Abstract
Rationale:
A 62-year-old male patient was admitted to our clinic in February 2016 with persistently elevated liver enzymes.
Patient concerns:
Clinical history involved a long time of poly-autoimmunity with a rheumatoid arthritis (in remission under tocilizumab therapy), an autoimmune thyroiditis, an eosinophilia as well as a hyper-immunoglobulin (IgG) 4-syndrome.
Diagnoses:
Laboratory studies revealed a significant increase in liver enzymes with an alanine aminotransferase (ALT) level of 574 U/L and an aspartate aminotransferase (AST) level of 864 U/L (normal <50 U/L). Furthermore, the patient was positive for anti-nuclear autoantibodies (ANA) with a titer of 1:320 (normal upper limit: 1:80).
Interventions:
Liver histology, obtained via mini-laparoscopy, demonstrated lobular hepatitis with markedly increased hepatocyte apoptosis, lymphoplasmatic cell infiltration, and 20% microvascular fat without significant fibrosis, which strengthened the diagnosis of autoimmune hepatitis (AIH). Pulse steroid treatment with 100 mg prednisolone for 3 days followed by a tapering down was initiated. Follow-up laboratory analysis demonstrated a decrease in liver enzymes and also of the ANA-titer.
Outcomes:
At that point, hepatitis E virus (HEV) infection was diagnosed with a positive anti-HEV immunoglobulin M (IgM) antibody and HEV-ribonucleotide acid (RNA) of 6280 copies/mL.
Lessons:
Despite the HEV infection and due to the strength of autoimmunity, we decided to continue immunosuppressive therapy and monitored HEV-PCR regularly. However, HEV-RNA became negative after 2 months and HEV-IgM turned negative after 13 months.
Keywords: autoimmune hepatitis, hepatitis E virus, immunosuppression
1. Introduction
Hepatitis E virus (HEV) is a small ribonucleotide acid (RNA) virus, which is considered to be the most usual cause of acute hepatitis in developing countries. The number of reported cases in Europe and especially in Germany is though increasing, so that hepatitis E should always be included in the differential diagnosis of unknown hepatitis. Hepatitis E infection varies from a simple acute hepatitis with spontaneous recovery to acute liver failure in patients with pre-existing liver disease.
Exceptionally interesting is therefore the course in immunosuppressed patients, since in these cases, hepatitis E can persist as a chronic infection. In immunocompromised patients HEV screening should be recommended in cases of persistently elevated liver enzymes. The diagnosis of a chronic hepatitis E infection is established by the presence of positive HEV-RNA for minimum of 3 to 6 months. However, reasons why hepatitis E becomes chronic and under which circumstances chronicity occurs still remains unclear.[1,2]
Autoimmune hepatitis is an immune-mediated liver disease, which is characterized by elevated liver enzymes, presence of certain auto-antibodies, and histological features with plasma cell infiltration as well as the absence of other causes of liver disease, especially viral hepatitis. The main backbone in treatment consists of immunosuppressive drugs such as corticosteroids in combination with azathioprine.
With the following case report we highlight the significance of HEV screening in an immunocompromised patient.
2. Case report
A 62-year-old male patient was admitted to our clinic in February 2016 with markedly increased liver enzymes. Clinical history of the patient involved a long time of autoimmunity with a rheumatoid arthritis (in remission under tocilizumab therapy), an autoimmune thyroiditis, an eosinophilia as well as a hyper-IgG4-syndrome. On admission, laboratory studies revealed an alanine aminotransferase (ALT) level of 574 U/L and an aspartate aminotransferase (AST) level of 864 U/L (normal <50 U/L). Cholestatic liver enzymes were also abnormal with an alkaline phosphatase (AP) level of 282 U//L (normal 25–124 U/L) and a gamma-glutamyl-transferase (γ-GT) value of 489 U/L (normal <55 U/L) (Fig. 1). Furthermore, the patient was positive for anti-nuclear autoantibodies (ANA) with a titer of 1:320 (normal upper limit: 1:80). Liver histology obtained via mini-laparoscopy demonstrated a moderate portal and periportal as well as distinct lobular hepatitis with infiltration of lymphoplasmatic cells, markedly increased hepatocyte apoptosis, and approximately 20% of pure microvascular fat without significant fibrosis, which strengthened the diagnosis of AIH (Fig. 2).
Figure 1.
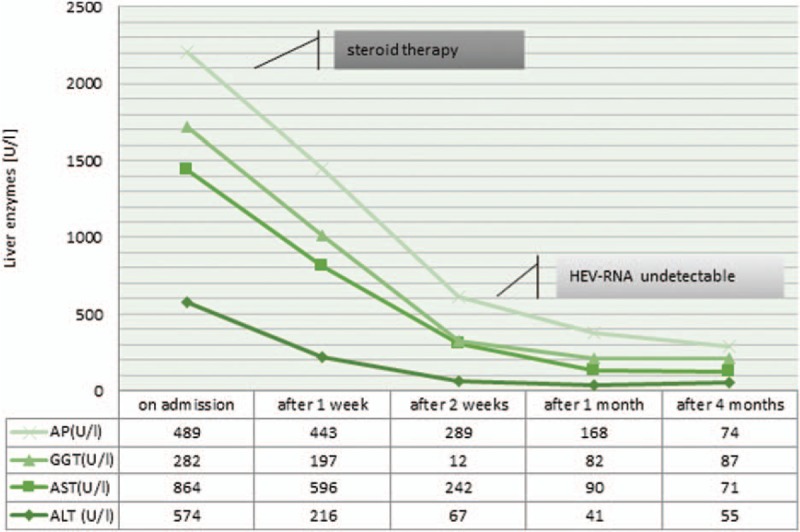
Course of liver enzymes following steroid therapy.
Figure 2.
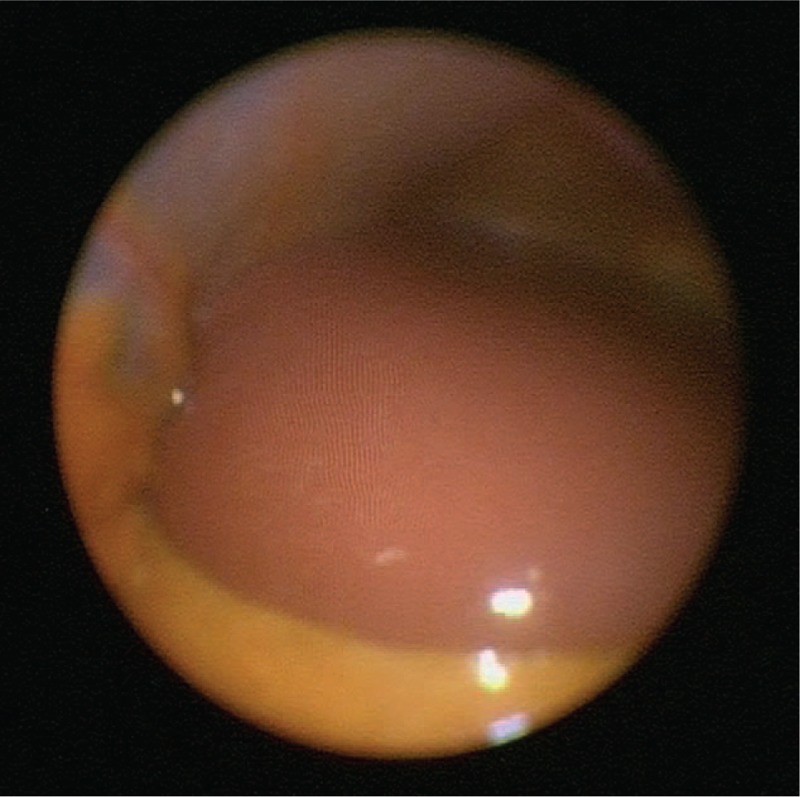
Mini-laparoscopy showing acute hepatitis with capsular swelling.
Under suspicion of a newly diagnosed AIH, immunosuppressive therapy with an initial steroid dose of 100 mg prednisolone for 3 days was started. Steroids were tapered down slowly by scheme to 12.5 mg/d prednisolone conservation dose per day. This resulted in a rapid decrease of AST while decrease of ALT-values was significantly delayed. However, liver enzymes turned to normal ranges after 6 months and the ANA-titer normalized after 1 month. Extended diagnostic work-up revealed an acute hepatitis E infection with the presence of immunoglobulin M (IgM) antibodies and a RNA-titer of 6280 copies/mL simultaneously with the diagnosis of AIH. On behalf of persistent autoimmunity, immunosuppressive therapy was continued despite these viral findings. HEV-RNA was no longer quantifiable during serological controls after 2 months. Interestingly, the IgM-titer became negative much later—after 13 months (Table 1). Antiviral therapy with ribavirin was not necessary any more.
Table 1.
Course of hepatitis E viral load, IgM, and IgG titers.

The University Clinic Essen ethics committee approved the data and the patient gave his written informed consent.
3. Discussion
In our case, an acute hepatitis E was simultaneously diagnosed with a boost of autoimmune hepatitis. The patient was referred to our department due to hepatitis of unknown origin. Liver biopsy revealed autoimmune hepatitis, which was treated by corticosteroids. Interestingly, the patient had a pre-existing immunosuppressive treatment with tocilizumab, a humanized monoclonal antibody against the interleukin-6 receptor due to rheumatoid arthritis. At that point an acute hepatitis E infection was diagnosed serologically. However, a fundamental differentiation between a newly diagnosed and acquired acute hepatitis E infection or a virus re-activation following immunosuppressive therapy with tocilizumab was unfortunately not possible.
Acute hepatitis E can lead to a chronic hepatitis in immunosuppressed patients (HIV-positive patients, patients with hematological malignancies or patients following organ transplantation).[3–5] This is defined by a HEV-RNA persistence for >6 months in serum. In case of an acute course, HEV-RNA becomes negative within 14 to 28 days. The prolonged detection of HEV-RNA defining chronicity may lead to liver cirrhosis or acute-on-chronic liver failure, especially in immunosuppressed patients.[6]
It should be mentioned that differentiation between drug-induced liver injury (DILI) and hepatitis E virus infection is still challenging, as both can cause elevated liver enzymes. Therefore, hepatitis E status must be monitored closely after an acute infection in patients receiving immunosuppressive drugs. In cases of chronicity, the initial immunosuppressive regime should be reduced, which is reported to result in reduction of the viral load in 30% of patients.[7] In our patient, immunosuppression was not reduced despite the presence of an acute hepatitis E, as the autoimmune condition was the major problem. However, IgM-titer remained positive for 13 months. This prolonged IgM detection was remarkable and reported by other groups likewise.[1,8]
If immunosuppressive therapy cannot be de-escalated, close monitoring of HEV-RNA is highly recommended. Due to HEV-RNA persistence, severe complications such as liver fibrosis or even cirrhosis can be observed. In this regard, a case of a liver transplant recipient who developed cirrhosis due to an undetected hepatitis E virus infection of the donor was published in 2012.[9] In such cases, the initiation of an antiviral therapy with ribavirin is indicated.[10,11] According to current data, antiviral treatment by use of the NS5A inhibitor Sofosbuvir, which is primarily administered in patients with chronic hepatitis C virus infection, also shows promising results.[12]
4. Conclusion
Hepatitis E is considered to be an acute infection. Rarely, it can although turn to a chronic form, especially in immunosuppressed patients. The chronic form of hepatitis E should be monitored closely, as it can progress to liver fibrosis or cirrhosis. In cases of positive HEV-RNA in immunosuppressed patient, immunosuppressive therapy should initially be reduced. If this reduction is not possible or promising, close monitoring of the HEV-RNA is strongly recommended. Antiviral treatment by use of ribavirin is highly recommended in patients with virus persistence.
Footnotes
Abbreviations: AIH = autoimmune hepatitis, ALT = alanine aminotransferase, ANA = anti-nuclear antibody, AP = alkaline phosphatase, AST = aspartate aminotransferase, DILI = drug-induced liver injury, γ-GT = gamma-glutamyl-transferase, HEV = hepatitis E virus, IgG = immunoglobulin G.
The authors report no conflicts of interest.
References
- [1].Singh A, Seth R, Gupta A, et al. Chronic hepatitis E - an emerging disease in an immunocompromised host. Gastroenterol Rep (Oxf) 2016;pii: gow024. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Murali AR, Kotwal V, Chawla S. Chronic hepatitis E: a brief review. World J Hepatol 2015;7:2194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kamar N, Selves J, Mansuy JM, et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. N Engl J Med 2008;358:811–7. [DOI] [PubMed] [Google Scholar]
- [4].Tavitian S, Péron JM, Huynh A, et al. Hepatitis E virus excretion can be prolonged in patients with hematological malignancies. J Clin Virol 2010;49:141–4. [DOI] [PubMed] [Google Scholar]
- [5].Dalton HR, Bendall RP, Keane FE, et al. Persistent carriage of hepatitis E virus in patients with HIV infection. N Engl J Med 2009;361:1025–7. [DOI] [PubMed] [Google Scholar]
- [6].Ingiliz P, Mayr C, Obermeier M, et al. Persisting hepatitis E virus infection leading to liver cirrhosis despite recovery of the immune system in an HIV-infected patient. Clin Res Hepatol Gastroenterol 2016;40:e23–5. [DOI] [PubMed] [Google Scholar]
- [7].Rathod SB, Das R, Thanapati S, et al. Suppressive activity and altered conventional phenotype markers/mediators of regulatory T cells in patients with self-limiting hepatitis E. J Viral Hepat 2014;21:141–51. [DOI] [PubMed] [Google Scholar]
- [8].Pischke S, Iking-Konert C. [Hepatitis E infections in rheumatology. A previously underestimated infectious disease?]. Z Rheumatol 2015;74:731–6. [DOI] [PubMed] [Google Scholar]
- [9].Schlosser B, Stein A, Neuhaus R, et al. Liver transplant from a donor with occult HEV infection induced chronic hepatitis and cirrhosis in the recipient. J Hepatol 2012;56:500–2. [DOI] [PubMed] [Google Scholar]
- [10].Pischke S, Greer M, Hardtke S, et al. Course and treatment of chronic hepatitis E virus infection in lung transplant recipients. Transpl Infect Dis 2014;16:333–9. [DOI] [PubMed] [Google Scholar]
- [11].Gerolami R, Borentain P, Raissouni F, et al. Treatment of severe acute hepatitis E by ribavirin. J Clin Virol 2011;52:60–2. [DOI] [PubMed] [Google Scholar]
- [12].Dao Thi VL, Debing Y, Wu X, et al. Sofosbuvir inhibits Hepatitis E virus replication in vitro and results in an additive effect when combined with ribavirin. Gastroenterology 2016;150:82.e4–5.e4. [DOI] [PubMed] [Google Scholar]


