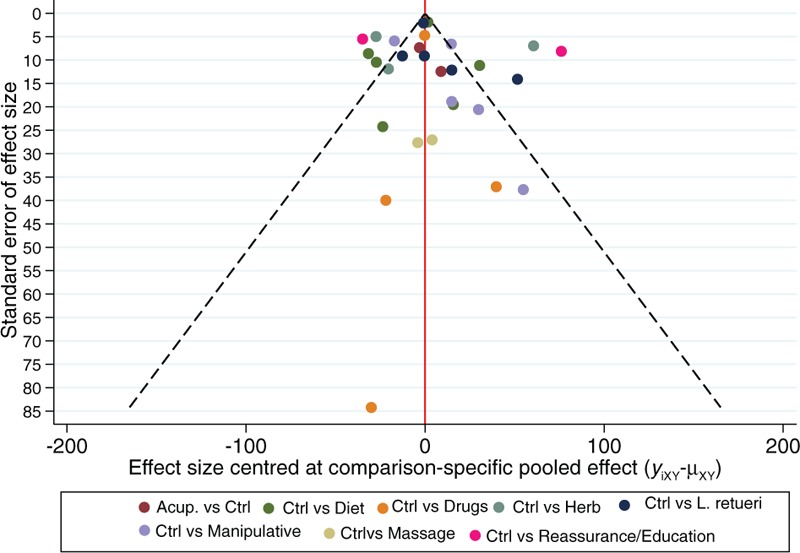
Comparison adjusted funnel plot of multiple treatments for infantile colic.
Figure 6.
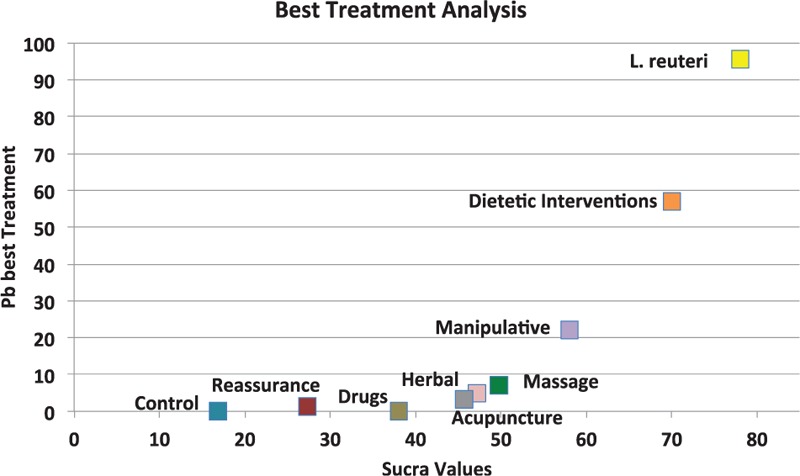
Ranking plot of multiple treatments for infantile colic.
4. Discussion
Infantile colic is a common condition worldwide, affecting 1 in 5 infants younger than 3 months. Although infantile colic is considered to be self-limiting, it is often a stressful problem for parents and a frequent and wrongly undervalued digestive disorder.[65] Recently, Indrio et al[66] demonstrated that preventive intervention in infants not only reduce the probability of colic episodes, but also reduce the number of pediatric visits or visits to the emergency department due to digestive symptoms, the parent's absenteeism and the use of unproved intervention such as simethicone, cimetroprium or herbal products. Although a significant number of papers on infantile colic have been published for more than 45 years ago, there is no adequate consensus about the most efficient way to treat these patients and many times the interventions are selected based on experience more than evidence or analyzing the evidence with some bias. Evidence-based analysis using traditional approaches and single meta-analysis had demonstrated conflicting results when the different therapeutic options for colic had been evaluated.[67] In this paper, we evaluated the evidence with the lowest risk of bias published regarding the treatment for infant with colic. We assembly a systematic review at first, searching the main databases around the world to reduce potential publication bias. After this approach and for the first time on this topic we used the NMA approach with the main purpose to establish direct and indirect comparisons, not only between active versus placebo, which is the most common analysis, but also establishing indirect comparison between active versus active treatments. From our point of view this is important because on the practical arena the clinical practitioners usually face the challenge to decide which treatment could be the best, but comparing one to other. We were able to demonstrate with this approach a superiority of the use of L reuteri DSM 17938 with a dose of 108 CFU/day for 21 to 28 days to significantly reduce the duration of crying episodes during the day. This statement was supported with 4 homogeneous studies[22,23,25,26] who consistently showed a reduction of the duration of colic in infants after the first 7 days of treatment. This superiority was demonstrated not only when we compared L reuteri DSM 17938 versus placebo, but also when we assembled the indirect comparisons with the other types of intervention, the superiority was maintained, as shown by the sucra analysis included in this paper. Additionally, we assembled a funnel plot analysis with the aim to demonstrate the absence of publication bias in this analysis. Multiple treatment analysis, assembled in this paper was important because, instead of different therapeutic options showed significant effects (i.e., dietetic, manipulative and herbal options), with P-values < 0.05, the NMA approach and the heterogeneity analysis demonstrated significant I2 values which reduced the possibility to recommend these types of treatments. Our findings about the superiority of L reuteri DSM 17938 for the treatment of infantile colic are strongly supported by some recently published hypothesis where different authors have identified different microbiota patterns in this type of children.[10–16] Thereby new avenues are opened for continuing to establish new evidence in this field to support the use of a specific strain of probiotic, with a specific dose at a specific frequency as a potentially cost-effective treatment for these infants. Additionally, when you observed the sucra analysis it is important to identify the limited evidence that exists regarding some therapeutic options which are used frequently (simethicone, diclyclomine, herbal interventions) which must be cautions for practitioners before they decide to continue using these non-evidence-based interventions. Regarding limitations of this study we are clear that NMA assumes that treatment arms are similar in rationale and procedure, allowing us to group them together as one node in the network.[61–64] However, we must be clear that decision to use for example reassurance or education could be slightly different when a decision to use probiotics or drugs are established. Additionally, instead of excluding studies with high risk of bias we identify some grades of heterogeneity and inconsistency among trials, which could have led to an overestimation of the effect size. Also, we did not establish a safety and/or cost-effectiveness approach which is also important at the moment of best make decisions.
5. Conclusions
Based on systematic analysis of evidence and networking meta-analysis approach use of L reuteri DSM 17938 seems to be the most evidence-based significant intervention to reduce the duration of crying time in infantile colic. [WMD −51.3 h (CI95% −72.2 to −30.5 h), P .0001]
Use of specialized infant formulas (i.e. partially hydrolyzed, whey-protein derivate) is the second most evidence-based intervention to reduce the clinical symptoms in this type of infants [WMD −37.4 h (CI95% −56.1 to −18.7 h), P 0.0001]
The associated evidence for the use of other interventions such as dicyclomine, cimetroprium, simethicone, herbals, acupuncture, or spinal massage is reduced or significantly biased to let us recommended as potential interventions.
Footnotes
Abbreviations: NMA = Network-meta-analysis, OR = Odds Ratio, RR = Risk Ratio, WMD = Weigthed mean differences.
Contributors’ Statement
Dr PG-C is the main and corresponding author of the manuscript. He has assembled the research protocol, contributes analyzing the evidence, write the paper, review the statistical analysis, and approved the final manuscript as submitted
Dr FI, Dr ABG, Dr IJ-E, Dr PV-V, Dr SV-C, Dr SW-M contribute analyzing the evidence, assembling the evidence tables, and approved the final manuscript as submitted
Dr CJ-G assembled the database and made the statistical analyzing
Dr GL-V participates in assembling the research protocol and submitting to IRB, contributes analyzing the evidence, assembling the manuscript, and approved the final manuscript as submitted.
The authors have no financial and conflicts of interest to disclose.
References
- [1].Brazelton TB. Crying in infancy. Pediatrics 1962;29:579–88. [PubMed] [Google Scholar]
- [2].Illingworth RS. Crying in infants and children. Br Med J 1955;1:75–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Anabrees J, Indrio F, Paes B, et al. Probiotics for infantile colic: a systematic review. BMC Pediatr 2013;13:186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Lucassen PL, Assendelft WJ, van Eijk JT, et al. Systematic review of the occurrence of infantile colic in the community. Arch Dis Child 2001;84:398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Steutel NF, Benninga MA, Langendam MW, et al. Reporting outcome measures in trials of infant colic. J Pediatr Gastroenterol Nutr 2014;59:341–6. [DOI] [PubMed] [Google Scholar]
- [6].Wessel MA, Cobb JC, Jackson EB, et al. Paroxysmal fussing in infancy, sometimes called colic. Pediatrics 1954;14:421–35. [PubMed] [Google Scholar]
- [7].Barr RG, Kramer MS, Boisjoly C, et al. Parental diary of infant cry and fuss behaviour. Arch Dis Child 1988;63:380–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hyman PE, Milla PJ, Benninga MA, et al. Childhood functional gastrointestinal disorders: neonate/toddler. Gastroenterology 2006;130:1519–26. [DOI] [PubMed] [Google Scholar]
- [9].Barr RG. The normal crying curve: what do we really know? Dev Med Child Neurol 1990;32:356–62. [DOI] [PubMed] [Google Scholar]
- [10].Barr RG. Colic and crying syndromes in infants. Pediatrics 1998;102:1282–6. [PubMed] [Google Scholar]
- [11].Poole SR. The infant with acute, unexplained, excessive crying. Pediatrics 1991;88:450–5. [PubMed] [Google Scholar]
- [12].de Weerth C, Fuentes S, Puylaert P, et al. Intestinal microbiota of infants with colic: development and specific signatures. Pediatrics 2013;131:e550–8. [DOI] [PubMed] [Google Scholar]
- [13].Lehtonen L, Korvenranta H, Eerola E. Intestinal microflora in colicky and noncolicky infants: bacterial cultures and gas-liquid chromatography. J Pediatr Gastroenterol Nutr 1994;19:310–4. [DOI] [PubMed] [Google Scholar]
- [14].Rhoads JM, Fatheree NY, Norori J, et al. Altered fecal microflora and increased fecal calprotectin in infants with colic. J Pediatr 2009;155:823–8. e1. [DOI] [PubMed] [Google Scholar]
- [15].Savino F, Cresi F, Pautasso S, et al. Intestinal microflora in breastfed colicky and non-colicky infants. Acta Paediatr 2004;93:825–9. [PubMed] [Google Scholar]
- [16].Savino F, Bailo E, Oggero R, et al. Bacterial counts of intestinal Lactobacillus species in infants with colic. Pediatr Allergy Immunol 2005;16:72–5. [DOI] [PubMed] [Google Scholar]
- [17].Savino F, Cordisco L, Tarasco V, et al. Molecular identification of coliform bacteria from colicky breastfed infants. Acta Paediatr 2009;98:1582–8. [DOI] [PubMed] [Google Scholar]
- [18].de Weerth C, Fuentes S, de Vos WM. Crying in infants: on the possible role of intestinal microbiota in the development of colic. Gut Microbes 2013;4:416–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Vandenplas Y, Gutierrez-Castrellon P, Velasco-Benitez C, et al. Practical algorithms for managing common gastrointestinal symptoms in infants. Nutrition 2013;29:184–94. [DOI] [PubMed] [Google Scholar]
- [20].Shergill-Bonner R. Infantile colic: practicalities of management, including dietary aspects. J Fam Health Care 2010;20:206–9. [PubMed] [Google Scholar]
- [21].Savino F, Emanuela Pelle, Elisabetta Palumeri, et al. Lactobacillus reuteri (American Type Culture Collection Strain 55730) versus simethicone in the treatment of infantile colic: a prospective randomized study. Pediatrics 2007;119:124–30. [DOI] [PubMed] [Google Scholar]
- [22].Savino F, Cordisco L, Tarasco V, et al. Lactobacillus reuteri DSM 17938 in infantile colic: a randomized, double-blind, placebo-controlled trial. Pediatrics 2010;126:e526–33. [DOI] [PubMed] [Google Scholar]
- [23].Szajewska H, Gyrczuk E, Horvath A. Lactobacillus reuteri DSM 17938 for the management of infantile colic in breastfed infants: a randomized, double-blind, placebo-controlled trial. J Pediatr 2013;162:257–62. [DOI] [PubMed] [Google Scholar]
- [24].Sung V, Hiscock H, Tang ML, et al. Treating infant colic with the probiotic Lactobacillus reuteri: double blind, placebo controlled randomized trial. BMJ 2014;348:g2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chau K, Lau E, Greenberg S, et al. Probiotics for infantile colic: a randomized, double-blind, placebo-controlled trial investigating Lactobacillus reuteri DSM 17938. J Pediatr 2015;166:74–8. [DOI] [PubMed] [Google Scholar]
- [26].Mi G-L, Zhao L, Qiao DD, et al. Effectiveness of Lactobacillus reuteri in infantile colic and colicky induced maternal depression: a prospective single blind randomized trial. Antonie Van Leeuwenhoek 2015;107:1547–53. [DOI] [PubMed] [Google Scholar]
- [27].Savino F, Brondello C, Cresi F, et al. Cimetropium bromide in the treatment of crisis in infantile colic. J Pediatr Gastroenterol Nutr 2002;34:417–9. [DOI] [PubMed] [Google Scholar]
- [28].Gomirato G, Bonomi A, Bensi G, et al. Effect of cimetropium bromide in the treatment of colic in the first 3 months of life. Randomized study. Minerva Pediatr 1989;41:259–62. [PubMed] [Google Scholar]
- [29].Oggero R, Garbo G, Savino F, et al. Dietary modifications versus dicyclomine hydrochloride in the treatment of severe infantile colics. Acta Paediatr 1994;83:222–5. [DOI] [PubMed] [Google Scholar]
- [30].Hwang CP. Dicyclomine hydrochloride in infantile colic. Br Med J 1985;291:1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Weissbluth M, Christoffel KK, Davis AT. Treatment of infantile colic with dicyclomine hydrochloride. J Pediatr 1984;104:951–5. [DOI] [PubMed] [Google Scholar]
- [32].Danielsson B, Hwang CP. Treatment of infantile colic with surface active substance (simethicone). Acta Paediatr Scand 1985;74:446–50. [DOI] [PubMed] [Google Scholar]
- [33].Treem WR, Hyams JS, Blankschen E, et al. Evaluation of the effect of a fiber-enriched formula on infant colic. J Pediatr 1991;119:695–701. [DOI] [PubMed] [Google Scholar]
- [34].Campbell JRM. Dietary treatment of infant colic: a double-blind study. J R Coll Gen Pract 1989;39:11–4. [PMC free article] [PubMed] [Google Scholar]
- [35].Lucassen PL, Assendelft WJ, Gubbels JW, et al. Infantile colic: crying time reduction with a whey hydrolysate: a double-blind, randomized, placebo-controlled trial. Pediatrics 2000;106:1349–54. [DOI] [PubMed] [Google Scholar]
- [36].Savino F, Palumeri E, Castagno E, et al. Reduction of crying episodes owing to infantile colic: a randomized controlled study on the efficacy of a new infant formula. Eur J Clin Nutr 2006;60:1304–10. [DOI] [PubMed] [Google Scholar]
- [37].Arikan D, Alp H, Gözüm S, et al. Effectiveness of massage, sucrose solution, herbal tea or hydrolysed formula in the treatment of infantile colic. J Clin Nurs 2008;17:1754–61. [DOI] [PubMed] [Google Scholar]
- [38].Berseth CL, Johnston WH, Stolz SI, et al. Clinical response to 2 commonly used switch formulas occurs within 1 day. Clin Pediatr 2009;48:58–65. [DOI] [PubMed] [Google Scholar]
- [39].Vandenplas Y, Leluyer B, Cazaubiel M, et al. Double-blind comparative trial with 2 antiregurgitation formulae. J Pediatr Gastroenterol Nutr 2013;57:389–93. [DOI] [PubMed] [Google Scholar]
- [40].Alexandrovich I, Rakovitskaya O, Kolmo E, et al. The effect of fennel (Foeniculum vulgare) seed oil emulsion in infantile colic: a randomized, placebo-controlled study. Altern Ther Health Med 2003;9:58–61. [PubMed] [Google Scholar]
- [41].Alves JG, de Brito Rde C, Cavalcanti TS. Effectiveness of Mentha piperita in the treatment of infantile colic: a crossover study. Evid Based Complement Alternat Med 2012;2012:981352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Savino F, Cresi F, Castagno E, et al. A randomized double blind placebo-controlled trial of a standardized extract of Matricariae recutita, Foeniculum vulgare and Melissa officinalis (ColiMil®) in the treatment of breastfed colicky infants. Phytother Res 2005;19:335–40. [DOI] [PubMed] [Google Scholar]
- [43].Weizman Z, Alkrinawi S, Goldfarb D, et al. Efficacy of herbal tea preparation in infantile colic. J Pediatr 1993;122:650–2. [DOI] [PubMed] [Google Scholar]
- [44].Landgren K, Kvorning N, Hallström I. Acupuncture reduces crying in infants with infantile colic: a randomized, controlled, blind clinical study. Acupunct Med 2010;28:174–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Skjeie H, Skonnord T, Fetveit A, et al. Acupuncture for infantile colic: a blinding-validated, randomized controlled multicentre trial in general practice. Scand J Prim Health Care 2013;31:190–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Wiberg JMM, Nordsteen J, Nilsson N. The short-term effect of spinal manipulation in the treatment of infantile colic: a randomized controlled clinical trial with a blinded observer. J Manipulative Physiol Ther 1999;22:517–25. [DOI] [PubMed] [Google Scholar]
- [47].Olafsdottir E, Forshei S, Fluge G, et al. Randomised controlled trial of infantile colic treated with chiropractic spinal manipulation. Arch Dis Child 2001;84:138–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Miller JE, Newell D, Bolton JE. Efficacy of chiropractic manual therapy on infant colic: a pragmatic single-blind, randomized controlled trial. J Manipulative Physiol Ther 2012;35:600–7. [DOI] [PubMed] [Google Scholar]
- [49].Hayden C, Mullinger B. A preliminary assessment of the impact of cranial osteopathy for the relief of infantile colic. Complement Ther Clin Pract 2006;12:83–90. [DOI] [PubMed] [Google Scholar]
- [50].Heber A, Senger U. Osteopathic Treatment of Infantile Colic (Master's Thesis), Akademie FüR Osteopathie (Afo), Germany, 2003 [Google Scholar]
- [51].Huhtala V, Lehtonen L, Heinonen R, et al. Infant massage compared with crib vibrator in the treatment of colicky infants. Pediatrics 2000;105:1–6. [DOI] [PubMed] [Google Scholar]
- [52].Çetinkaya B, Başbakkal Z. The effectiveness of aromatherapy massage using lavender oil as a treatment for infantile colic. Int J Nurs Pract 2012;18:164–9. [DOI] [PubMed] [Google Scholar]
- [53].Taubman B. Clinical trial of the treatment of colic by modification of parent-infant interaction. Pediatrics 1984;74:998–1003. [PubMed] [Google Scholar]
- [54].Keefe MR, Lobo ML, Froese-Fretz A, et al. Effectiveness of an intervention for colic. Clinic Pediatr 2006;6:123–33. [DOI] [PubMed] [Google Scholar]
- [55].Hall B, Chesters J, Robinson A. Infantile colic: a systematic review of medical and conventional therapies. J Paediatr Child Health 2011;22:1–0. [DOI] [PubMed] [Google Scholar]
- [56].St James-Roberts I. Infant crying and sleeping: helping parents to prevent and manage problems. Prim Care 2008;35:547–67. [DOI] [PubMed] [Google Scholar]
- [57].Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med 2015;162:777–84. [DOI] [PubMed] [Google Scholar]
- [58].Schulz KF, Altman DG, Moher D. CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. PLoS Med 2010;7:e1000251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Lu G, Ades AE. Combination of direct and indirect evidence in mixed treatment comparisons. Stat Med 2004;23:3105–24. [DOI] [PubMed] [Google Scholar]
- [60].Ades AE, Sculpher M, Sutton A, et al. Bayesian methods for evidence synthesis in cost-effectiveness analysis. Pharmacoeconomics 2006;24:1–9. [DOI] [PubMed] [Google Scholar]
- [61].Salanti G, Higgins JP, Ades AE, et al. Evaluation of networks of randomized trials. Stat Methods Med Res 2008;17:279–301. [DOI] [PubMed] [Google Scholar]
- [62].Salanti G, Ades AE, Ioannidis JP. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: an overview and tutorial. J Clin Epidemiol 2011;64:163–71. [DOI] [PubMed] [Google Scholar]
- [63].Salanti G, Marinho V, Higgins JP. A case study of multiple-treatments meta-analysis demonstrates that covariates should be considered. J Clin Epidemiol 2009;62:857–64. [DOI] [PubMed] [Google Scholar]
- [64].Dias S, Welton NJ, Caldwell DM, et al. Checking consistency in mixed treatment comparison meta-analysis. Stat Med 2010;29:932–44. [DOI] [PubMed] [Google Scholar]
- [65].Radesky JS, Zuckerman B, Silverstein M, et al. Inconsolable infant crying and maternal postpartum depressive symptoms. Pediatrics 2013;131:e1857–64. [DOI] [PubMed] [Google Scholar]
- [66].Indrio F, Di Mauro A, Riezzo G, et al. Prophylactic use of a probiotic in the prevention of colic, regurgitation, and functional constipation a randomized clinical trial. JAMA Pediatr 2014;168:228–33. [DOI] [PubMed] [Google Scholar]
- [67].Dobson D, Lucassen PLBJ, Miller JJ, et al. Manipulative therapies for infantile colic. Cochrane Database Syst Rev 2012;12:CD004796. [DOI] [PMC free article] [PubMed] [Google Scholar]


