Supplemental Digital Content is available in the text
Keywords: breast cancer, Ki-67, meta-analysis, neoadjuvant chemotherapy, pathological complete response
Abstract
Background:
A pathological complete response (pCR) after neoadjuvant chemotherapy (NCT) is a strong indicator of the benefit of therapy and presents an early surrogate for a favorable long-term outcome. It remains unclear whether Ki-67, a marker for tumor proliferation, can function as a predictor of the response to NCT in breast cancer. The objective of this meta-analysis was to compare the pCR rate and clinical outcomes in breast cancer patients with different Ki-67 labeling indexes (Ki-67 LI) who received NCT.
Methods:
Clinical studies were retrieved from the electronic databases of PubMed, Embase, Clinical Trials, Wanfang, and the Chinese National Knowledge Infrastructure, from their inception to July 31, 2017. Meta-analysis was performed on pool eligible studies to determine whether Ki-67 LI was associated with the pCR rate and clinical outcomes of breast cancer patients who were treated with NCT. Pooled analyses were performed using fixed effects models. Two reviewers screened all titles and abstracts and independently assessed all articles.
Results:
A total of 36 studies involving 6793 patients were included in the meta-analysis. Pooled analysis results revealed that patients with high Ki-67 LI exhibited significantly higher pCR rates (odds ratio [OR] = 3.94, 95% confidence interval [CI]: 3.33–4.67, P <.001) but poorer relapse-free survival (OR = 1.99, 95% CI: 1.39–2.85, P <.001) than those with low Ki-67 LI, but there was no significant difference in objective tumor response rate.
Conclusion:
The meta-analysis reported here demonstrates that pretherapeutic Ki-67 LI is associated with pCR in breast cancer patients undergoing NCT. More phase III randomized clinical trials will be required to confirm our findings.
1. Introduction
The most common cancer in women in 2016 was breast cancer, which is expected in the near future to account for approximately 29% of all newly diagnosed cancers in females.[1] Neoadjuvant chemotherapy (NCT) has been established as a standard treatment for patients with not only locally advanced breast cancer but also operable breast cancer. The objectives of NCT for operable breast cancers are to downstage tumors, making inoperable tumors operable, to render tumors amenable to breast conserving surgery, and to improve the survival time.[2,3] Biomarkers have been used in the past to monitor cancer treatment and increasing evidence indicates that tumor biomarker levels can help clinicians to assess the effectiveness of NCT.[4–8]
Ki-67 is a nuclear protein expressed during all phases of the cell cycle, except G0, and its expression has been reported to be correlated with the tumor cell proliferation rate. Many studies have investigated immunohistochemical expression of Ki-67 as a prognostic and predictive marker for breast cancer.[9–11] But previous studies did not report completely consistent results regarding the impact of NCT on the status of tumor biomarkers.[12–17]
One of the main objectives of NCT is to achieve a pathological complete response (pCR) because pCR has been found to be associated with longer disease-free and overall survival rates.[18,19] Several studies have associated high levels of Ki-67 with higher pCR rates.[20,21] However, other studies failed to confirm these findings.[22,23] A recently published meta-analysis involving 44 articles that investigated the relationship between Ki-67 expression levels and the pCR rate indicated that a high Ki-67 level was associated with a high pCR rate (OR = 3.10, 95% CI: 2.52–3.81, P <.001).[24] However, many of these articles did not explore the relationship between Ki-67 levels and the clinical response, nor did they discuss the prognostic value of Ki-67 in breast cancer. Therefore, the primary purpose of our study was to evaluate the function of pretherapeutic Ki-67 labeling index (LI) as a predictive marker for pCR to NCT using meta-analytical methodology. We also investigated the predictive value of Ki-67 for the clinical response and the prognostic value of Ki-67 in breast cancer patients receiving NCT.
2. Materials and methods
2.1. Literature search strategy
To identify studies involving the association between Ki-67 expression and the pCR in breast cancer, a literature search was conducted among 3 English databases (PubMed, Embase, and Clinical Trials), and 2 Chinese databases (Wanfang and Chinese National Knowledge Infrastructure databases) from their inception to July 31, 2017. We checked these electronic databases using the search terms “Ki-67” and “breast cancer” and “NCT”. Additionally, we performed a computerized search of abstracts presented at the Annual Meetings of the American Society of Clinical Oncology (ASCO). Finally, we screened the references in all relevant articles to identify additional articles that were not retrieved during the initial literature search. The search strategy used for PubMed is shown in Table 1.
Table 1.
PubMed search strategies.

2.2. Selection criteria
Our meta-analysis included all studies meeting the following criteria: patients were pathologically diagnosed with breast cancer; all patients received NCT; results were stratified according to the level of pretherapeutic Ki-67 expression; pCR was the end point in trials and could be calculated directly; the results were part of an original analysis; papers were published in Chinese or English. We only selected the articles published in peer-reviewed journals and excluded reviews, letters, and meeting abstracts. Patients who received preoperative chemotherapy concomitant endocrine therapy or local treatment were excluded.
2.3. Data extraction
Information from each study was abstracted independently by 2 investigators using a standardized data extraction form, predesigned on the basis of the Cochrane Consumers and Communication Review Group data extraction template. Any disagreement over extracted data was resolved through discussion until the 2 investigators reached a consensus opinion. The following information was recorded for each publication: first author's name, publication year, study type, country of origin, the cut-off value of Ki-67 LI, numbers of patients in study sample, clinical stage, NCT regimens and cycles, molecular subtypes, numbers of patients with “high” Ki-67 LI, and numbers of patients with “low” Ki-67 LI. When key pieces of information were not present in articles, the corresponding author was contacted. In the event that we still could not obtain the whole dataset, the missing information was classified as “not reported”. The primary endpoint was the pCR rate of NCT. pCR was defined as complete disappearance of invasive carcinoma in both breast and axillary lymph nodes. Residual ductal carcinoma in situ was included in the pCR category. The objective tumor response was assessed according to modified Response Evaluation Criteria in Solid Tumors.[57] In other words, “complete response” or “partial response” was classified as “response”, while “stable” or “progressive disease” as “nonresponse”. Relapse-free survival (RFS) was defined as the elapsed time between the date of first diagnosis and the date of the first relapse. Overall survival (OS) was calculated from the date of diagnosis to the date of death or the last follow-up.
2.4. Quality assessment
The initial relevance evaluation was implemented by 2 reviewers through independently screening of titles and abstracts. If either reviewer considered any titles or abstracts met the eligibility criteria, the full text was obtained. The quality and bias risk of the selected papers were critically appraised separately by 2 reviewers. Quality assessment was conducted for each of the eligible studies by using the validated Newcastle–Ottawa Quality Assessment Scale (NOS).[58] This scale is composed of 8 items that assess patient selection, study comparability, and outcome with scores ranging from 0 to 9. In our meta-analysis, studies with a score no <6 were graded as high quality.[59] Eventual consensus governance resolved disagreements.
2.5. Statistical methods
Dichotomous results were summarized as pooled odds ratios (ORs) and 95% confidence intervals (95% CIs) around the point estimates. OR was abstracted or calculated to quantitatively evaluate the association between pretherapeutic Ki-67 LI and the response rate. The overall pooled effect was assessed using the z-statistic with a P-value ≤.05 representing statistical significance.
Heterogeneity between the studies was assessed by χ2 statistics and expressed as an “I2” value. When I2 ≥50% or the P-value for the I2 statistic was <.05, which indicated significant heterogeneity across the studies, the pooled estimate was calculated using a random effects model and if the data were contrary, a fixed effect model was adopted. In subgroup analysis on the basis of patients’ populations, studies were divided into an “Asian population” and a “European population”. In the subgroup analysis by cut-off values of Ki-67, studies were classified according to the levels of “≤ 14%,” “15% to 29%,” and “≥ 30%”. And in the subgroup analysis by molecular subtypes, studies were divided into “TNBC,” “HER2+,” “HR+,” “HR–,” and “unclassified” (contains all molecular subtypes). All statistical analyses were carried out using RevMan V.5.3 software.
All analyses were based on previous published studies, thus no ethical approval or patient consent was required.
3. Results
3.1. Search results
The search strategy yielded 849 potentially relevant references in the electronic databases. We initially excluded 321 duplicated publications. Upon review of the remaining abstracts, we further removed 433 more articles for reasons of ineligibility. According to the inclusion criteria established for the present study, an additional 59 articles were excluded. We thus finally selected 36 studies,[20–23,25–56] which consisted of a cohort of 6793 patients with breast cancer (shown in the flow diagram).
All of the 36 selected studies assessed the association analysis between pretherapeutic Ki-67 LI and pCR, 4 of them contained the association analysis between Ki-67 LI and clinical response,[28,31,33,35] 7 of them reported the relationships between pretherapeutic Ki-67 LI and RFS,[20,33,35,43,45,50,51] and 3 of them explored the relationships between Ki-67 LI and OS.[20,35,45] Based on the type of study, there were 17 prospective observational studies, and the 19 remaining studies were retrospective. A summary of the available information included in the present meta-analysis is provided in Table 2. Quality assessment with the NOS, shown in Table 3, demonstrated that the combined scores of selection, comparability, and outcome aspects was >6 in each of the selected studies.
Table 2.
Summary of studies included in the meta-analysis.
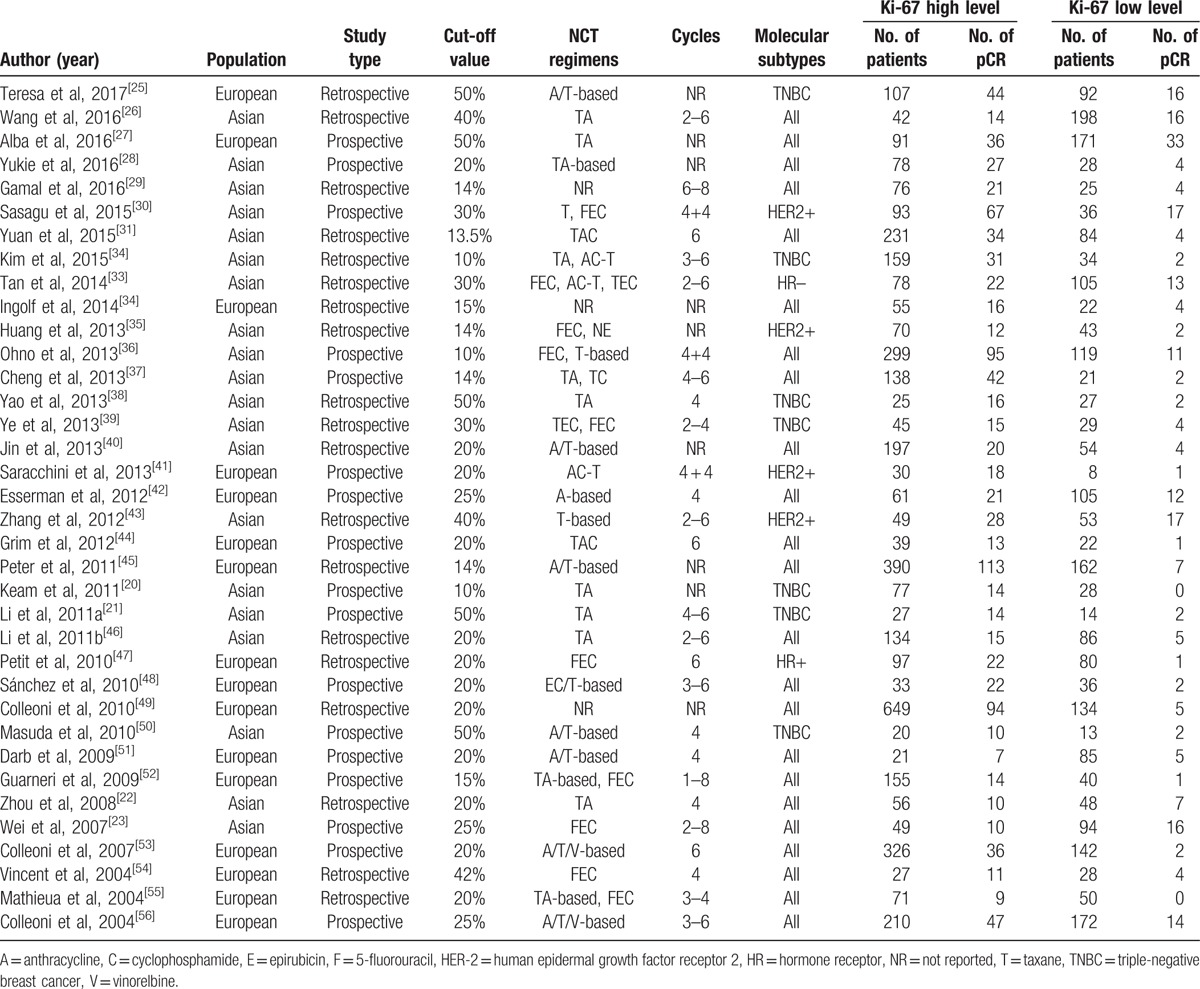
Table 3.
Quality of literature included in the meta-analysis.
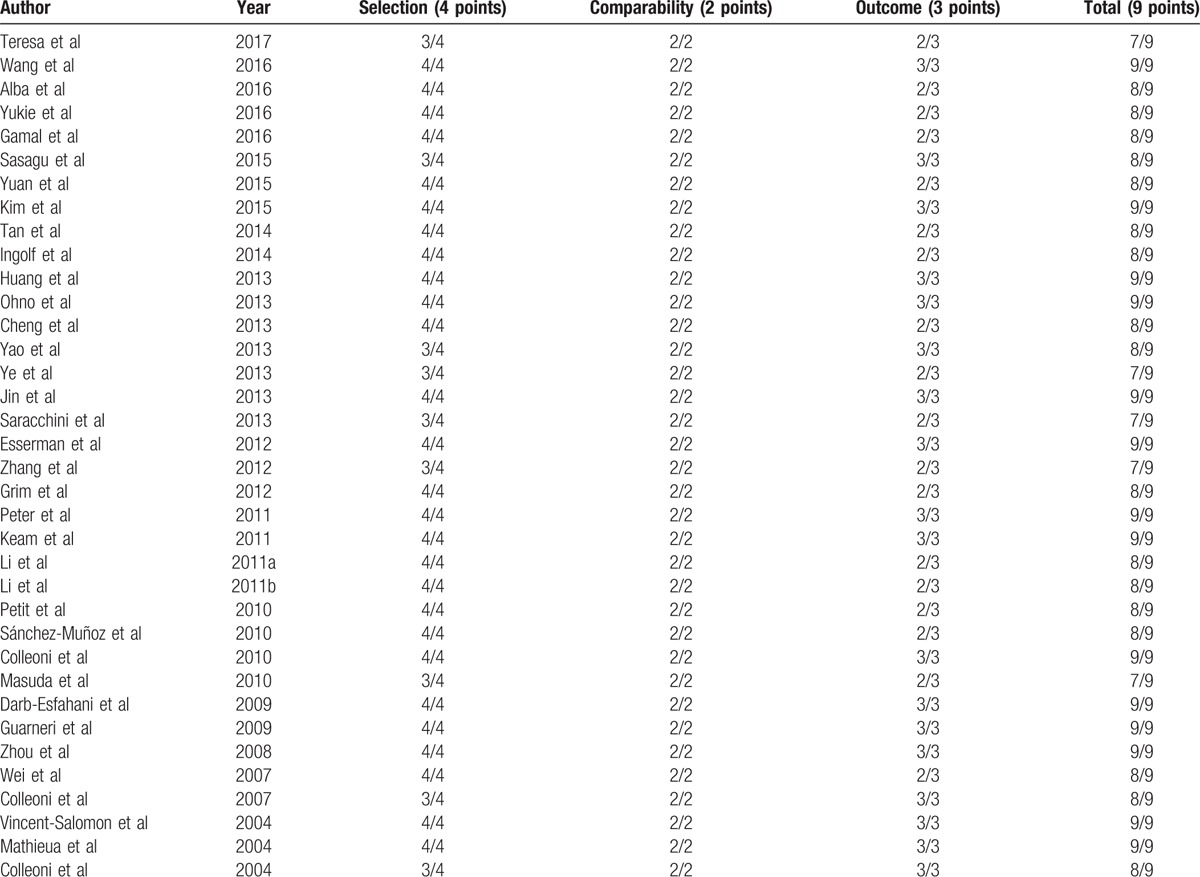
3.2. Clinical and methodological heterogeneity
The included studies utilized either retrospective or prospective observational designs. In addition, they also varied in ways that could affect pCR, including the populations of the study samples, NCT strategies and cycles, proportions of patients with different molecular subtypes, and cut-off values of Ki-67. Therefore, there was considerable clinical and methodological heterogeneity among the included studies.
3.3. Statistical pooling
3.3.1. The pCR rate of patients with high Ki-67 LI was significantly higher than that of patients with low Ki-67 LI
The pooled results from the analysis of the association between pretherapeutic Ki-67 LI and pCR are shown in Figure 1. Since there was low heterogeneity between studies (χ2 = 48.34, P = .07, I2 = 28%), the fixed effects model was applied to perform the meta-analysis. As shown in Figure 1, the pCR rate of patients with high Ki-67 LI (n = 4305) was significantly higher than that of patients with low Ki-67 LI (n = 2488) (OR: 3.94, 95% CI: 3.33–4.67, P <.001), and the OR values of prospective and retrospective studies were 4.02 (95% CI: 3.16–5.12, P <.001) and 3.88 (95% CI: 3.06–4.91, P <.001) respectively. These results indicated that the pretherapeutic Ki-67 level is indeed a determinant of the pCR rate to NCT in breast cancer.
Figure 1.
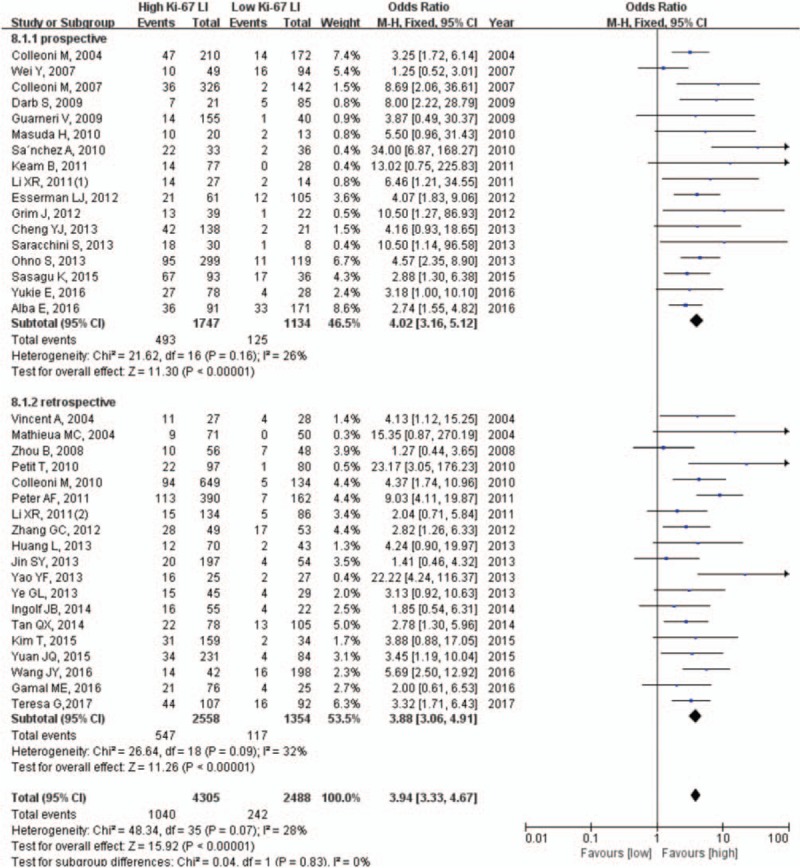
Pooled analysis of Ki-67 LI and pCR. Ki-67 LI = Ki-67 labeling index, pCR = pathological complete response.
Taking into account the heterogeneity between studies, we conducted a sensitivity analysis. The pooled results did not differ substantially between the fixed and random effects models. By recalculating ORs with 1 study removed and all others included from the pooled estimate, we assessed the influence of each study on the overall estimate. Influence analysis showed no substantial difference in pooled ORs when any single study was excluded, which indicated that the conclusion was robust.
Then we utilized the fixed effects model to calculate results in a sub-group analysis on the basis of patients’ population type and found that the pCR rate was significantly higher in patients with high Ki-67 LI than those with low Ki-67 LI, in both European (22.1% vs 8.0%, OR = 4.90, 95% CI: 3.83–6.28, P <.001) and Asian (26.6% vs 11.7%, OR = 3.18, 95% CI: 2.52–4.02, P <.001) subgroups (Fig. 2).
Figure 2.
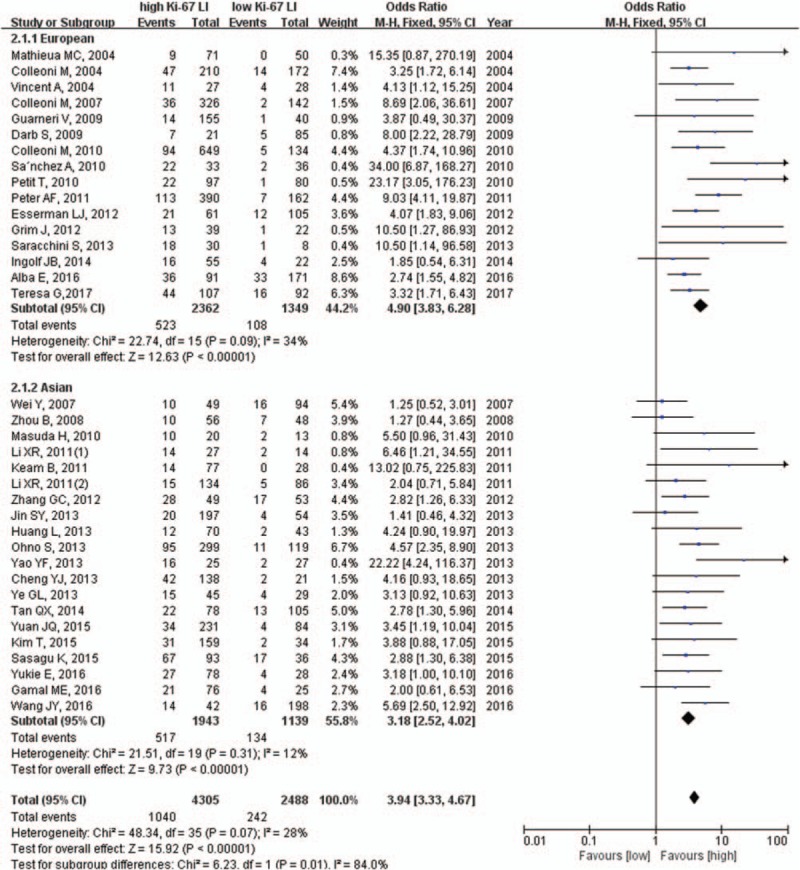
Subgroup analysis of patient population type.
Taking into account the effects of different cut-off values of Ki-67 LI on the results, we performed a subgroup analysis based on specified cut-off values. The results showed that patients with high Ki-67 LI were more likely to achieve pCR no matter what the cut-off value; Ki-67 LI was ≤14% (25.1% vs 6.2%, OR = 5.03, 95% CI: 3.45–7.34, P <.001), 15% to 29% (17.7% vs 7.0%, OR = 3.76, 95% CI: 2.88–4.91, P <.001), or ≥30% (45.9% vs 16.4%, OR = 3.51, 95% CI: 2.69–4.57, P <.001) (Fig. 3).
Figure 3.
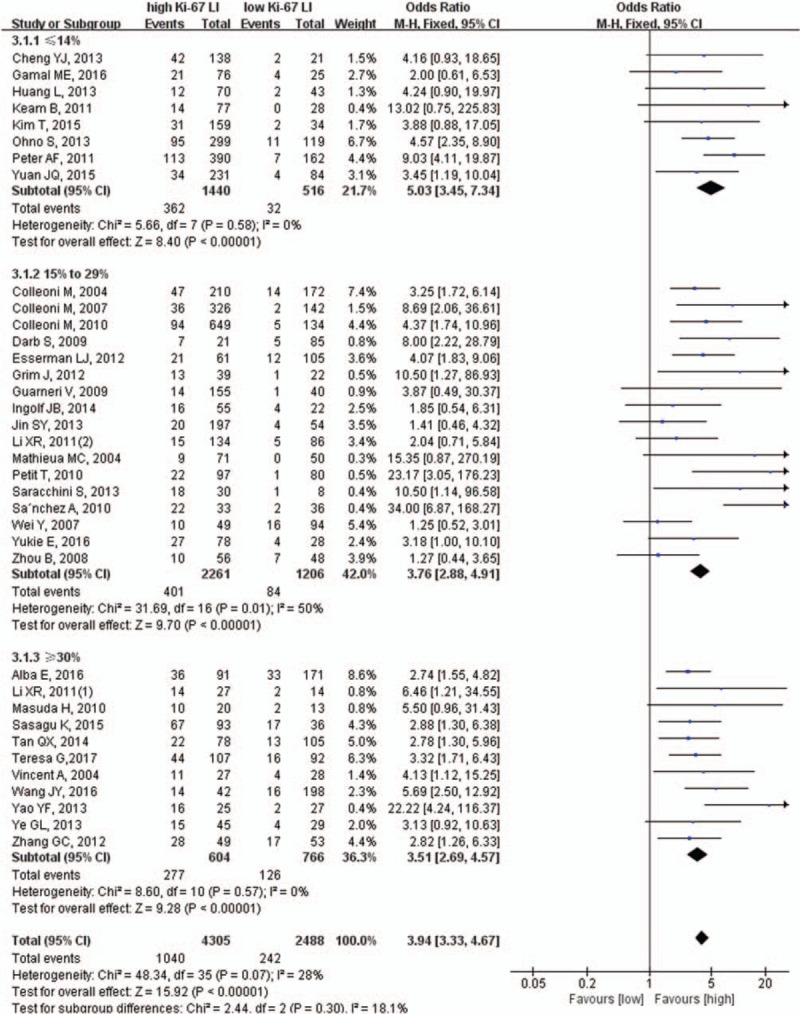
Subgroup analysis of the cut-off value of Ki-67.
Considering the influence of the molecular subtypes, a subgroup analysis was performed. We found that the pCR rate of patients with high Ki-67 LI was significantly higher than those with low Ki-67 LI even when the included patients were triple-negative breast cancer (31.3% vs 11.8%, OR = 4.65, 95% CI: 2.93–7.38, P <.001), HER+ (51.7% vs 26.4%, OR = 3.32, 95% CI: 1.99–5.54, P <.001), or unclassified (21.2% vs 8.5%, OR = 3.85, 95% CI: 3.15–4.72, P <.001) (Fig. 4).
Figure 4.
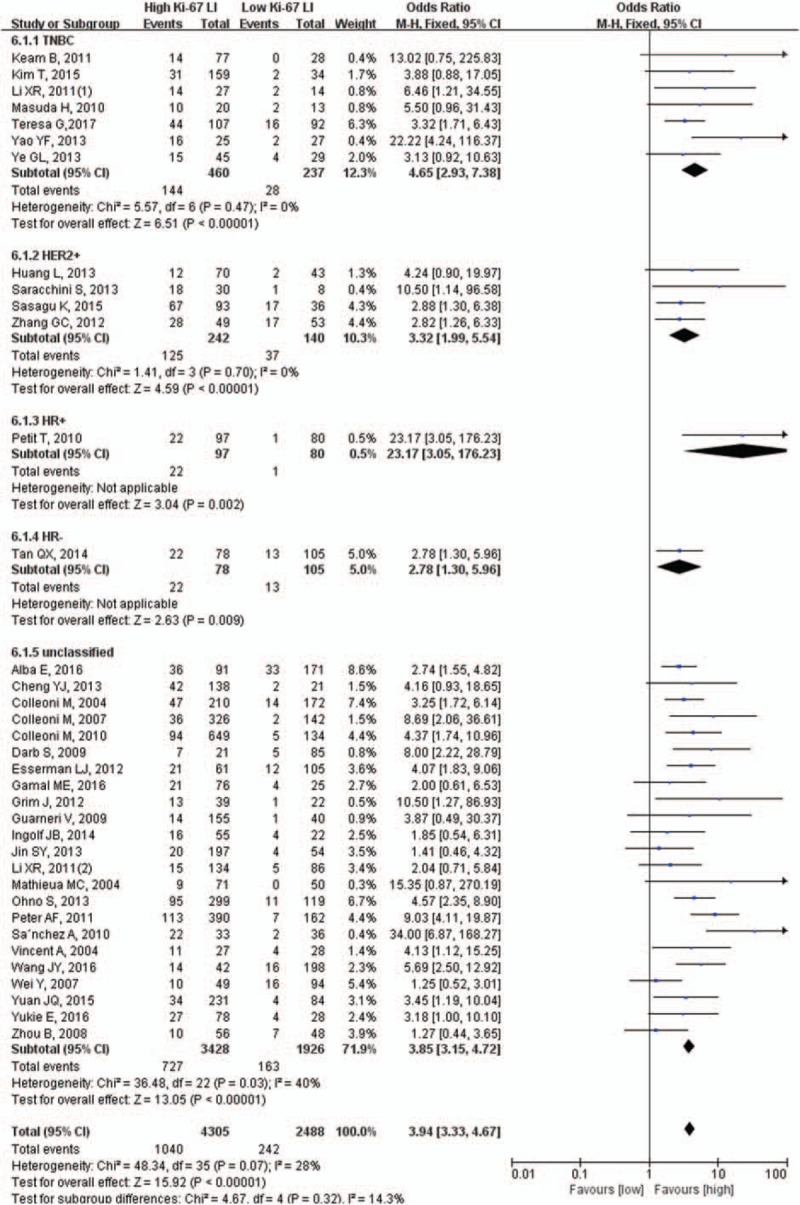
Subgroup analysis of molecular subtypes.
3.3.2. Patients with Ki-67 LI tended to have a better objective tumor response
We next assessed objective tumor response in 4 studies, which included 717 patients. We performed meta-analysis using the random effects model because of the heterogeneity among studies (χ2 = 8.75, P = .03, I2 = 66%). We found that patients with a Ki-67 LI tended to have a better objective tumor response (83.8% vs 75.8%, OR = 1.57, 95% CI: 0.72–3.42, P = .26; Fig. 5). However, the result did not reach statistical significance.
Figure 5.
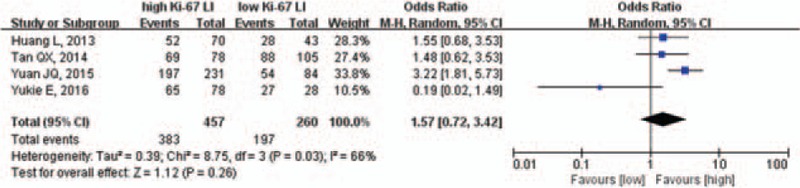
Pooled analysis of Ki-67 LI and objective tumor response. Ki-67 LI = Ki-67 labeling index.
Because of the significant heterogeneity, we performed a sensitivity analysis and found a substantial difference in pooled OR when the study of Yukie et al[28] was excluded. The adjusted results showed that patients with a high Ki-67 LI had a better objective tumor response than those with a low Ki-67 LI (84.0% vs 73.3%, OR = 2.19, 95% CI: 1.45–3.33, P <.001; supplemental Fig. 2).
3.3.3. Patients with a high Ki-67 LI have a poorer RFS
The results of the pooled analysis of the association between pretherapeutic Ki-67 LI and RFS are shown in Figure 6. Patients with a high Ki-67 LI have a poorer RFS than those with a low Ki-67 LI (OR = 1.99, 95% CI: 1.39–2.85, P <.001).
Figure 6.
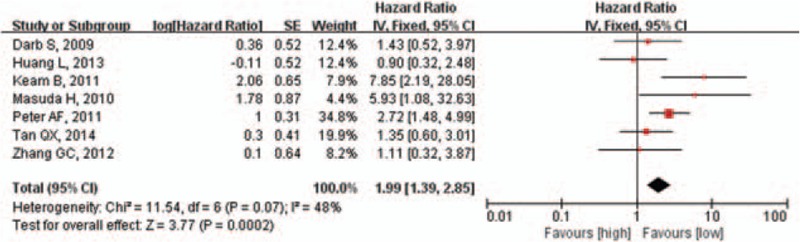
Pooled analysis of Ki-67 LI and RFS. Ki-67 LI = Ki-67 labeling index, RFS = relapse-free survival.
3.3.4. Publication bias
In the meta-analysis, funnel plots were generally symmetrical (Fig. 7). These results indicated that publication bias was insignificant across the included studies.
Figure 7.
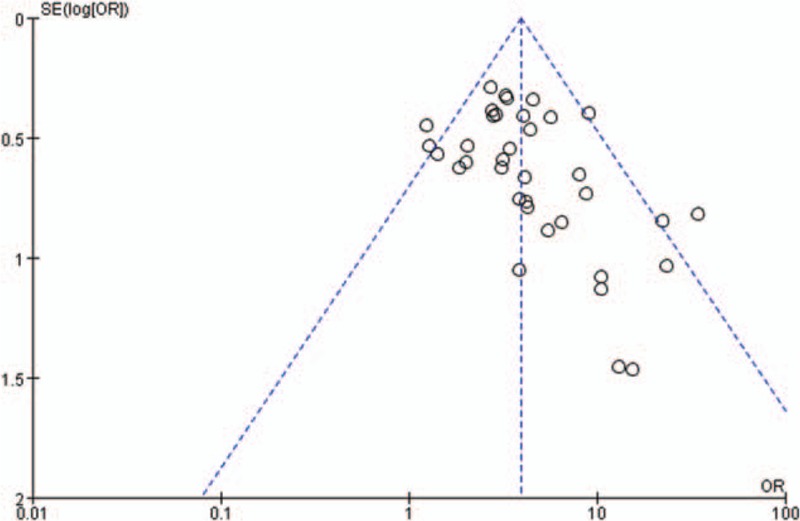
Funnel plot for detection of publication bias.
4. Discussion
A recently published meta-analysis reported that a high Ki-67 level was associated with a high pCR rate.[24] Although the selection criteria and pooling methods were not exactly the same, our study came to a similar conclusion. However, in addition we not only explored the predictive value of Ki-67 for NCT in breast cancer, but also investigated its prognostic value. Our results demonstrate that patients with a Ki-67 LI are more sensitive to NCT, have higher pCR rates, and benefit more from NCT compared to those with a low Ki-67 LI (P <.001). Conversely, patients with a high Ki-67 LI have a worse RFS.
In a subgroup analysis of patients’ population, we found that the pCR rate of patients with a high Ki-67 LI was significantly higher than in patients with a low Ki-67 LI in both European and Asian subgroups. However, it remains unclear whether other factors such as therapy regimens and cycles of NCT, the clinical stage, and tumor location have an impact on Ki-67-based health outcomes. Our study's design did not allow for the evaluation of these relationships, so further research will need to be carried out.
Numerous studies have shown a positive correlation between the expression of Ki-67 and the response to chemotherapy.[60–62] However, threshold values for dividing high and low Ki-67 LI are not clearly defined and vary between laboratories, ranging from 10% to 50%. The St Gallen Consensus Meeting declared that Ki-67 LI is chiefly important for distinguishing between luminal A and luminal B subtypes of breast cancer with a cut-off value of 14%.[63] In a previous study, researchers found that the expression of Ki-67 was the only independent predictor of pCR and also discovered that a Ki-67 value >25% was a significant predictive factor for pCR.[60] The latter results were supported by another study in which a cut-off value of Ki-67 of circa 30% was suitable for predicting pCR.[33] Therefore, we performed a subgroup analysis based on this factor with 14% and 30% as the cut-off points and found that the pCR rate of patients with a high Ki-67 LI was significantly higher than in patients with a low Ki-67 LI regardless of whether the cut-off value was ≤14%, 15% to 29%, or ≤ 30%. Interestingly, when we performed a subgroup analysis according to a cut-off value of Ki-67, the heterogeneity among subgroups varied greatly, the I2 values being 0%, 50%, and 0%, respectively, indicating that the cut-off value of Ki-67 may be one of the sources of heterogeneity.
Patients with different types of breast cancer have different responses to NCT regimens. Previous studies have shown that patients with hormone receptor-positive breast cancer, which were categorized into luminal subtypes, are less likely to achieve pCR.[64,65] In a retrospective study, 240 patients with breast cancer received 4 to 6 weeks of NCT before surgery and it was found that patients with luminal A (1.6%) and luminal B (13.4%) cancer types had the lowest pCR rates followed by the human epidermal growth factor receptor 2 (HER2) overexpression (22.6%) and triple negative (23.8%) forms.[58] This result is consistent with that from another study in which the authors found that the odds of achieving pCR in HER2+ cancers were 3.6 times higher than that in luminal cancers.[66] All of these findings suggest that patients with luminal type tumors gained less benefit from NCT. We next performed a subgroup analysis based on molecular types, and found that the pCR rate of patients with a high Ki-67 LI was significantly higher than those with a Ki-67 LI regardless of the molecular type of cancer. Unfortunately, the vast majority of selected articles (23/36) were not classified into molecular subtypes, so the results do not fully reflect the real clinical situation.
In exploring the relationship between Ki-67 LI and objective remission rates, we found that the Yukie et al's study had a significant impact on outcomes.[28] The study included 183 patients, 120 of whom came from Hyogo College of Medicine, and the others from Yao Municipal Hospital. However, for some reason, further analyses were performed only for patients treated at the Hyogo College of Medicine, which can lead to significant experimental errors. When we excluded this study from the pooled analysis, the results showed that patients with a high Ki-67 LI had a better objective tumor response (P <.001). More studies will be needed to confirm this finding.
Several studies have demonstrated that patients who achieve pCR to NCT tend to have improved RFS and OS compared with those with residual invasive disease.[67,63] However, few studies have explored the relationship between Ki-67 LI and RFS or OS. Our study suggested that high Ki-67 LI was significantly associated with poor RFS (P <.001). We explored the relationship between Ki-67 LI and OS using the random effects model and found that patients with a high Ki-67 LI had a worse OS than patients with a low Ki-67 LI (OR = 3.44, 95% CI: 0.57–15.8, P = .11, data shown in supplemental fig. 1). But these results may not be reliable due to the small number of included studies (3/36). High Ki-67 LI was significantly associated with a high pCR rate but poor RFS. In other words, patients who did not achieve a pCR to NCT maintained a good prognosis even in the presence of residual disease. The good outcome of these patients was largely dependent on the efficacy of surgery and postoperative therapy. In other words, whether the patients achieved pCR or not, all of them underwent surgery and adjuvant therapy, thus weakening the impact of pCR on survival.
There are several limitations to the present meta-analysis. First, our analysis was based mainly on findings from observational studies, which might contain a higher number of confounding factors than randomized controlled clinical trials. Second, our analysis only contained published studies. Since reports with positive results are more likely to be published than those with negative observations, potential publication bias represents a concern. Furthermore, among the selected studies, the patients’ populations and treatment measures differed widely, and the cut-off values for Ki-67 to designate high and low levels varied widely, which may influence the pooled analysis. Therefore, more detailed data such as NCT regimens and cycles are needed for future analyses.
In conclusion, our findings support the hypothesis that Ki-67 LI is associated with the pCR of patients with breast cancer. Ki-67 LI is a crucial predictive biomarker for pCR in patients with breast cancer who received NCT, indicating that this marker could help select patients who will benefit from NCT. However, it is more difficult to translate pathological response results into a clinical benefit. Large-scale prospective and randomized trials will be required before Ki-67 testing can be widely used as a prognostic tool in the clinic.
Supplementary Material
Footnotes
Abbreviations: HER2 = human epidermal growth factor receptor 2, HR = hormone receptor, Ki-67 LI = Ki-67 labeling index, NCT = neoadjuvant chemotherapy, NR = not reported, OR = odds ratio, OS = overall survival, pCR = pathological complete response, RFS = relapse-free survival, TNBC = triple-negative breast cancer.
The authors have no conflict of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2016. CA Cancer J Clin 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Mamounas EP, Fisher B. Preoperative (neoadjuvant) chemotherapy in patients with breast cancer. Semin Oncol 2001;4:389–99. [DOI] [PubMed] [Google Scholar]
- [3].Fisher B, Gunduz N, Saffer EA. Influence of the interval between primary tumor removal and chemotherapy on kinetics and growth of metastases. Cancer Res 1983;43:1488–92. [PubMed] [Google Scholar]
- [4].Ragaz J, Baird R, Rebbeck P, et al. Neoadjuvant (preoperative) chemotherapy for breast cancer. Cancer 1985;56:719–24. [DOI] [PubMed] [Google Scholar]
- [5].Fisher B, Bryant J, Wolmark N, et al. Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 1998;16:2672–85. [DOI] [PubMed] [Google Scholar]
- [6].Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol 2008;26:778–85. [DOI] [PubMed] [Google Scholar]
- [7].van der Hage JA, van de Velde CJ, Julien JP, et al. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. J Clin Oncol 2001;19:4224–37. [DOI] [PubMed] [Google Scholar]
- [8].Mauri D, Pavlidis N, Ioannidis JP. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. J Natl Cancer Inst 2005;97:188–94. [DOI] [PubMed] [Google Scholar]
- [9].de Azambuja E, Cardoso F, de Castro G, et al. Ki67 as prognostic marker in early breast cancer: a meta-analysis of published studies involving 12,155 patients. Br J Cancer 2007;96:1504–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Yerushalmi R, Woods R, Ravdin PM, et al. Ki67 in breast cancer: prognostic and predictive potential. Lancet Oncol 2010;11:174–83. [DOI] [PubMed] [Google Scholar]
- [11].von Minckwitz G, Untch M, Nuesch E, et al. Impact of treatment characteristics on response of different breast cancer phenotypes: pooled analysis of the German neo-adjuvant chemotherapy trials. Breast Cancer Res Treat 2011;125: 145e56. [DOI] [PubMed] [Google Scholar]
- [12].Shet T, Agrawal A, Chinoy R, et al. Changes in the tumor grade and biological markers in locally advanced breast cancer after chemotherapy—implications for a pathologist. Breast J 2007;13:457–64. [DOI] [PubMed] [Google Scholar]
- [13].Neubauer H, Gall C, Vogel U, et al. Changes in tumour biological markers during primary systemic chemotherapy (PST). Anticancer Res 2008;28:1797–804. [PubMed] [Google Scholar]
- [14].Hirata T, Shimizu C, Yonemori K, et al. Change in the hormone receptor status following administration of neoadjuvant chemotherapy and its impact on the long-term outcome in patients with primary breast cancer. Br J Cancer 2009;101:1529–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Faneyte IF, Schrama JG, Peterse JL, et al. Breast cancer response to neoadjuvant chemotherapy: predictive markers and relation with outcome. Br J Cancer 2003;88:406–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Varga Z, Caduff R, Pestalozzi B. Stability of the HER2 gene after primary chemotherapy in advanced breast cancer. Virchows Arch 2005;446:136–41. [DOI] [PubMed] [Google Scholar]
- [17].Adams AL, Eltoum I, Krontiras H, et al. The effect of neoadjuvant chemotherapy on histologic grade, hormone receptor status, and HER2/neu status in breast carcinoma. Breast J 2008;14:141–6. [DOI] [PubMed] [Google Scholar]
- [18].Scholl SM, Pierga JY, Asselain B, et al. Breast tumor response to primary chemotherapy predicts local and distant control as well as survival. Eur J Cancer 1995;31A:1969–75. [DOI] [PubMed] [Google Scholar]
- [19].Kuerer HM, Newman LA, Smith TL, et al. Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol 1999;17:460–9. [DOI] [PubMed] [Google Scholar]
- [20].Keam B, Im SA, Lee KH, et al. Ki-67 can be used for further classification of triple negative breast cancer into two subtypes with different response and prognosis. Breast Cancer Res 2011;13:R22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Li XR, Liu M, Zhang YJ, et al. CK5/6, EGFR, Ki-67, cyclin D1, and nm23-H1 protein expressions as predictors of pathological complete response to neoadjuvant chemotherapy in triple-negative breast cancer patients. Med Oncol 2011;28:S129–34. [DOI] [PubMed] [Google Scholar]
- [22].Zhou B, Yang DQ, Xie F. Biological markers as predictive factors of response to neoadjuvant taxanes and anthracycline chemotherapy in breast carcinoma. Chin Med J (Engl) 2008;121:387–91. [PubMed] [Google Scholar]
- [23].Wei Y, Li JF, Wang TF, et al. Association between hormone receptors and response to neoadjuvant anthracycline-based chemotherapy in breast cancer patients (Chinese). Beijing Da Xue Xue Bao 2007;5:481–3. [PubMed] [Google Scholar]
- [24].Chen X, He C, Han D, et al. The predictive value of Ki-67 before neoadjuvant chemotherapy for breast cancer: a systematic review and meta-analysis. Future Oncol 2017;9:843–57. [DOI] [PubMed] [Google Scholar]
- [25].Teresa G, Laura P, Isabella S, et al. Neoadjuvant chemotherapy in triple-negative breast cancer: a multicentric retrospective observational study in real-life setting. J Cell Physiol 2017;233:2313–23. doi:10.1002/jcp.26103. [DOI] [PubMed] [Google Scholar]
- [26].Wang JY, Sang D, Xu BH, et al. Value of breast cancer molecular subtypes and ki67 expression for the prediction of efficacy and prognosis of neoadjuvant chemotherapy in a Chinese population. Medicine (Baltimore) 2016;18:e3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Alba E, Lluch A, Ribelles N, et al. High proliferation predicts pathological complete response to neoadjuvant chemotherapy in early breast cancer. Oncologist 2016;21:150–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Yukie E, Takashi M, Arisa N, et al. Impact of biomarker changes during neoadjuvant chemotherapy for clinical response in patients with residual breast cancers. Int J Clin Oncol 2016;21:254–61. [DOI] [PubMed] [Google Scholar]
- [29].Gamal ME, Ahmed HE, Ahmed HO, et al. Response of triple negative breast cancer to neoadjuvant chemotherapy: correlation between Ki-67 expression and pathological response. Asian Pac J Cancer Prev 2016;17:807–13. [DOI] [PubMed] [Google Scholar]
- [30].Sasagu K, Kenichi I, Hiroyuki T, et al. ER, PgR, Ki67, p27(Kip1), and histological grade as predictors of pathological complete response in patients with HER2-positive breast cancer receiving neoadjuvant chemotherapy using taxanes followed by fluorouracil, epirubicin, and cyclophosphamide concomitant with trastuzumab. BMC Cancer 2015;15:622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yuan JQ, Wang SM, Tang LL, et al. Relative dose intensity and therapy efficacy in different breast cancer molecular subtypes: a retrospective study of early stage breast cancer patients treated with neoadjuvant chemotherapy. Breast Cancer Res Treat 2015;151:405–13. [DOI] [PubMed] [Google Scholar]
- [32].Kim T, Han W, Kim MK, et al. Predictive significance of p53, Ki-67, and Bcl-2 expression for pathologic complete response after neoadjuvant chemotherapy for triple-negative breast cancer. J Breast Cancer 2015;18:16–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tan QX, Qin QH, Yang WP, et al. Prognostic value of Ki67 expression in HR-negative breast cancer before and after neoadjuvant chemotherapy. Int J Clin Exp Pathol 2014;7:6862–70. [PMC free article] [PubMed] [Google Scholar]
- [34].Ingolf JB, Russalina M, Simona M, et al. Can Ki-67 play a role in prediction of breast cancer patients’ response to neoadjuvant chemotherapy? Biomed Res Int 2014;2014:628217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Huang L, Chen TW, Chen CM, et al. Prognostic and predictive value of Phospho-p44/42 and pAKT in HER2-positive locally advanced breast cancer patients treated with anthracycline-based neoadjuvant chemotherapy. World J of Surg Oncol 2013;11:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Ohno S, Chow LWC, Sato N, et al. Randomized trial of preoperative docetaxel with or without capecitabine after 4 cycles of 5-fluorouracil-epirubicin-cyclophosphamide (FEC) in early-stage breast cancer: exploratory analyses identify Ki67 as a predictive biomarker for response to neoadjuvant chemotherapy. Breast Cancer Res Treat 2013;142:69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cheng YJ, Ye JM, Xu L, et al. Factors related with pathological complete response of neoadjuvant chemotherapy in primary breast cancer (Chinese). Zhonghua Wai Ke Za Zhi 2013;4:339–43. [PubMed] [Google Scholar]
- [38].Yao YF, Gong JP, Tang JH, et al. Value of Ki-67 predicting the effectiveness of neoadjuvant chemotherapy in patients with triple-negative breast cancer (Chinese). J Basic Clin Oncol 2013;5:403–5. [Google Scholar]
- [39].Ye GL, Yang J, Gu WQ, et al. The Influence of Ki-67 expression on triple-negative breast cancer with neo-adjuvant chemotherapy (Chinese). Hebei Medicine 2013;6:832–4. [Google Scholar]
- [40].Jin SY, Kim SB, Ahn JH, et al. 18F-Fluorodeoxyglucose uptake predicts pathological complete response after neoadjuvant chemotherapy for breast cancer: a retrospective cohort study. J Surg Oncol 2013;107:180–7. [DOI] [PubMed] [Google Scholar]
- [41].Saracchini S, Foltran L, Tuccia F, et al. Phase II study of liposome-encapsulated doxorubicin plus cyclophosphamide, followed by sequential trastuzumab plus docetaxel as primary systemic therapy for breast cancer patients with HER2 overexpression or amplification. Breast 2013;22:1101–7. [DOI] [PubMed] [Google Scholar]
- [42].Esserman LJ, Berry DA, DeMichele A, et al. Pathologic complete response predicts recurrence-free survival more effectively by cancer subset: results from the I-SPY 1 TRIAL-CALGB 150007/150012, ACRIN 6657. J Clin Oncol 2012;26:3242–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Zhang GC, Qian XK, Guo ZB, et al. Pre-treatment hormonal receptor status and Ki67 index predict pathologic complete response to neoadjuvant trastuzumab/taxanes but not disease-free survival in HER2-positive breast cancer patients. Med Oncol 2012;29:3222–31. [DOI] [PubMed] [Google Scholar]
- [44].Grim J, JandÍk P, SlÁnskÁ. I, et al. Low expression of NQO1 predicts pathological complete response to neoadjuvant chemotherapy in breast cancer patients treated with TAC regimen. Folia Biologica (Praha) 2012;58:185–92. [PubMed] [Google Scholar]
- [45].Peter AF, Katharina H, Lothar H, et al. Ki67, chemotherapy response, and prognosis in breast cancer patients receiving neoadjuvant treatment. BMC Cancer 2011;11:486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Li XR, Liu M, Zhang YJ, et al. Evaluation of ER, PgR, HER-2, Ki-67, cyclin D1, and nm23-H1 as predictors of pathological complete response to neoadjuvant chemotherapy for locally advanced breast cancer. Med Oncol 2011;28:S31–8. [DOI] [PubMed] [Google Scholar]
- [47].Petit T, Wilt M, Velten M, et al. Semi-quantitative evaluation of estrogen receptor expression is a strong predictive factor of pathological complete response after anthracycline-based neo-adjuvant chemotherapy in hormonal-sensitive breast cancer. Breast Cancer Res Treat 2010;124:387–91. [DOI] [PubMed] [Google Scholar]
- [48].Sánchez-Muñoz A, Dueñas-García R, Jaén-Morago A, et al. Is it possible to increase pCR in the neoadjuvant treatment with a dose-dense/sequential combination?: results from a phase II Trial combining epirubicin and cyclophosphamide followed by paclitaxel and gemcitabine ± trastuzumab in stage II and III breast cancer patients. Am J Clin Oncol 2010;5:432–7. [DOI] [PubMed] [Google Scholar]
- [49].Colleoni M, Bagnardi V, Rotmensz N, et al. A nomogram based on the expression of Ki-67, steroid hormone receptors status and number of chemotherapy courses to predict pathological complete remission after preoperative chemotherapy for breast cancer. Eur J Cancer 2010;46:2216–24. [DOI] [PubMed] [Google Scholar]
- [50].Masuda H, Masuda N, Kodama Y, et al. Predictive factors for the effectiveness of neoadjuvant chemotherapy and prognosis in triple-negative breast cancer patients. Cancer Chemother Pharmacol 2011;67:911–7. [DOI] [PubMed] [Google Scholar]
- [51].Darb-Esfahani S, Sibylle Loibl S, Müller BM, et al. Identification of biology-based breast cancer types with distinct predictive and prognostic features: role of steroid hormone and HER2 receptor expression in patients treated with neoadjuvant anthracycline/taxane-based chemotherapy. Breast Cancer Res 2009;11:R69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Guarneri V, Piacentini F, Ficarra G, et al. A prognostic model based on nodal status and Ki 67 predicts the risk of recurrence and death in breast cancer patients with residual disease after preoperative chemotherapy. Ann Oncol 2009;20:1193–8. [DOI] [PubMed] [Google Scholar]
- [53].Colleoni M, Viale G, Zahrieh D, et al. Expression of ER, PgR, HER1, HER2, and response: a study of preoperative chemotherapy. Ann Oncol 2008;19:465–72. [DOI] [PubMed] [Google Scholar]
- [54].Vincent-Salomon A, Rousseau A, Jouve M, et al. Proliferation markers predictive of the pathological response and disease outcome of patients with breast carcinomas treated by anthracycline-based preoperative chemotherapy. Eur J Cancer 2004;40:1502–8. [DOI] [PubMed] [Google Scholar]
- [55].Mathieu MC, Rouzier R, Llombart-Cussac A, et al. The poor responsiveness of infiltrating lobular breast carcinomas to neoadjuvant chemotherapy can be explained by their biological profile. Eur J Cancer 2004;40:342–51. [DOI] [PubMed] [Google Scholar]
- [56].Colleoni M, Viale G, Zahrieh D, et al. Chemotherapy is more effective in patients with breast cancer not expressing steroid hormone receptors: a study of preoperative treatment. Clin Cancer Res 2004;10:6622–8. [DOI] [PubMed] [Google Scholar]
- [57].Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 2000;92:205–16. [DOI] [PubMed] [Google Scholar]
- [58].Wells G, Shea B, O’Connell D, et al. The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. 2003. Available at: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed January 15, 2017. [Google Scholar]
- [59].Fu XY, Zhou Q, Zhang XQ. Efficacy comparison between total laryngectomy and nonsurgical organ-preservation modalities in treatment of advanced stage laryngeal cancer: a meta-analysis. Medicine (Baltimore) 2016;14:e3142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Kim KI, Lee KH, Kim TR, et al. Ki67 as a predictor of response to neoadjuvant chemotherapy in breast cancer patients. J Breast Cancer 2014;17:40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Fasching PA, Heusinger K, Haeberle L, et al. Ki67, chemotherapy response, and prognosis in breast cancer patients receiving neoadjuvant treatment. BMC Cancer 2011;11:486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Urruticoechea A, Smith IE, Dowsett M. Proliferation marker Ki67 in early breast cancer. J Clin Oncol 2005;23:7212–20. [DOI] [PubMed] [Google Scholar]
- [63].Goldhirsch A, Wood W, Coates A, et al. Strategies for subtypes-dealing with the diversity of breast cancer: highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer. Ann Oncol 2011;22:1736–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Ring AE, Smith IE, Ashley S, et al. Oestrogen receptor status, pathological complete response and prognosis in patients receiving NAC for early breast cancer. Br J Cancer 2004;91:2012–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Yoo C, Ahn JH, Jung KH, et al. Impact of immunohistochemistry based molecular subtypes on chemosensitivity and survival patients with breast cancer following NAC. J Breast Cancer 2012;15:203–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Li XX(Bill), Krishnamurti U, Bhattarai S, et al. Biomarkers predicting pathologic complete response to neoadjuvant chemotherapy in breast cancer. Am J Clin Pathol 2016;145:871–8. [DOI] [PubMed] [Google Scholar]
- [67].von Minckwitz G, Fontanella C. Selecting the neoadjuvant treatment by molecular subtype: how to maximize the benefit? Breast 2013;22(suppl 2):S149–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


