Abstract
Usually, after osteoporotic vertebral fracture (OVF), bone healing follows a normal clinical course leading to bone union with conservative treatment using a brace. However, some patients with OVF do not undergo the normal fracture healing process for a few months, possibly leading to delayed union and/or pseudoarthrosis. In these cases, we performed posterior surgery with combined decompression, vertebroplasty, and posterior spinal fusion with spinal instrumentation. This study aimed to determine the clinical results of posterior surgery for delayed neural disorder secondary to OVF over a 5-year follow-up.
Forty-one Japanese patients who had posterior surgery for delayed paralysis secondary to OVF were enrolled in this study. All patients were followed for ≥5 years (mean, 67 months; range, 61–86 months). Patients comprised 12 men and 29 women with an average age of 76.3 ± 6.2 years (range 63–87 years) at the time of operation. We performed posterior fixation from 2 levels above to 1 level below the decompression and vertebroplasty as an all in one procedure. Vertebral height index (VHI) and kyphotic angle (KA) were evaluated on radiogram. For clinical symptoms, a visual analog scale of back and leg pain and the Frankel classification and Japanese Orthopaedic Association scores were used.
During the operation and perioperative period, no serious complications occurred. In all patients, symptoms improved within 1 month and were maintained for 5 years postoperatively. In all patients, VHI and KA improved after surgery; however, reduction losses of 7.7% of VHI and 23% of KA were recognized. Five of 41 patients required reoperation due to adjacent vertebral fracture (AVF) and recollapse of the vertebral body.
Operation time and blood loss were acceptable, even for elderly patients. In all patients, alignment and subjective symptoms improved. However, reoperation owing to AVF and recollapse was necessary within 1 year in 5 of 41 (12%) patients. Careful follow-up is required within 1 year after surgery for OVF.
Keywords: adjacent vertebral fracture, clinical result, delayed paralysis, instrument failure, laminectomy, osteoporotic vertebral fracture, posterior surgery
1. Introduction
Osteoporosis is characterized by low bone mass and microarchitectural deterioration of bone tissue, leading to decreased bone fragility and a consequent increase in fracture risk.[1] It is a common problem in older people in developed countries[2] and vertebral fracture is one of the most common fractures caused by osteoporosis.[3] Usually, after osteoporotic vertebral fracture (OVF), bone healing follows a normal clinical course leading to bone union with conservative treatment using a brace. However, some patients with OVF do not undergo the normal fracture healing process for a few months, which leads to delayed union and/or pseudoarthorosis.[4,5] Moreover, some of these patients experience delayed paresthesia secondary to OVF.[6] In these cases, surgical treatment is highly recommended as the patients have severe low back pain and/or neurological complications.[7,8] However, there are controversies about the surgical procedures, which include anterior spinal fixation,[9] combined anterior-posterior procedures,[7] and posterior shortening.[10]
However, to minimize surgical invasiveness in elderly patients, a 1-stage posterior instrumentation technique with or without vertebroplasty (VP) has also been tried, according to several reports.[7–9,11] For delayed neural disorder secondary to OVF, we performed posterior surgery with combined decompression, VP,, and posterior spinal fusion with spinal instrumentation. The purpose of this study was to determine the clinical results of posterior approach surgery for delayed paralysis secondary to OVF during a 5-year follow-up.
2. Methods
2.1. Ethical approval
The study was approved by the Ethics Committee of Toyama University Hospital. Patients gave their written consent for this analysis.
2.2. Design
The design of this study was a case–control group.
2.3. Patient population
Forty-one Japanese patients who had posterior surgery for delayed paralysis secondary to OVF from 2007 to 2012 were enrolled in this study. All patients were followed for 5 years (mean, 67 months; range, 61–86 months). Osteoporosis was diagnosed by radiography and/or bone mineral density (BMD) based on the Clinical Practice Guideline of the Japanese Osteoporosis Society.[12] BMD of the vertebral body (VB) and the femoral neck was measured by dual-energy x-ray absorptiometry using Lunar enCORETM (GE Healthcare, Tokyo, Japan) preoperatively. Cases consisted of 14 men and 27 women with an average age of 76.3 ± 6.2 years (range, 63–87 years) at the time of operation. Delayed union and/or pseudoarthrosis was diagnosed based on presence of an intravertebral vacuum cleft on plain radiography and/or computed tomography (CT) as well as fluid collection within the VB on T2-weighted sagittal magnetic resonance imaging (MRI) using a 1.5T system (MAGNETOM Avanto, Siemens Co., München, Germany).[13] All patients had continued to complain of symptoms for >3 months (range, 3–36 months; mean, 6.9 months). Inclusion criteria for the surgery were as follows: those who could not maintain spinal alignment owing to repositioning of affected VB, had the conditions of delayed union and/or pseudoarthrosis according to OVF classification,[13] and had a cleft in the affected VB. Exclusion criteria were as follows: those who were treated with anterior surgery and those who underwent reduction operation for severe spinal deformity.
During the 5-year follow-up, 3 subjects died as a result of other factors within 1 year, 2 within 3 years, and 1 within 5 years after surgery (Fig. 1). Moreover, 3 subjects developed difficulty standing, owing to cerebral infarction in 1 and dementia in 2, within 1 year after surgery. One subject developed difficulty standing because of dementia within 3 years. Two subjects developed difficulty standing because of dementia within 5 years. Seven subjects died, and 6 nonstanding subjects were excluded sequentially. Therefore, 28 of 41 subjects were followed for the entire 5 years (final follow-up ratio, 71%).
Figure 1.
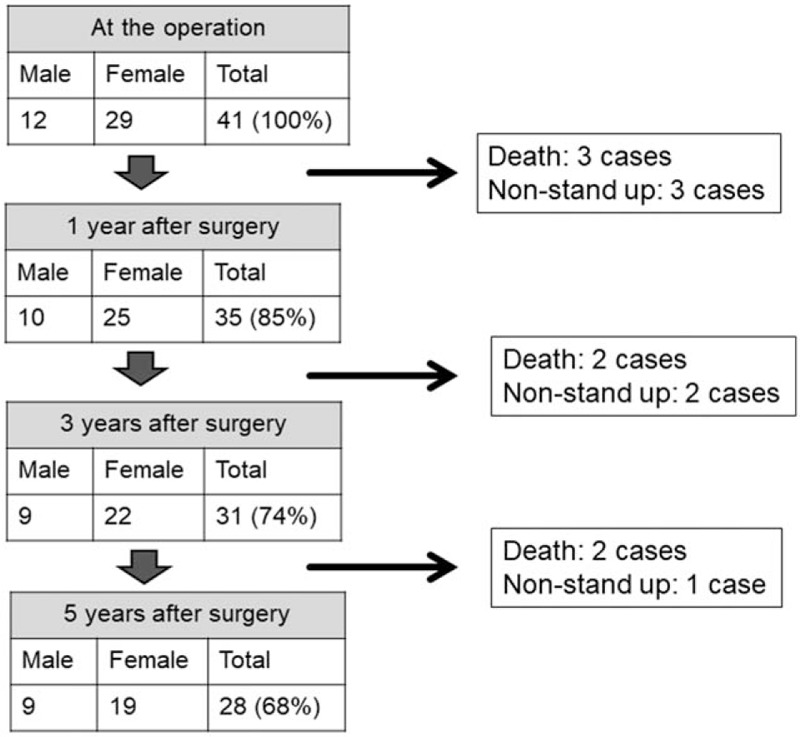
Demographic data of patients during follow-up.
2.4. Surgical procedure
The surgical technique was performed according to previous reports.[9,11] Briefly, initial reduction of the kyphotic deformity was obtained by placing the patient in the prone position. VP was performed using a hydroxyapatite (HA) block (Apaceram, HOYA Co, Tokyo, Japan).[14] Pedicle screws (PS) were placed promptly into the vertebrae, 2 levels above and 1 level below, the affected vertebra. A wide decompressive laminectomy was performed. Nesplon tape (ultrahigh-molecular-weight polyethylene tape; Alfresa Pharma, Osaka, Japan) and/or a hook were used to obtain more rigid fixation (Fig. 2). The surgeries were performed by multiple surgeons in one institute.
Figure 2.
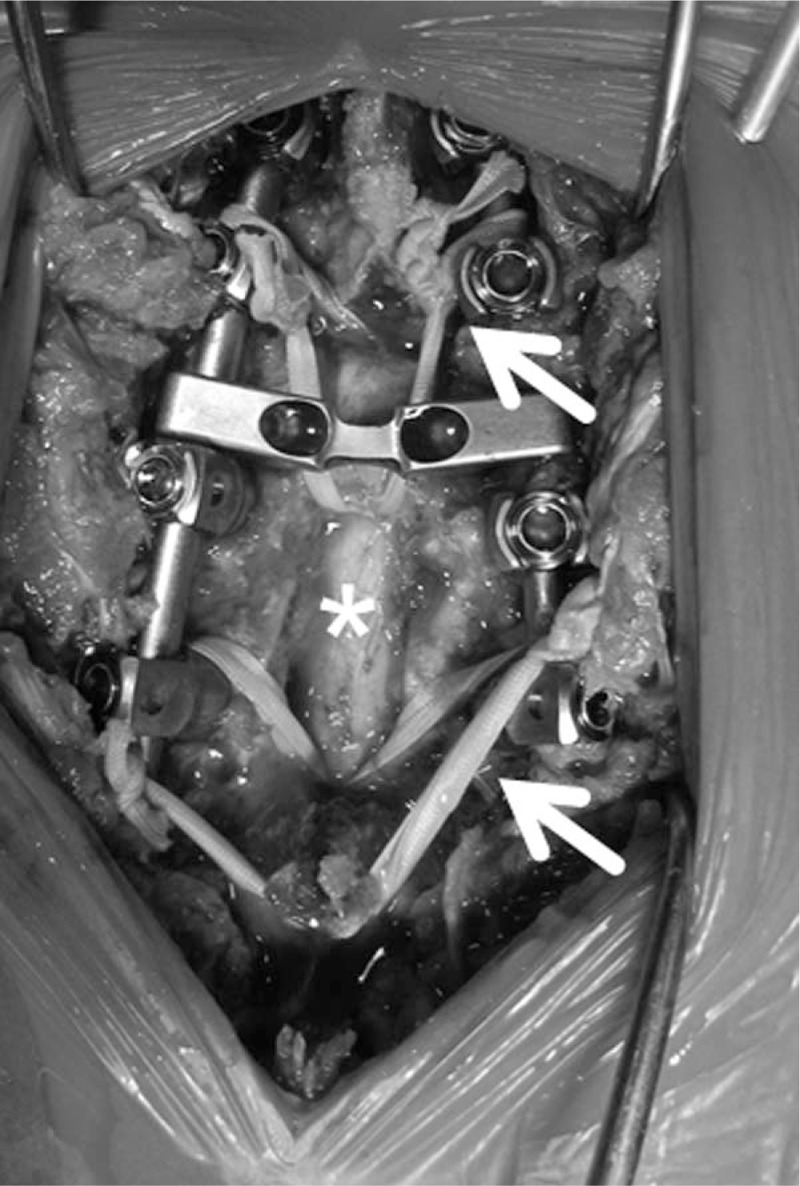
Intraoperative findings. Pedicle screws are placed promptly into the vertebrae 2 levels above and 1 level below the fracture. Dura mater (asterisk) is seen during laminectomy at the fracture level. Sublaminar taping is performed using Nesplon tape (arrows).
The patients were allowed to move freely, wearing a hard orthosis on the second day after surgery. All patients wore a hard brace for 2 months and then a soft brace for 2 months.
2.5. Imaging evaluation
To analyze the deformity of the VB, we serially measured the vertebral height index (VHI) on a lateral radiograph acquired in the neutral decubitus position and expressed it as the ratio of the vertebral height (sum of measurements at anterior, middle, and posterior regions) to its longitudinal diameter[15,16] preoperatively, at 1 month, and at 1, 3, and 5 years after surgery. We also measured the kyphotic angle (KA) between the upper end plate line and lower end plate line as an evaluation of kyphotic deformity preoperatively, at 1 month, and 1, 3, and 5 years after surgery.
2.6. Clinical evaluation
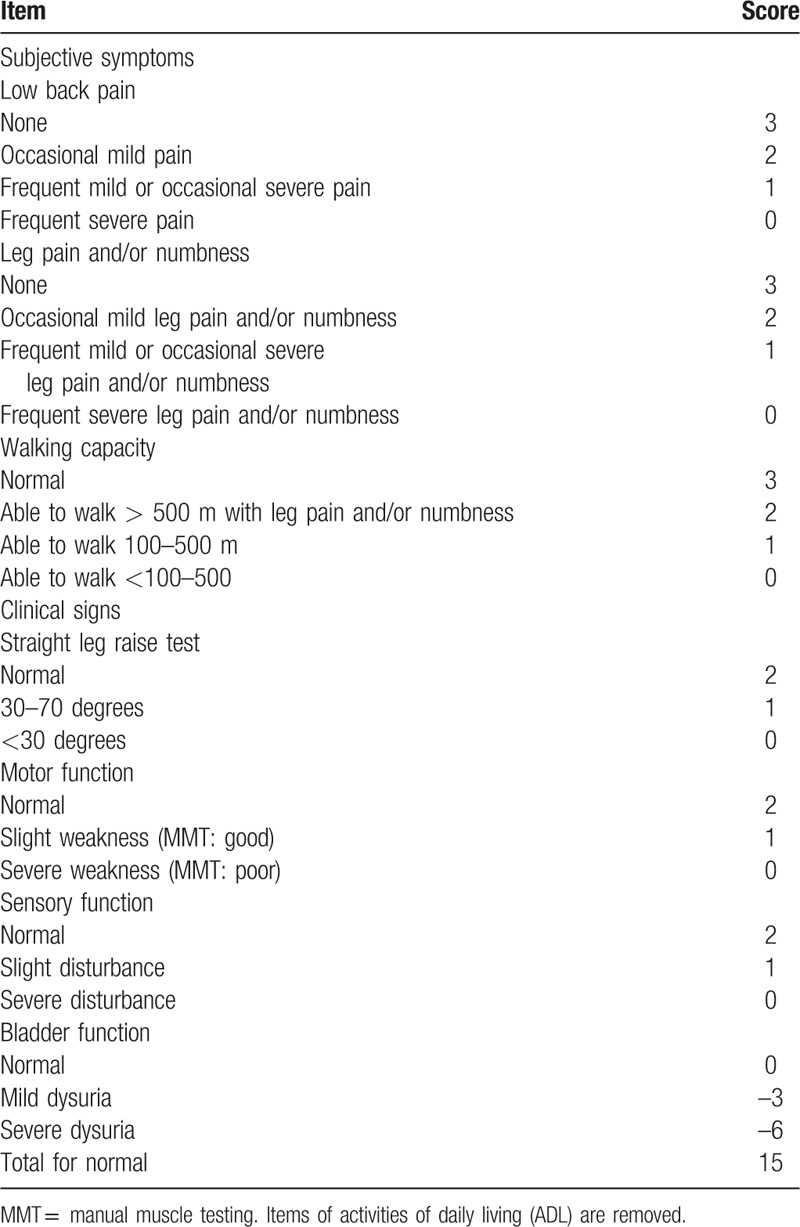
Clinical records were reviewed for operative time, intraoperative blood loss, complications during the operation and perioperative period (within 1 week after surgery), and radiological and neurological improvements. With regard to the subjective symptoms, back pain (BP) and leg pain (LP) were evaluated using the visual analogue scale (VAS, 0–10 cm) preoperatively, at 1 month, and at 1, 3, and 5 years after surgery. Neural function was evaluated using the Frankel classification. Furthermore, surgical outcomes were evaluated by the Japanese Orthopaedic Association (JOA) score, with the exception of the activities of daily living (ADL) items (Table 1).[17] Briefly, subjective symptom items (9 points: low BP, LP, and walking ability) and clinical signs (6 points: sensory and motor disturbance and angle of positive straight leg raising test) were evaluated. In addition, we investigated the causes and treatments in reoperative and/or complicated cases.
Table 1.
Evaluation system for the treatment of low back disorders devised by the Japanese Orthopaedic Association.
2.7. Statistical analysis
Values were expressed as means ± standard deviation (SD). Significant differences between means were analyzed using the Student t test, χ2 test, Kruskal-Wallis test, and repeated measurement with analysis of variance, when appropriate. A P value of <0.05 was considered statistically significant.
3. Results
3.1. Patient profiles
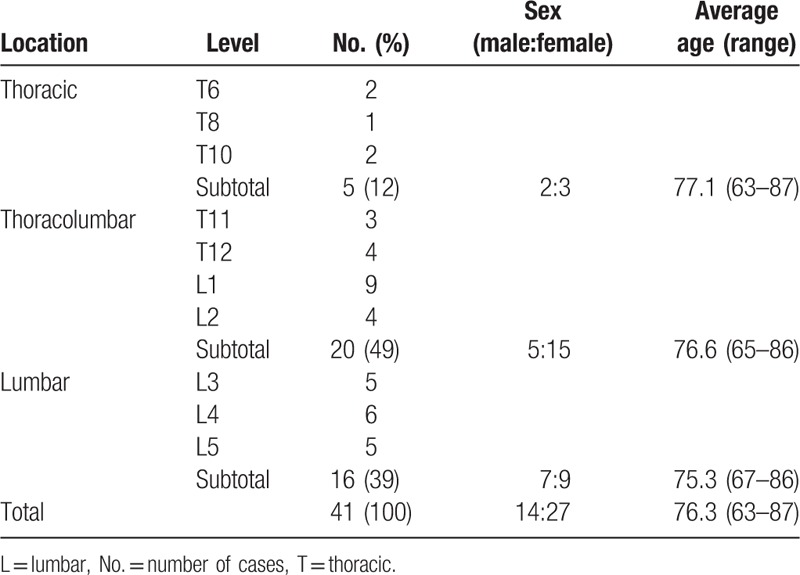
We performed the surgery in 41 patients, including 5 thoracic levels (2 at T6, 1 at T8, and 2 at T10), 20 thoracolumbar (3 at T11, 4 at T12, 9 at L1, and 4 at L2), and 16 lumbar (5 at L3, 6 at L4, and 5 at L5). Table 2 summarizes the characteristics of the patients. There was no significant difference in sex (χ2 test, P = .419) and age (Kruskal-Wallis test, P = .727) in relationship to fracture level.
Table 2.
Characteristics of patients.
3.2. Surgical results
Blood loss averaged 384 ± 214 (range, 70–970) mL during the operation. Operative times were 217 ± 148 (range, 106–281) minutes. Complications during the surgeries included dural laceration in 3 and leakage of HA block into the spinal canal in 1 in the early cases. In that case the HA block was removed 6 days after surgery because the patient developed LP. Delirium was a perioperative complication in 4 patients (9.5%), all of whom recovered within 1 week.
3.3. Clinical course
In all subjects, the subjective symptoms improved within 1 month. The mean preoperative VAS for BP was 8.3 ± 3.2, which improved to 3.8 ± 1.3 at 1 month after surgery (Fig. 3). Postoperative VAS for BP at 1 year, 3 years, and 5 years were 2.1 ± 0.7, 2.7 ± 0.6, and 3.1 ± 0.9, respectively. The mean preoperative VAS score for LP was 7.8 ± 2.9, which improved to 3.4 ± 1.9 at 1 month after surgery. Postoperative VAS for LP at 1 year, 3 years, and 5 years were 1.5 ± 0.9, 1.2 ± 0.8, and 1.8 ± 0.5, respectively. During the follow-up period, VAS for BP and LP significantly improved in comparison with preoperative VAS (P < .001).
Figure 3.
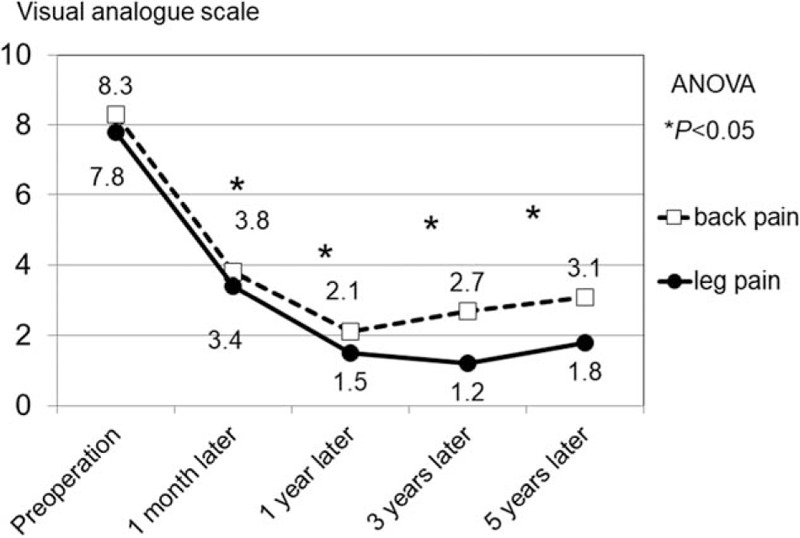
Change in visual analog scale scores for low back pain and leg pain. Preoperative symptoms are shown to statistically significantly improve postoperatively, (asterisk, P < .001). BP = back pain, LP = leg pain, VAS = visual analog scale.
Preoperative Frankel Grade was classified as C in 35, D in 5, and E in 1. In all cases, the deficit was stable or recovered by >1 step after surgery, and no subjects experienced worsening after surgery. The mean preoperative JOA score was 7.5 ± 2.6. At 1 month, 1 year, 3 years, and 5 years after surgery, mean JOA scores were 11.2 ± 3.7, 12.9 ± 4.1, 12.5 ± 3.3, and 11.5 ± 2.3 points, respectively. During the follow-up period, JOA scores were significantly improved in comparison with preoperative JOA scores (P < .001).
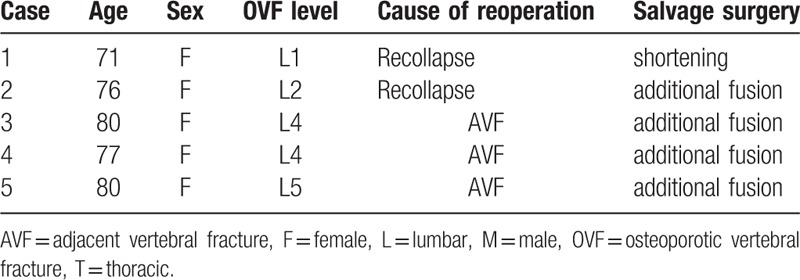
During follow-up, we found adjacent vertebral fracture (AVF) in 7 (17%) subjects. The fracture site was proximal to the VB in all cases. As salvage treatment, balloon kyphoplasty (BKP, Medtronic Sofamor Danek Japan Co, Osaka, Japan) was performed in 1 case, conservative therapy with hard brace in 4 cases, and additional fusion in 2 cases. Recollapse of the VB with backout of spinal instrumentation was seen in 2 cases. As salvage treatment, spinal shortening was performed in 1 case and an additional fusion was performed in 1 case. Loosening of PS was recognized in 14 cases (34%); however, we did not need additional therapy to avoid contributing to symptoms during follow-up. Finally, reoperation was necessary in 5 cases (12%) (Table 3).
Table 3.
Reoperation cases.
3.4. Imaging course
The mean preoperative VHI was 1.38 ± 0.32, which improved to 1.82 ± 0.56 at 1 month after surgery (Fig. 4). Postoperative VHIs at 1 year, 3 years, and 5 years were 1.76 ± 0.49, 1.73 ± 0.45, and 1.70 ± 0.48, respectively. VHI was significantly improved in comparison with preoperative VHI (P < .001). However, the mean ratio of reduction loss was 7.7%, recognized at 5 years in comparison to that at 1 month.
Figure 4.
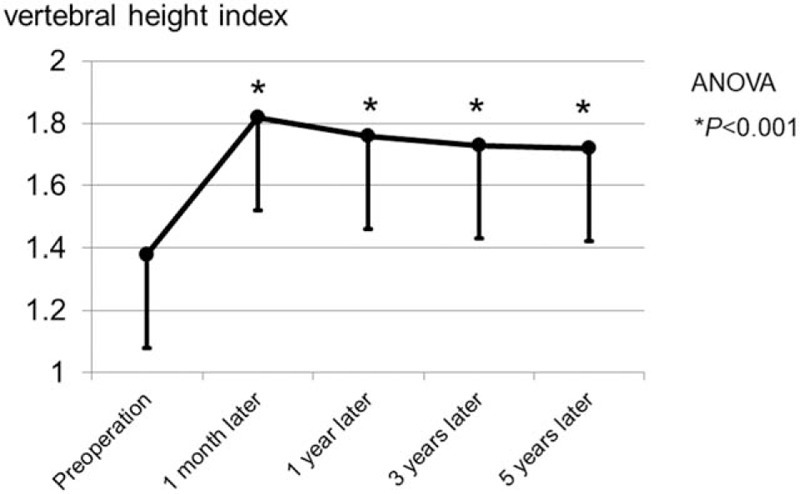
Course of vertebral body height. Although the vertebral height index was significantly improved after operation, the ratio of reduction loss was 7.7% recognized at 5 years in comparison to that at 1 month.
The mean preoperative KA was 19.3 ± 6.7, which improved to 3.9 ± 1.4 at 1 month after surgery. Postoperative KAs at 1 year, 3 years, and 5 years were 4.5 ± 1.2, 4.7 ± 1.7, and 4.8 ± 1.8, respectively. KAs were significantly improved in comparison with preoperative KA (P < .001). However, the mean ratio of reduction loss was 23% recognized at 5 years in comparison to that at 1 month.
3.5. Illustrative case
3.5.1. Case 1: 75-year-old man
The patient fell on his buttocks 6 months before presentation. He gradually developed low BP and LP. He could not stand or walk because of severe low BP and right LP (L5 area) at admission. Manual muscle testing (MMT) revealed muscle weakness of the tibialis anterior muscle and extensor hallucis longus, classified as Frankel Grade D. Radiogram showed L4 OVF and flexion-extension myelography showed abnormal instability of the VB and compression of the dural sac by the posterior wall of the VB (Fig. 5A and B). VHI was 0.77 and kyphotic angle was 13 degrees before surgery. CT myelography showed a cleft in the L4 VB (Fig. 5C) and T2-weighted MRI showed high signal change in the L4 VB (Fig. 5D). Surgery was performed. Blood loss was 310 mL and operative time was 156 minutes. There were no complications during the operation or perioperative period. The subjective and neurological symptoms improved remarkably within 1 week after surgery. VHI was 1.38 and KA was 2 degrees just after surgery (Fig. 5E). Because of reduction loss, his VHI was 1.22 and KA was 4 degrees 5 years later (Fig. 5F). Although loosening of the PS was recognized, he was ambulatory with only slight low BP.
Figure 5.
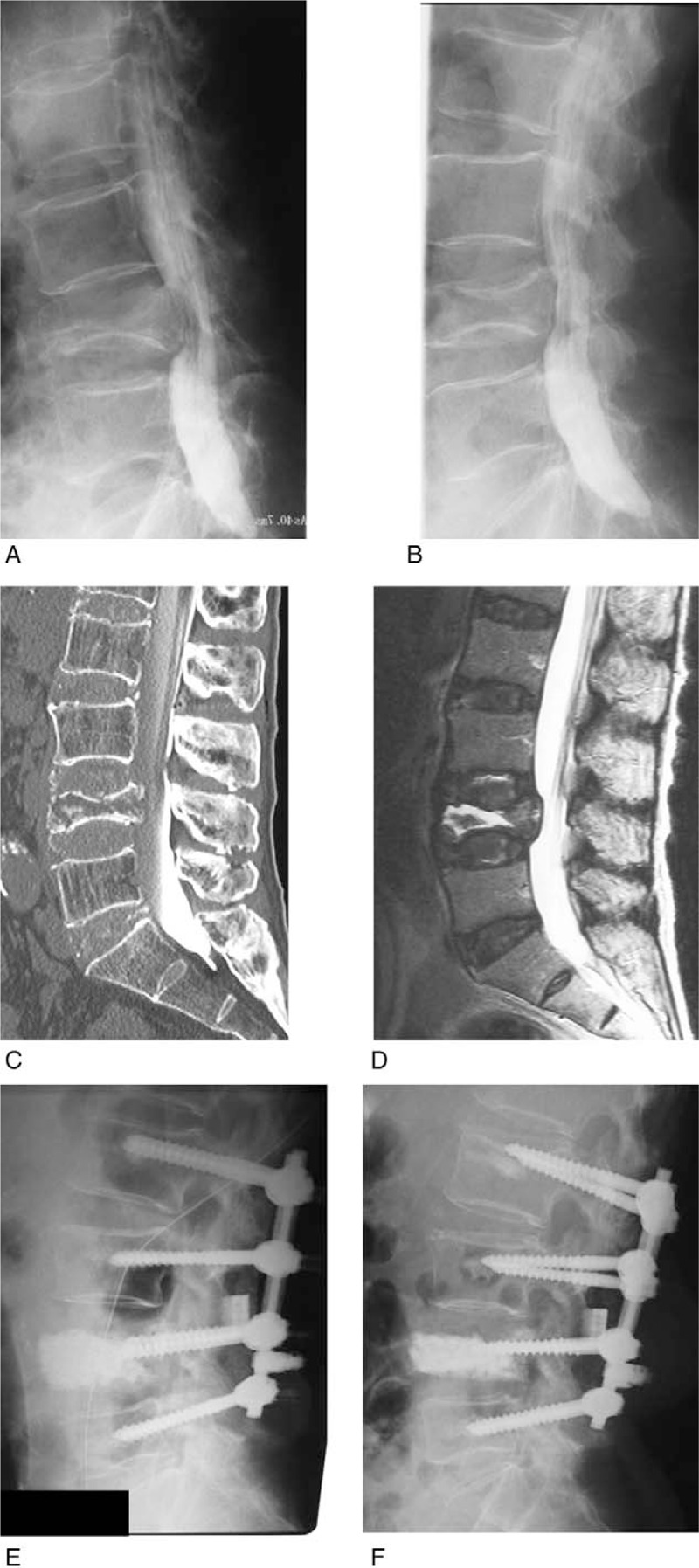
Case 1. (A) Lateral myelogram in flexion, (B) lateral myelogram in extension; abnormal instability of the L4 vertebral body (VB) is shown. In the flexed position, the dural sac is compressed by the posterior wall of the L4 VB. (C) Reconstructed sagittal computed tomography after myelography; the cleft is shown in the L4 VB. (D) T2-weighted midsagittal magnetic resonance imaging; high signal area is shown in the L4 VB. (E) Lumbar lateral radiogram just after operation. (F) Lumbar lateral radiogram at 5 years after surgery. Although VB height is reduced compared to just after surgery, the augmented hydroxyapatite block is solidified.
3.5.2. Case 2: 76-year-old woman
The patient fell on her buttocks 3 months before presentation. She gradually developed low BP and LP. She could not stand and walk because of severe low BP and muscle weakness at admission. She was classified as Frankel Grade C. Her radiogram showed an L2 OVF and flexion-extension views showed abnormal instability of the VB (Fig. 6A and B). VHI was 0.77 and kyphotic angle was 8 degrees before surgery. CT showed a cleft in the L2 VB (Fig. 6C), and T2-weighted MRI showed high signal change in the L2 VB and compression of the dural sac (Fig. 6D). Surgery was performed. Blood loss was 455 mL and operative time was 204 minutes. There were no complications during the operation or perioperative period. Her symptoms and the neurological signs improved remarkably immediately after surgery. VHI was 1.73 and KA was 2 degrees at 1 month after surgery (Fig. 6E). After 6 months, recollapse with backout of the spinal instrumentation was seen (Fig. 6F). We performed repositioning and an additional fusion 7 months after the initial operation (Fig. 6G). After reoperation, her clinical course improved, and she was able to ambulate with a cane.
Figure 6.
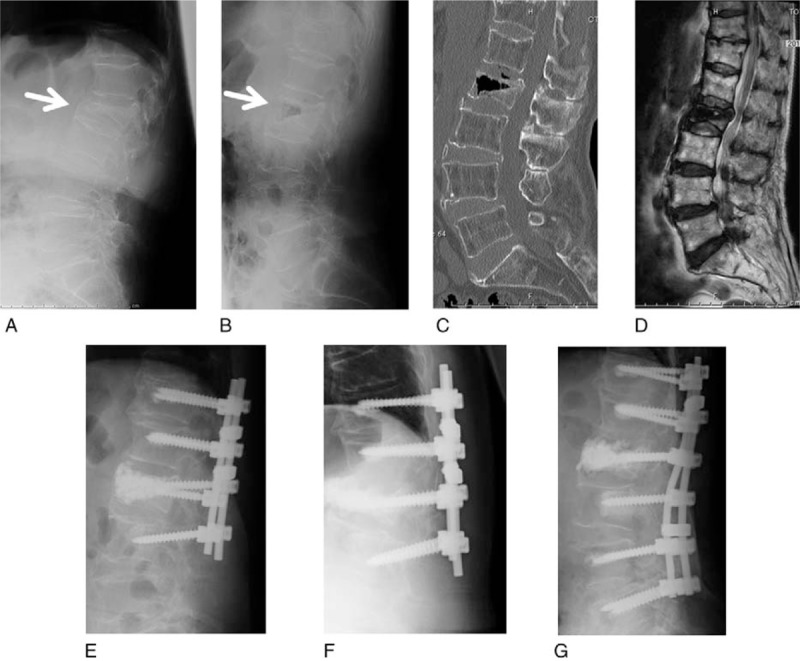
Case 2. (A) Lateral radiogram in flexion before surgery. (B) Lateral radiogram in extension before surgery. The abnormal instability of L2 vertebral body (VB) is shown (arrows). (C) Reconstructed sagittal computed tomography. The cleft is shown in L2 VB. (D) T2-weighted midsagittal magnetic resonance imaging. (E) Lateral radiogram at 1 month after surgery. Lower pedicle screws beginning to back out. (F) Lateral radiogram 6 months after surgery. Recollapse of the L2 VB with backout of spinal instrumentation is shown. (G) Lateral radiogram after reoperation (5 years after initial operation). Good reposition is maintained.
4. Discussion
OVFs usually heal without development of severe BP and/or LP or neuropathy with conventional conservative treatment. However, some OVF patients do not experience the normal fracture healing process, leading to delayed union and/or pseudarthrosis.[4,5] OVF pseudarthrosis, also known as Kummell disease, was first described by Kummel in 1895.[18] Of patients with OVF, 3.5% develop pseudarthrosis[19] and 3% to 5.3% develop late-onset paresis.[20,21] Risk factors for delayed union and/or pseudarthrosis, such as trauma with slight external force and a diffuse low-intensity area on T1-weighted MRI at the time of trauma, have been reported.[19]
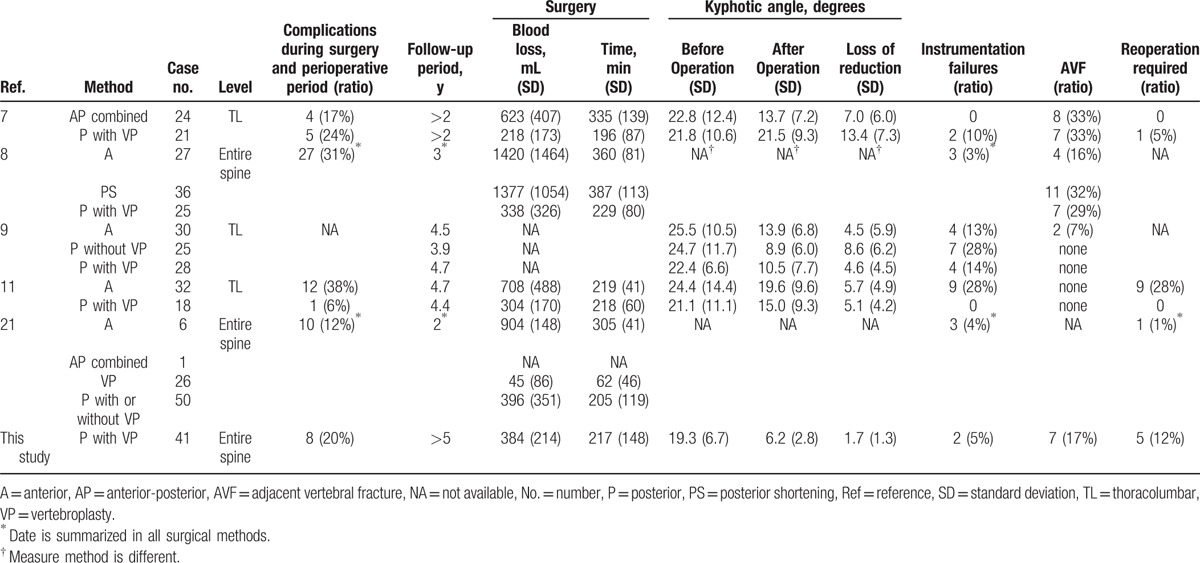
When OVF causes the neural disorder, surgery is necessary to achieve spinal stability and improve the neural disorder.[6–11,21] However, there are many older patients who may experience, depending on the operative method chosen, residual general functional decline, presence of various kinds of complications, and local bone fragility.[7,11] Therefore, surgeons must take several factors into consideration when choosing the surgical procedure best suited for each patient. For OVF, anterior decompression and fusion,[8,9,11,21] posterior decompression and fusion,[7–9,11,21] anterior and posterior combined surgery,[7,21] spinal shortening,[8,10] and VP[21] have been reported (Table 4). Anterior surgery is the direct method that can resect the pseudarthrosis VB and replace with bone. However, respiratory and cardiovascular complications during the perioperative period are of concern in elderly patients.[11] Furthermore, additional posterior surgery may be required for sinking or transplantation of grafted bone owing to osteoporosis.[8,9,11,21] Although VP for nonunion may provide relief, it is difficult to maintain the postoperative kyphotic correction and height restoration at the final follow-up.[21–23] On the contrary, this surgical method, which involves posterior decompression, VP, and rigid posterior fixation with instrumentation in one procedure, is technically common, but is a little aggressive for elderly patients. However, each surgical method has advantages and disadvantages as described above. Therefore, the important question regarding how to improve surgical outcomes for patients with OVF accompanied by neurological deficits remains somewhat unanswered.
Table 4.
Various surgical methods for osteoporotic vertebral fracture with neurological deficit.
This study is one of the longest follow-up reports of this kind. As others have reported to date,[7–9,11,21] this procedure is effective for improving pain and paralysis. However, 7 of 41 subjects died and 6 were unable to stand due to other factors at the 5-year follow-up. To our knowledge, 5-year survival rate and walking ability after surgery for delayed neural disorder following OVF has not yet been reported. In this study, 5-year survival was 83%. Although the validity of this result is not clear, systematic management is required for OVF after surgery.
During 5 years of follow-up, 5 of 41 subjects required reoperation. The main causes were AVF and backout of spinal instrumentation. AVF and backout of spinal instrumentation are considered to be contradictory complications. AVF can occur because of complete fusion. However, backout of spinal instrumentation can occur because of residual instability as a result of incomplete fusion. Toyone et al[24] reported that patients who underwent spinal instrumentation surgery were susceptible to the development of subsequent vertebral fractures within 2 years after surgery. As the mechanism, change in postoperative immobilization and altered biomechanics and initial low bone density were assumed. Therefore, careful follow-up and aggressive treatment for osteoporosis are required, especially within 1 year of surgery. Recently, we actively treated osteoporosis preoperatively and early postoperatively to prevent AVF and backout of spinal instrumentation.[25] In the study, perioperative administration of teriparatide was effective to prevent AVF and implant failure in osteoporotic patients with spinal deformities undergoing surgery.
4.1. Limitations
The present study has several limitations. The first is there is no control group. However, the positioning of this surgical procedure was evaluated by comparing with other previously reported methods as shown in Table 4. The limitation and long-term results of this procedure revealed. The second is restriction of the vertebral level performing this surgery. The thoracic spine may be poses special neuroanatomical challenges, considering the relatively small pedicle size and severe angulation from physiological kyphosis in the mid- and upper thoracic spine. In such cases, it is necessary to devise to change the approach to the VB.[26] Finally, the population size is relatively small and future studies would need a larger sample size.
5. Conclusion
We investigated the clinical results of posterior surgery for delayed paralysis secondary to OVF during 5 years. Operative time and blood loss were acceptable, even for elderly patients. In all subjects, imaging results and subjective symptoms improved. However, reoperation because of AVF and backout of spinal instrumentation was necessary within 1 year in 5 of 41 (12%) subjects. Careful follow-up is required for the first year after surgery for OVF.
Footnotes
Abbreviations: AVF = adjacent vertebral fracture, KA = kyphotic angle, OVF = osteoporotic vertebral fracture, VHI = vertebral height index.
This work was supported in part by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 17K10961.
The authors report no conflicts.
References
- [1].Alexeeva L, Burkhardt P, Christiansen C, et al. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis Report of a WHO study group. World Health Organ Tech Rep Ser 1994;843:1–29. [PubMed] [Google Scholar]
- [2].Wade SW, Strader C, Fitzpatrick L A, et al. Estimating prevalence of osteoporosis: examples from industrialized countries. Arch Osteoporos 2014;9:182. [DOI] [PubMed] [Google Scholar]
- [3].Golob AL, Laya MB. Osteoporosis: screening, prevention, and management. Med Clin North Am 2015;99:587–606. [DOI] [PubMed] [Google Scholar]
- [4].Steel HH. Kümmell's disease. Am J Surg 1951;81:161–7. [DOI] [PubMed] [Google Scholar]
- [5].Salomon C, Chopin D, Benoist M. Spinal cord compression: an exceptional complication of spinal osteoporosis. Spine 1988;13:222–4. [PubMed] [Google Scholar]
- [6].Ito Y, Hasegawa Y, Toda K, et al. Pathogenesis and diagnosis of delayed vertebral collapse resulting from osteoporotic spinal fracture. Spine J 2002;22:101–6. [DOI] [PubMed] [Google Scholar]
- [7].Nakashima H, Imagama S, Yukawa Y, et al. Comparative study of 2 surgical procedures for osteoporotic delayed vertebral collapse: anterior and posterior combined surgery versus posterior spinal fusion with vertebroplasty. Spine 2015;40:E120–126. [DOI] [PubMed] [Google Scholar]
- [8].Kashii M, Yamazaki R, Yamashita T, et al. Surgical treatment for osteoporotic vertebral collapse with neurological deficits: retrospective comparative study of three procedures–anterior surgery versus posterior spinal shorting osteotomy versus posterior spinal fusion using vertebroplasty. Eur Spine J 2013;22:1633–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Uchida K, Kobayashi S, Matsuzaki M, et al. Anterior versus posterior surgery for osteoporotic vertebral collapse with neurological deficit in the thoracolumbar spine. Eur Spine J 2006;15:1759–67. [DOI] [PubMed] [Google Scholar]
- [10].Saita K, Hoshino Y, Kikkawa I, et al. Posterior spinal shortening for paraplegia after vertebral collapse caused by osteoporosis. Spine 2000;25:2832–5. [DOI] [PubMed] [Google Scholar]
- [11].Sudo H, Ito M, Kaneda K, et al. Anterior decompression and strut graft versus posterior decompression and pedicle screw fixation with vertebroplasty for osteoporotic thoracolumbar vertebral collapse with neurologic deficits. Spine J 2013;13:1726–32. [DOI] [PubMed] [Google Scholar]
- [12].Orihashi H, Sugioka Y, Fukunaga M. The Japanese society for bone and mineral research 1996 revised criteria for the diagnosis of primary osteoporosis. J Jpn Soc Bone Miner Res 1997;14:219–33. [Google Scholar]
- [13].Takahashi S, Hoshino M, Takayama K, et al. Time course of osteoporotic vertebral fractures by magnetic resonance imaging using a simple classification: a multicenter prospective cohort study. Osteoporos Int 2017;28:473–82. [DOI] [PubMed] [Google Scholar]
- [14].Oshima M, Matsuzaki H, Tokuhashi Y, et al. Evaluation of biomechanical and histological features of vertebrae following vertebroplasty using hydroxyapatite blocks. Orthopedics 2010;33:89–93. [DOI] [PubMed] [Google Scholar]
- [15].Bouxsein ML, Genant HK. International Osteoporosis Foundation. The breaking spine. 2010; available at: https://www.iofbonehealth.org/breaking-spine-report-2010. Accessed September 13, 2017. [Google Scholar]
- [16].Genant HK, Wu CY, van Kuijk C, et al. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 1993;8:1137–48. [DOI] [PubMed] [Google Scholar]
- [17].Izumida S, Inoue S. Assessment of treatment for low back pain. J Jpn Orthop Assoc 1986;60:391–4. (in Japanese). [Google Scholar]
- [18].Yang H, Pan J, Wang G. A review of osteoporotic vertebral fracture nonunion management. Spine 2014;39:B4–6. [DOI] [PubMed] [Google Scholar]
- [19].Tsujio T, Nakamura H, Terai H, et al. Characteristic radiographic or magnetic resonance images of fresh osteoporotic vertebral fractures predicting potential risk for nonunion: a prospective multicenter study. Spine 2011;36:1229–35. [DOI] [PubMed] [Google Scholar]
- [20].Taneichi H, Kaneda K, Oguma T, et al. Risk factor analysis for osteoporotic vertebral collapse and pseudarthrosis (in Japanese). Rinsho Seikei Geka 2002;37:437–42. [Google Scholar]
- [21].Ito M, Harada A, Nakano T, et al. Retrospective multicenter study of surgical treatments for osteoporotic vertebral fractures. J Orthop Sci 2010;15:289–93. [DOI] [PubMed] [Google Scholar]
- [22].Ha KY, Lee JS, Kim KW, et al. Percutaneous vertebroplasty for vertebral compression fractures with and without intravertebral clefts. J Bone Joint Surg Br 2006;88:629–33. [DOI] [PubMed] [Google Scholar]
- [23].Garfin SR, Yuan HA, Reiley MA. New technologies in spine: kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine 2001;26:1511–5. [DOI] [PubMed] [Google Scholar]
- [24].Toyone T, Ozawa T, Kamikawa K, et al. Subsequent vertebral fractures following spinal fusion surgery for degenerative lumbar disease: a mean ten-year follow-up. Spine 2010;35:1915–8. [DOI] [PubMed] [Google Scholar]
- [25].Seki S, Hirano N, Kawaguchi Y, et al. Teriparatide versus low-dose bisphosphonates before and after surgery for adult spinal deformity in female Japanese patients with osteoporosis. Eur Spine J 2017;26:2121–7. [DOI] [PubMed] [Google Scholar]
- [26].Filis AK, Aghayev K, Schaller B, et al. Transdiscal mid- and upper thoracic vertebroplasty: first description of 2 exemplary cases. J Neurosurg Spine 2016;25:193–7. [DOI] [PubMed] [Google Scholar]


