Abstract
Rationale:
Mucoepidermoid carcinoma (MEC) of the breast is a rare entity comprising specific morphological and immunohistochemical features, and has been previously only reported in 33 cases.
Patient concerns:
Four cases of MEC of the breast are reported in this study. All patients were women with ages ranging from 39 to 66 years. The lesions consisted of neoplastic solid nests and cystic spaces sometimes filled with mucoid material.
Diagnoses:
At high power, the tumors were composed of various proportions of basaloid, intermediate, epidermoid, and mucinous cells in different cases. All cases were classified as low-grade MEC of the breast. Tumor cells exhibited low levels of hormonal receptor expression in two cases (cases 1 and 3), and immunonegativity in one case (case 2). On the contrary, estrogen receptors (ER) were positively expressed in 60% of tumor cells in case 4. Tumor cells did not express human epidermal growth factor receptor 2 (HER-2)/neu protein in all the cases.
Interventions:
Modified radical mastectomy (Auchincloss) was performed in the first two cases, while the remaining two patients underwent mastectomy plus sentinel lymph node biopsy.
Outcomes:
All patients were alive and well without evidence of recurrent disease after a period ranging from 4 months to 156 months.
Lessons:
MEC of the breast is a rare primary carcinoma that is difficult to diagnose. Multiple tissue blocks are necessary before obtaining all cell types. Special stains for mucin and electron microscopy would be helpful in suspected cases. Hormonal factors might have an impact on the biological behavior of tumors, but further studies are needed to draw conclusions.
Keywords: breast, hormonal factors, immunohistochemistry, mucoepidermoid carcinoma, prognosis
1. Introduction
Mucoepidermoid carcinoma (MEC) is a common malignant tumor of the minor salivary glands with standard grading criteria and prognostic features. MEC of the breast shares similar morphologic features with MEC of the salivary gland. However, the former is a rare entity with an incidence of 0.2% to 0.3%.[1] Only 33 cases have been reported to date. Patchefsky et al[2] were the first to present 2 cases of low-grade MEC of the breast.
Salivary gland-like tumors of the breast have been divided into 2 categories: tumors with myoepithelial differentiation (myoepithelioma, pleomorphic adenoma, adenoid cystic carcinoma, adenomyoepithelioma) and tumors with scanty myoepithelial differentiation (acinic cell carcinoma, oncocytic carcinoma, mucoepidermoid carcinoma).[3] Histologically, MECs are composed of 4 cell types in varying proportions. These are basaloid, intermediate, epidermoid, and mucinous cells. Clinical features, therapeutic strategies, and the prognosis of MEC are related to its histological grading and the accuracy of existing literature.
In this study, we report 4 cases of MEC of the breast and present a review of the literature.
2. Methods
Data from 4 cases of MEC of the breast were retrieved from the consultation files of the Breast Center of the Fourth Hospital of Hebei Medical University between 2004 and 2016. All the patients were confirmed by histopathology and underwent surgeries after diagnosis.
The postoperative specimens were fixed in 10% formalin, routinely processed, and embedded in paraffin. Selected blocks were serially cut and stained with hematoxylin and eosin and Alcian blue (AB) (pH 2.5) after diastase digestion. For immunohistochemistry, a routine EnVison method was used.[4] The tumors were graded according to the Elston–Ellis grading system for breast carcinoma.[5] All procedures were supervised and approved by the Ethics Committee of Fourth Hospital of Hebei Medical University (No. SCXK2017-0025).
3. Results
3.1. Clinical findings
Clinical data are summarized in Table 1. All patients were females with ages ranging from 39 to 66 years. The first 3 patients presented with short medical histories of not more than 3 months, while the fourth patient harbored a palpable mass in her left breast for nearly 37 years, which became enlarged with the time coursing.
Table 1.
Clinical findings of the herein reported cases.
In 3 cases, the lesion presented as a solid nodule, 2 of which had poorly defined boundaries (cases 1 and 3), while the other was well-circumscribed (case 2). The fourth patient harbored an irregular, solid, cystic mass in the breast. Computed tomography revealed only a few solid tissue masses within the septa-divided cystic spaces (Fig. 1). An ultrasound-guided core biopsy was performed during which purulent fluid was withdrawn. Cytological examination showed a small amount of proliferation of epithelial cells among a large number of blood cells. Excision biopsy revealed a circumscribed cyst measuring 30 mm in maximum diameter, and only a few solid tissue masses were present.
Figure 1.
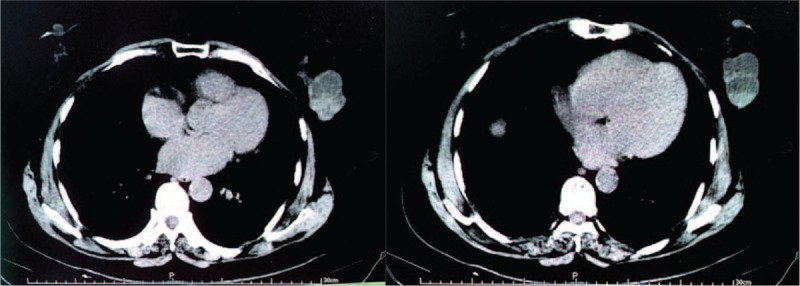
Computed tomography revealed only a few solid tissue masses within the septa-divided cystic spaces.
Modified radical mastectomy (Auchincloss) was performed in the first 2 cases, while the remaining 2 patients underwent mastectomy plus sentinel lymph node biopsy. Three of the 18 lymph nodes contained metastatic carcinoma in case 1, while no lymph node metastases were found in other cases.
Follow-up information was available for all the cases: patients were alive and well without evidence of recurrent disease after a period ranging from 4 months to 156 months.
3.2. Histopathological findings
The lesions comprised neoplastic solid nests and cystic spaces sometimes filled with mucoid material. At high power, the tumors were composed of various proportions of basaloid, intermediate, epidermoid, and mucinous cells in different cases. A prominent lymphocytic infiltrate was observed around the tumor lobules. AB stains showed numerous mucinous cells in the invasive component (Fig. 2A).
Figure 2.
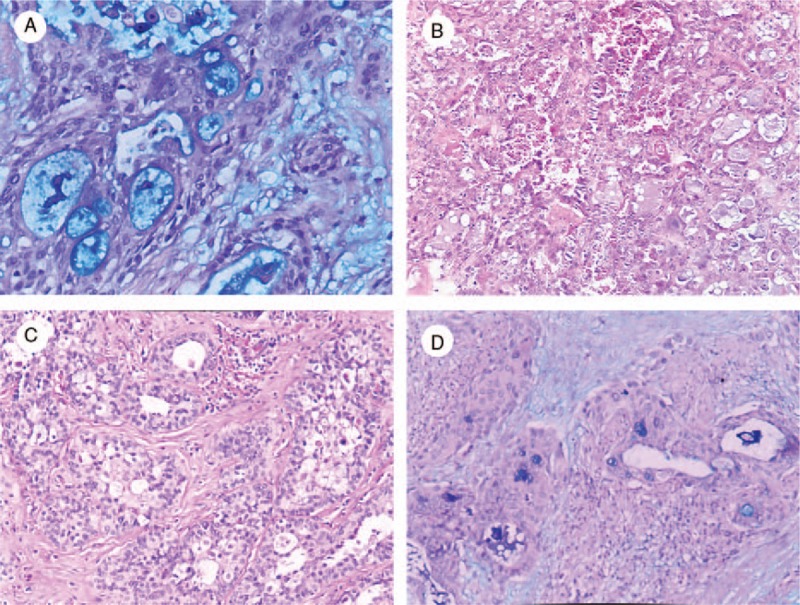
Histopathological features of mucoepidermoid carcinoma of the breast. A, Alcian blue stains showed numerous mucinous cells in the invasive component (Alcian blue stain, ×200). B, Scattered microcystic spaces, foamy cells, and vacuolated cells were also observed (HE, ×200). C, The nuclei were large with mild chromatin clearing and prominent nucleoli (HE, ×200). D, Cystic spaces were mainly lined by flat cells (Alcian blue stain, ×100).
Tumor cells were plump with granular eosinophilic cytoplasm and eosinophilic secretory material. Scattered microcystic spaces, foamy cells, and vacuolated cells were also observed (Fig. 2B). The nuclei were large with mild chromatin clearing and prominent nucleoli (Fig. 2C). Cystic spaces were mainly lined by flat cells. The latter were devoid of cytoplasmic vacuoles, but their cytoplasm was stained with AB (Fig. 2D).
Mitoses were infrequent in 3 cases [1/10 high-power field (HPF) cases 2 and 4, 2/10 HPF case 3]. Case 1 showed a moderate number of mitoses (3/10 HPF) with mild cytological atypia, while no perineural or lymphovascular invasion was observed.
All the cases were classified as low-grade (grade 1), according to the Elston–Ellis grading system.[5]
3.3. Immunohistochemical findings
Immunohistochemical findings are summarized in Table 2. Most intermediate and epidermoid cells expressed cytokeratin (CK) 5/6 (Fig. 3A). CK 7 was mainly observed in the cells composing the central part of the neoplastic nests and in the cells lining glandular and cystic spaces (Fig. 3B). In addition, both basaloid and intermediate cells were positive for p63 (Fig. 3C). Tumor cells exhibited low levels of hormonal receptor expression in 2 cases (cases 1 and 3), and immunonegativity in 1 case (case 2). On the contrary, estrogen receptors (ERs) were positively expressed in 60% of tumor cells in case 4. Tumor cells did not express human epidermal growth factor receptor 2 (HER-2)/neu protein in all the cases.
Table 2.
Immunohistochemical findings of the herein-reported cases.
Figure 3.
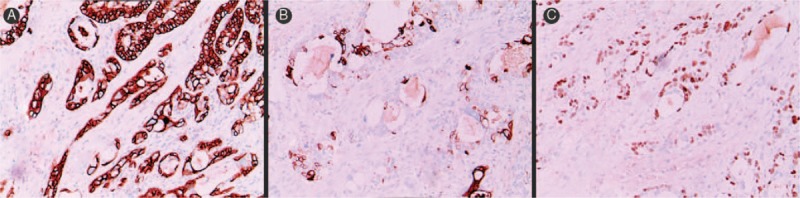
Immunohistochemical features of mucoepidermoid carcinoma of the breast. A, Most intermediate and epidermoid cells expressed CK 5/6 (immunohistochemistry reactions, ×200). B, CK 7 was mainly observed in the cells composing the central part of the neoplastic nests and in the cells lining glandular and cystic spaces (immunohistochemistry reactions, ×200). C, Both basaloid and intermediate cells were positive for p63 (immunohistochemistry reactions, ×200). CK = cytokeratin.
4. Discussion
MEC is a malignant tumor usually associated with the salivary glands. Primary MEC of the breast is extremely rare, having an incidence of 0.2% to 0.3%.[1] In our study, the incidence was 0.03% (4 cases of MEC of the breast out of 15,344 cases of breast cancer between 2004 and 2016), which is much lower than that reported previously. Fisher et al[1]suggested that the true frequency of MEC in the breast is higher than previously realized since MEC may masquerade under other diagnoses, such as atypical squamous metaplasia. This possibility must be taken into consideration, especially when only 1 cell type is observed. Multiple tissue blocks are necessary before obtaining all cell types and their true ratio of constituents. Special stains for mucin and electron microscopy may be helpful in suspected cases. On the other hand, studies on the incidence of MEC of the breast in Asian populations are not available, so we cannot exclude population susceptibility factors.
The breast and major salivary glands are derived from the embryonal ectoderm and their basic tubuloalveolar structures, probably explaining the similar morphologic features of tumors arising at these different sites.[6] MEC is described in other organs besides the salivary gland and breast including the esophagus, pleura, forearm, penis, tonsils, thyroid, colon, lacrimal gland, and thymus.[7–14] The 4 current cases of MEC were all primary MEC of the breast.
All tumors located outside the salivary glands share the same morphological and even immunohistochemical features as MEC of the major salivary glands.[15–18] Histologically, MEC is composed of 4 cell types in varying proportions. These are basaloid, intermediate, epidermoid, and mucinous cells. The tumor in the fourth patient was initially considered to be a pure mucinous adenocarcinoma of the breast, but on further examination, the diagnosis was changed to MEC.
The parameters for the grading criteria are the relative proportion of cystic components, the presence of neural invasion and necrosis, mitotic rate, and anaplasia.[5,19] All 4 cases were classified as low-grade (grade 1), according to the Elston–Ellis grading system. Patients with high-grade tumors that are highly aggressive should be treated by radical surgery with lymph node sampling and dissection, and patients with low-grade tumors may be cured by complete resection as low-grade tumors are usually considered to be potentially curable. All the patients underwent surgery; the first 2 cases underwent modified radical mastectomy (Auchincloss), and the others underwent mastectomy with sentinel lymph node biopsy. Three of the 18 lymph nodes contained metastatic carcinoma in case 1, while no lymph node metastases were observed in the other 3 cases. All the patients were alive and well without evidence of recurrent disease, with follow-up ranging from 4 to 156 months.
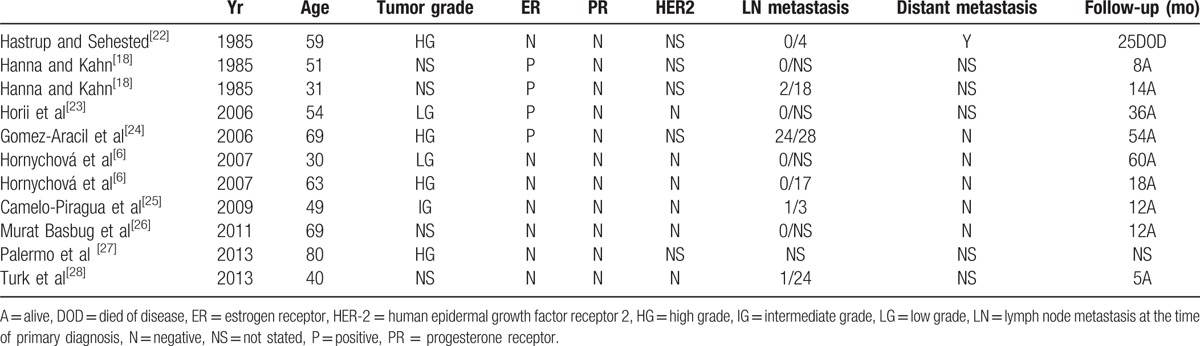
Tumors with the basal cell phenotype represent 15% to 25% of invasive breast carcinomas. They are usually high-grade; comprise areas of necrosis; are often ER-, progesterone receptor (PR)-, and HER2/neu-negative (triple negative breast cancer, TNBC); and affected patients harbor BRCA1 mutations more frequently than other types of breast carcinoma.[20,21] Some studies have shown that most cases of MEC of the breast are characterized by negative expression for ER, PR, and HER2. However, unlike other TNBCs, they exhibit a relatively good prognosis.[3] The absence of the expression of hormonal receptors (estrogen and progesterone) is found in the literature for most metaplastic carcinomas, including MEC.[22] According to previous reports, hormonal receptor status was studied in only 11 cases (Table 3).[6,18,22–28] Of these, 4 patients expressed ERs, while the other 7 cases were ER-negative. PRs were immunonegative in all the cases. In the cases reported herein, tumor cells exhibited low levels of hormonal receptor expression in 2 cases (cases 1 and 3), and immunonegativity in 1 case (case 2). No HER-2/neu protein was detected, but all the cases presented with good prognosis.
Table 3.
Summary of previously reported cases of mucoepidermoid carcinoma of breast.
The effect of hormonal factor expression in MEC is controversial; a few studies have mentioned the role of hormonal factors. Liang et al[9] described a MEC located in the left forearm of a 39-year-old pregnant woman, in which tumor growth accelerated with increasing hormone levels, suggesting that hormonal factors might influence the biological behavior of tumors. In case 4, we observed strong ER immunopositivity; the patient was diagnosed with low-grade MEC and no metastasis was identified despite a 37-year medical history without treatment. Hormonal factors may influence the prognosis of MEC of the breast, although the number of cases is far too small to draw conclusions. Follow-up is necessary to determine the biological behavior.
5. Conclusion
MEC of the breast is a rare primary carcinoma that is difficult to diagnose. Multiple tissue blocks are necessary before obtaining all cell types. Special stains for mucin and electron microscopy may be helpful in suspected cases. Most cases of MEC of the breast are characterized by negative expression of ER, PR, and HER2. However, unlike other TNBCs, they exhibit a relatively good prognosis. Hormonal factors might influence the biological behavior of tumors, but further studies are needed to draw conclusions.
Acknowledgments
The pathology department must be thanked for their kind help in analyzing the pathological sections.
Footnotes
Abbreviations: AB = alcian blue, CK = cytokeratin, ER = estrogen receptor, HER-2 = human epidermal growth factor receptor 2, HPF = high-power field, MEC = Mucoepidermoid carcinoma, PR = progesterone receptor, TNBC = triple negative breast cancer.
The authors have no conflicts of interest to disclose.
References
- [1].Fisher ER, Palekar AS, Gregorio RM, et al. Mucoepidermoid and squamous cell carcinomas of breast with reference to squamous metaplasia and giant cell tumors. Am J Surg Pathol 1983;7:15–27. [DOI] [PubMed] [Google Scholar]
- [2].Patchefsky AS, Frauenhoffer CM, Krall RA, et al. Low-grade mucoepidermoid carcinoma of the breast. Arch Pathol Lab Med 1979;103:196–8. [PubMed] [Google Scholar]
- [3].Pia-Foschini M, Reis-Filho JS, Eusebi V, et al. Salivary gland-like tumours of the breast: surgical and molecular pathology. J Clin Pathol 2003;56:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Haroon S, Hashmi AA, Khurshid A, et al. Ki67 Index in breast cancer: correlation with other prognostic markers and potential in pakistani patients. Asian Pac J Cancer Prev 2013;14:4353–8. [DOI] [PubMed] [Google Scholar]
- [5].Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 1991;19:403–10. [DOI] [PubMed] [Google Scholar]
- [6].Hornychová H, Ryska A, Betlach J. Mucoepidermoid carcinoma of the breast. Neoplasma 2007;54:168–72. [PubMed] [Google Scholar]
- [7].Koide N, Hamanake K, Igarashi J. Co-occurrence of mucoepidermoid carcinoma and squamous cell carcinoma of the esophagus: report of a case. Surg Today 2000;30:636–42. [DOI] [PubMed] [Google Scholar]
- [8].Moran CA, Suster S. Primary mucoepidermoid carcinoma of the pleura: a clinicopathologic study of two cases. Am J Clin Pathol 2003;120:381–5. [DOI] [PubMed] [Google Scholar]
- [9].Liang YF, Lin XY, Ruan JB, et al. A case of mucoepidermoid carcinoma located in the left forearm of a middle-aged pregnant woman. Int J Clin Exp Med 2014;7:2377–9. [PMC free article] [PubMed] [Google Scholar]
- [10].Jarvis SJ, Giangrande V, Brennan PA. Mucoepidermoid carcinoma of the tonsil: a very rare presentation. Acta Otorhinolaryngol Ital 2013;33:286–8. [PMC free article] [PubMed] [Google Scholar]
- [11].Minagawa A, Iitaka M, Suzuki M, et al. A case of primary mucoepidermoid carcinoma of the thyroid: molecular evidence of its origins. Clin Endocrinol 2002;57:551–6. [DOI] [PubMed] [Google Scholar]
- [12].Sato H, Kuroda M, Maruta M, et al. Mucoepidermoid carcinoma of the ascending colon: report of a case. Surg Today 2002;32:1004–7. [DOI] [PubMed] [Google Scholar]
- [13].Williams JD, Agrawal A, Wakely PE., Jr Mucoepidermoid carcinoma of the lacrimal sac. Ann Diagn Pathol 2003;7:31–4. [DOI] [PubMed] [Google Scholar]
- [14].Tanaka T, Morishita Y, Mori Y, et al. Fine needle aspiration cytology of the mucoepidermoid carcinoma of the thymus. Cytopathology 1990;1:49–53. [DOI] [PubMed] [Google Scholar]
- [15].Di Tommaso L, Foschini MP, Ragazzini T. Mucoepidermoid carcinoma of the breast. Virchows Arch 2004;444:13–9. [DOI] [PubMed] [Google Scholar]
- [16].Cameselle-Teijeiro J, Febles-Perez C, Sobrinho-Simoes M. Papillary and mucoepidermoid carcinoma of the thyroid with anaplastic transformation: a case report with histologic and immunohistochemical findings that support a provocative histogenetic hypothesis. Pathol Res Pract 1995;191:1214–21. [DOI] [PubMed] [Google Scholar]
- [17].Choi D, Kim H, Lee KS, et al. Mucoepidermoid carcinoma of the liver diagnosed as a liver abscess: report of a case. Surg Today 2004;34:968–72. [DOI] [PubMed] [Google Scholar]
- [18].Hanna W, Kahn HJ. Ultrastructural and immunohistochemical characteristics of mucoepidermoid carcinoma of the breast. Hum Pathol 1985;16:941–6. [DOI] [PubMed] [Google Scholar]
- [19].Ellis GL, Auclair PL. Ellis GL<Eds>, Auclair PL<Eds>. Tumors of the salivary glands. Atlas of Tumor Pathology, 3rd series, fascicle 17. Washington, DC: Armed Forces Institute of Pathology; 1996. 155–75. [Google Scholar]
- [20].ABD El-rehim DM, Pinder SE, Palsh CE. Expression of luminal and basal sytokeratins in human breast carcinoma. J Pathol 2004;203:661–71. [DOI] [PubMed] [Google Scholar]
- [21].Jones C, Ford E, Gillett C. Molecular cytogenetic identification of subgroups of grade III invasive ductal breast carcinomas with different clinical outcomes. Clin Cancer Res 2004;10:5988–97. [DOI] [PubMed] [Google Scholar]
- [22].Hastrup N, Sehested M. High-grade mucoepidermoid carcinoma of the breast. Histopathology 1985;9:887–92. [DOI] [PubMed] [Google Scholar]
- [23].Horii R, Akiyama F, Ikenaga M, et al. Mucoepidermoid carcinoma of the breast. Pathol Int 2006;56:549–53. [DOI] [PubMed] [Google Scholar]
- [24].Gomez-Aracil V, Mayayo Artal E, Azua-Romeo J, et al. Fine needle aspiration cytology of high grade mucoepidermoid carcinoma of the breast: a case report. Acta Cytol 2006;50:344–8. [DOI] [PubMed] [Google Scholar]
- [25].Sandra I, Camelo-Piragua, Claudine Habib, et al. Mucoepidermoid carcinoma of the breast shares cytogenetic abnormality with mucoepidermoid carcinoma of the salivary gland: a case report with molecular analysis and review of the literature. Hum Pathol 2009;40:887–92. [DOI] [PubMed] [Google Scholar]
- [26].Murat B, Sami A, Zulfu A, et al. Mucoepidermoid carcinoma in a breast affected by burn scars: comprehensive literature review and case report. Breast Care 2011;6:293–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Palermo MH, Pinto MB, Zanetti JS, et al. Primary mucoepidermoid carcinoma of the breast: a case report with immunohistochemical analysis and comparison with salivary gland mucoepidermoid carcinomas. Pol J Pathol 2013;64:210–5. [DOI] [PubMed] [Google Scholar]
- [28].Turk E, Karagulle E, Erinanc OH, et al. Mucoepidermoid carcinoma of the breast. Breast J 2013;19:206–8. [DOI] [PubMed] [Google Scholar]


