Abstract
Background:
Studies on stroke and lipoprotein-associated phospholipase A2 (Lp-PLA2) have produced conflicting results.
Objective:
The aim of the study was to assess the associations of Lp-PLA2 levels (mass and activity) with recurrent vascular events in patients with transient ischemic attack (TIA) and/or first ischemic stroke and with stroke in the general population.
Methods:
The MEDLINE, Embase, the Cochrane Library, Web of Science, Science Direct, China National Knowledge Infrastructure, China Biology Medical Disc (CBMdisc), and WanFang were searched for prospective observational studies reported until January 2017. Eligible studies reported Lp-PLA2 levels and adjusted risk estimates of recurrent vascular events and/or stroke. Risk ratio (RR) with corresponding 95% confidence intervals (CIs) were used to express the pooled data in a random-effects model.
Results:
A total of 11 studies that comprised 20,284 participants (4,045 were TIA and/or first ischemic stroke patients and 16,239 were residents in general population) were identified, which reported either Lp-PLA2 mass levels (4 studies) or Lp-PLA2 activity levels (10 studies). The pooled RR of recurrent vascular events (467 cases) in TIA and/or first ischemic group was 2.24 (95% CI, 1.33–3.78), whereas the pooled RR of stroke (1604 cases) in the general population was 1.47 (95% CI, 1.10–1.97). The pooled RRs of Lp-PLA2 mass and activity levels with the risk of stroke in the general population were 1.69 (95% CI, 1.03–2.79) and 1.28 (95% CI, 0.88–1.85), respectively.
Conclusions:
In patients with TIA and first ischemic stroke, elevated Lp-PLA2 activity levels were associated with recurrent vascular events. And in the general population elevated Lp-PLA2 levels were associated with the risk of stroke, although the association between Lp-PLA2 activity levels and the risk of stroke was less profound compared with the corresponding association of stroke risk with the Lp-PLA2 mass levels.
Keywords: ischemic stroke, lipoprotein-associated phospholipase A2, meta-analysis, recurrent vascular event, stroke, systematic review, TIA
1. Introduction
Stroke is considered as the main cause of death and neurologic disability worldwide. A considerably high number of cases worldwide are reported to suffer from stroke and transient ischemic attack (TIA) annually, of which more than one-fifth will experience a recurrent stroke within a short period of time.[1] Management of vascular risk factors is of great importance for primary and secondary prevention of stroke.[2,3] As conventional risk factors such as hyperglycemia, hyperlipidemia, and blood pressure are inadequate in predicting stroke,[4,5] novel predictive biomarkers were investigated to predict high-risk subjects.[6,7]
Approximately 87% of all stroke incidents are ischemic stroke incidents,[8] most of which are highly associated with the etiology of atherosclerosis. Inflammation is postulated to play a significant role in the process of atherosclerosis.[9] Lipoprotein-associated phospholipase A2 (Lp-PLA2) is an enzyme derived from inflammatory cells that is mainly bound to low-density lipoproteins (LDL) in the circulation.[10] Lp-PLA2 has been reported to be associated with atherosclerotic plaque inflammation and instability.[11] Lp-PLA2 plays a role in hydrolysis of oxidized LDLs by the production of proinflammatory mediators, namely, lysophosphatidylcholine and oxidized nonesterified fatty acids that are involved in the migration of vascular smooth muscle cells, endothelial dysfunction, expression of adhesion molecules and cytokines, as well as the formation of necrotic core in plaques.[12–14]
A majority of studies have confirmed the relationship between Lp-PLA2 mass and/or activity levels and the risk of subsequent cardiovascular diseases (CVD), but investigations of Lp-PLA2 mass and/or activity levels and the risk of stroke have produced conflicting results. The available data in a previous review indicated that the determination of Lp-PLA2 levels was associated with the risk of stroke risk which approximately doubled the increase noted in the occurrence of stroke.[15] A meta-analysis of 32 prospective studies suggested that circulating Lp-PLA2 mass and activity levels demonstrated an association with the risk of coronary heart disease, but the association of Lp-PLA2 was less evident with regard to ischemic stroke.[16] Certain studies further included stroke as a combined endpoint[17,18] although a single meta-analysis of the risk of TIA and stroke was not conducted.
The objective of the present meta-analysis was to assess the available evidence of associations of Lp-PLA2 levels (mass and activity) with TIA and/or stroke-related recurrent vascular events and with the incidence of stroke in the general population, respectively.
2. Methods
This study was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement.[19] All analyses were based on previous published studies, thus no ethical approval and patient consent was required.
2.1. Search strategy
The following databases were used as online sources: MEDLINE, Embase, the Cochrane Library, Web of Science, Science Direct, China National Knowledge Infrastructure, China Biology Medical Disc (CBMdisc), and WanFang, and the time period included the initial inspection till January 2017. The languages of the studies investigated were restricted to English and Chinese. The search terms and their related combinations were the following: “lipoprotein-associated phospholipase A2” OR “Lp-PLA2” OR “Lp-PLA2 and platelet-activating factor acetylhydrolase” OR “PAF acetylhydrolase” AND “Stroke” OR “ischemic stroke” OR “hemorrhagic stroke” OR “cerebrovascular disease” OR “brain infarction” OR “intracranial vascular disorder” OR “apoplexy” OR “cerebrovascular insufficiency” OR “cerebrovascular accident” OR “transient ischemic attack” OR “TIA” OR “recurrent vascular event” OR “recurrent stroke.” The reference lists of all selected studies, conference abstracts, and reviews were further manually screened to supplement for eligible studies.
2.2. Study selection
Studies were considered eligible if they accorded with the following criteria: the present study was a prospective observational study. Participants with TIA/first ischemic stroke were classified into recurrent group and participants from general population were allocated to the stroke group. Blood levels of Lp-PLA2 mass and/or activity were measured. Adjusted hazard ratio (HR) and risk ratio (RR) with 95% confidence intervals (CIs) of recurrent vascular events and/or stroke were reported with a cutoff value, defined as the highest quantile vesus the bottom quantile and/or per 1 SD change in Lp-PLA2 levels. Reviews, editorials, case reports, correspondences, experimental studies, and studies with <50 participants were excluded. Studies that were published with similar population sizes were selected based on the most complete presentation of data and the date of publication.
2.3. Data extraction
Data from eligible studies were extracted by 2 independent reviewers (Ye Tian and Huan Jia) using the same standard extraction form. Certain discrepancies were resolved by discussion and/or consulting to a senior author (Bin Li). The variables extracted from eligible studies were the following: first author's name, publication date, original country, study design, sampling frame, number of participants, mean age and/or age range, endpoint event, measurement of Lp-PLA2, fully adjusted risk estimates and corresponding CIs, follow-up duration, and adjusted covariates. Some of the authors were contacted for information that was unclear and data that were unpublished. Stroke was defined as a focal neurological defect confirmed by physical examination and CT scan and/or magnetic resonance imaging (MRI), which lasted >24 hours with a rapid onset.[20] Recurrent vascular events included TIA, ischemic and/or hemorrhage stroke, myocardial infarction, and vascular death.
2.4. Quality assessment
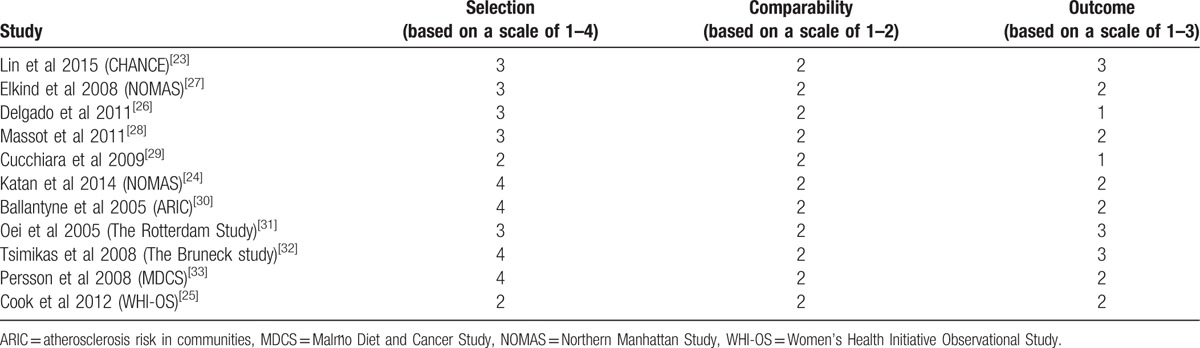
The methodological qualities of included studies were assessed according to the Newcastle-Ottawa Scale (NOS), which is commonly used to evaluate the quality of nonrandomized studies.[21] A total of 3 domains were judged in NOS, including the selection of the subjects, the comparability of groups, and the assessment of exposure and outcomes. Each study was evaluated using a scoring system of 0 to 9 stars, based on the aforementioned 3 domains. The studies that achieved 6 or more stars were regarded as high quality studies.
2.5. Statistical analyses
The present meta-analysis was conduced using STATA (version 12.0 College Station, TX; StataCorp LP). A P < .05 was considered statistically significant. The variables adjusted HR and RR with 95% CIs were further used to calculate the pooled risk estimates. In addition, pooled risk ratios were used as risk estimates due to the relatively low morbidity of the diseases investigated and the similarity in the interpretation of these parameters. The Cochran Q tests and I2 statistics were used to examine the heterogeneity between studies. A random-effects model was used if P was <.1 in Q tests and/or at I2 score of 50% or higher, which indicated larger heterogeneity across studies. A fixed-effects model was used for the remaining studies that deviated from these cutoff values.[22] Subgroup analyses were carried out to compare recurrent vascular events in TIA and/or primary ischemic stroke and the different types of Lp-PLA2 assays with the risk of stroke in the general population. Sensitivity analysis was carried out to identity the study responsible for the heterogeneity and/or to test the validity of the conclusions by omitting one study at sequentially. Begg rank correlation and Egger linear regression tests were used to screen for the potential publication bias. When the P value was >.05 in the combined Begg and Egger tests, the publication bias was regarded as absent.
3. Results
3.1. Literature search
A total number of 572 studies were identified via electronic database search. After omission of duplicated studies, the titles and abstracts of 338 studies were screened. A further assessment of 57 full-text articles and 16 studies was in alignment with the inclusion criteria. A total of 5 articles were further removed and manual examination of reference lists did not produce additional articles for evaluation. Eventually 11 studies[23–33] were included in the final analysis (Fig. 1).
Figure 1.
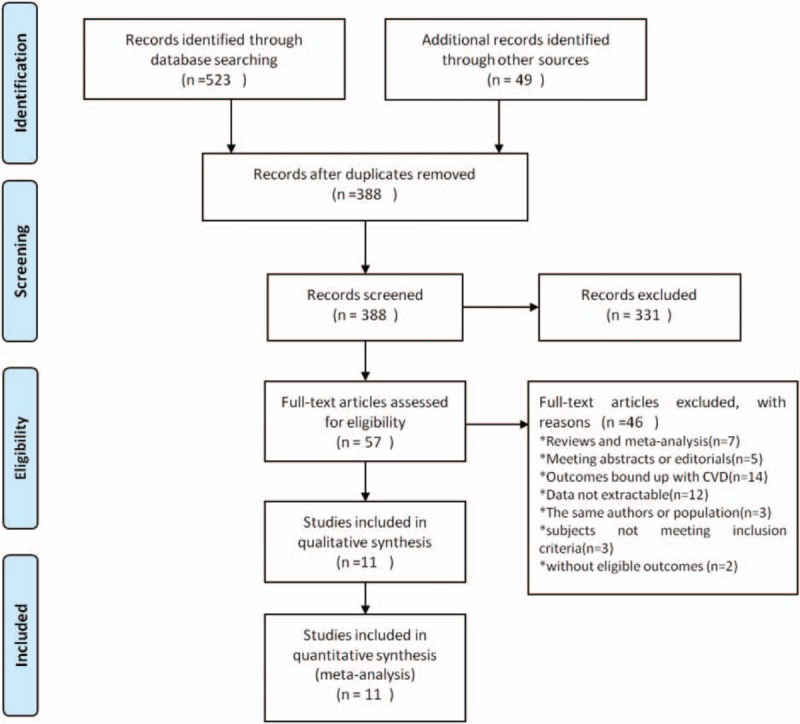
Flow diagram of study selection.
3.2. Characteristics of eligible studies and quality assessment
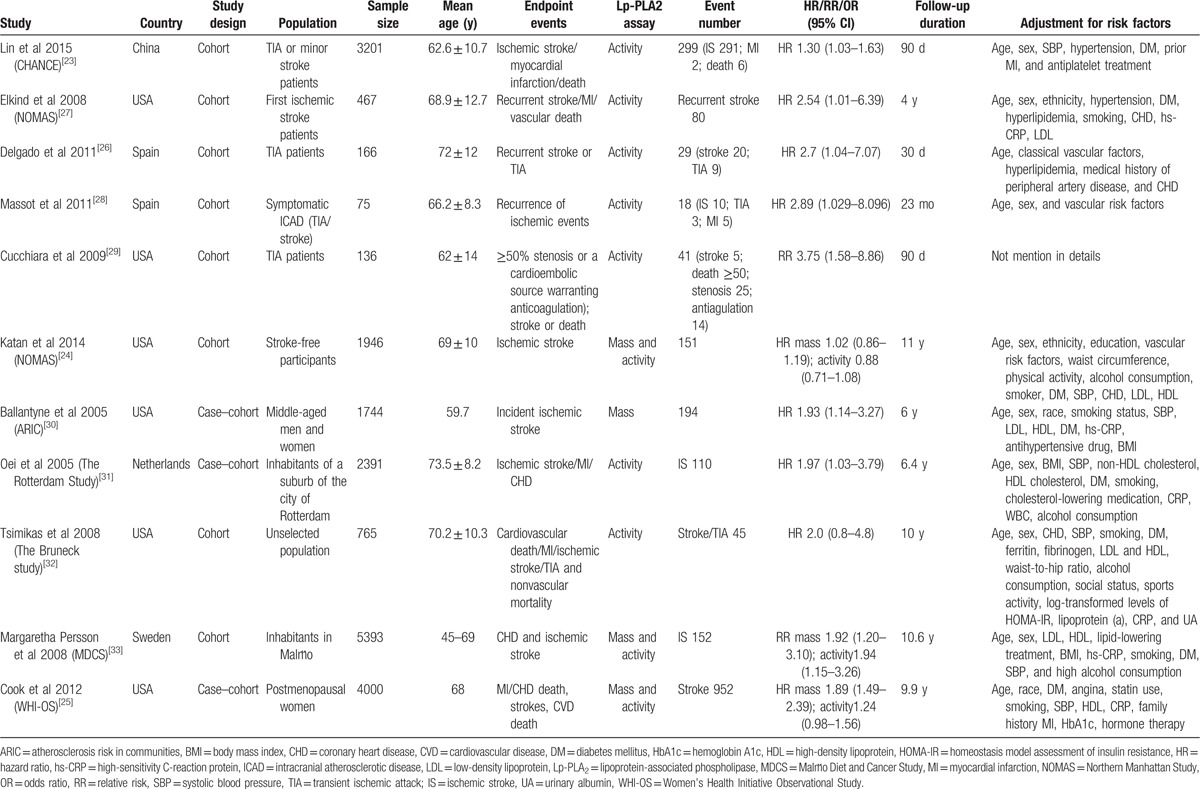
The characteristics of the eligible studies are summarized in Table 1. A total of 20,284 participants were included in 11 studies, of which 4,045 were TIA and/or first ischemic stroke patients reported in 5 studies[33,26–29] and 16,239 were residents in the general population reported in 6 studies.[24,25,30–33] Within all eligible studies, 3 were prospective case–cohort studies,[25,30,31] whereas the remaining 8 were prospective cohort studies.[23,24,26–29,32,33] The sample size ranged from 75 to 5393 and the follow-up duration period varied from 30 days to 11 years. A total of 4 studies reported Lp-PLA2 mass levels,[24,25,30,33] whereas 10 studies reported Lp-PLA2 activity levels.[23–29,31–33] A total of 3 studies included results of both Lp-PLA2 assays.[24,25,33] In addition, 2 studies further reported the results of the etiological classification of stroke.[24,26]
Table 1.
Characteristics of included studies in the meta-analysis.
The methodological qualities of the included studies are shown in Table 2 with an acceptable range of results. The qualities of all eligible studies included in the present analysis were between moderate to high and the score ranged from 5 to 9 stars according to the NOS.
Table 2.
Quality assessment of eligible studies.
3.3. Lp-PLA2 activity is associated with the risk of TIA and/or stroke-related recurrent vascular events
Patients with TIA and/or primary ischemic stroke at baseline were reported in 5 studies.[23,26–29] A total number of 467 recurrent vascular events that were related to TIA and/or stroke occurred in 4045 participants. The pooled RR of further adjustment was 2.24 (95% CI, 1.33–3.78, P = .002) with high heterogeneity (I2 = 60.2%, P = .039). Furthermore, a random-effects model was used (Fig. 2). Subgroup analysis was conducted to compare the recurrent vascular events in TIA patients with the incidence of stroke. A total of 3 studies[23,27,28] that included patients with stroke exhibited further adjustment of pooled RR of 1.78 (95% CI, 1.02–3.09, P = .042) with moderate score (I2 = 48.7%; P = .142) in a random-effects model (Fig. 3A). Patients with TIA were solely reported in 2 studies[26,29] (Fig. 3B) with a pooled RR of further adjustment of 3.24 (95% CI, 1.71–6.15, P < .001) and no evidence of heterogeneity (I2 = 0, P = .617).
Figure 2.
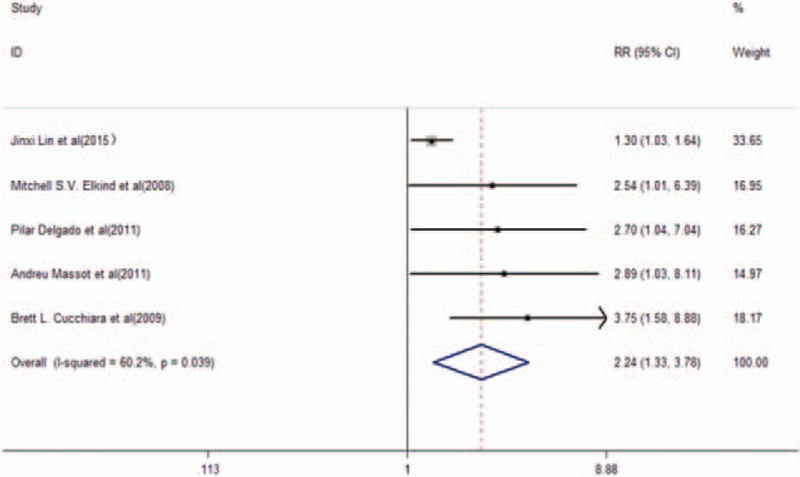
RR and 95% CI of Lp-PLA2 activity levels and recurrent vascular events in TIA/primary ischemic stroke patients in a random-effects model.
Figure 3.
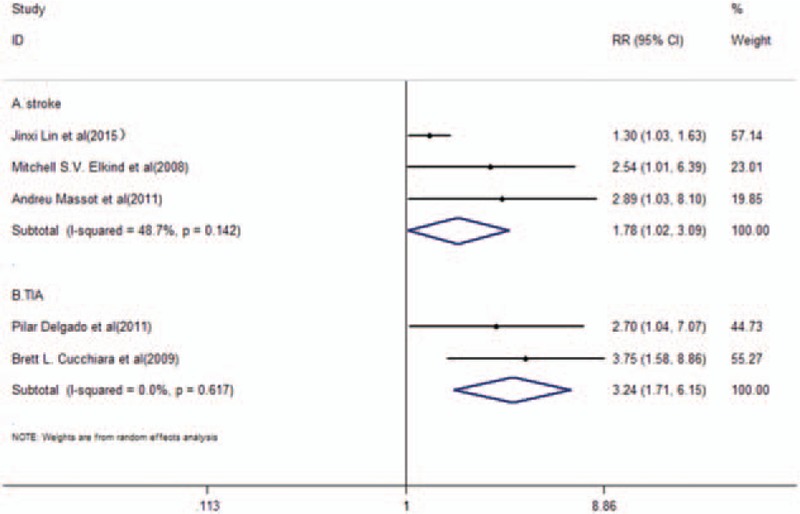
RR and 95% CI of Lp-PLA2 activity levels and recurrent vascular events in stroke patients (A) or TIA patients (B) in a random-effects model.
3.4. Lp-PLA2 levels were associated with the risk of stroke
Stroke was reported as an endpoint in the general population in 6 studies[24,25,30–33] that further included the determination of Lp-PLA2 mass and activity levels. A total of 16,239 participants were included in the meta-analysis, of which 1,604 were stroke cases. The pooled RR of further adjustment was 1.47 (95% CI, 1.10–1.97, P = .01) with high heterogeneity (I2 = 84.3%, P < .001) in a random-effects model (Fig. 4). Subgroup analysis was carried out based on the different assays (mass and/or activity levels) of Lp-PLA2. A total of 4 studies[24,25,30,33] that included reported Lp-PLA2 mass levels revealed pooled RR of further adjustment to 1.69 (95% CI, 1.03–2.79, P = .039) with high heterogeneity (I2 = 89.9%; P < .001) in a random-effects model (Fig. 5A). Lp-PLA2 activity levels were reported in 5 included studies of this group[24,25,31–33] (Fig. 5B). The pooled RR of further adjustment was 1.28 (95% CI, 0.88–1.85, P = .198) with high heterogeneity (I2 = 72.8%; P = .005) in a random-effects model (Fig. 5B).
Figure 4.
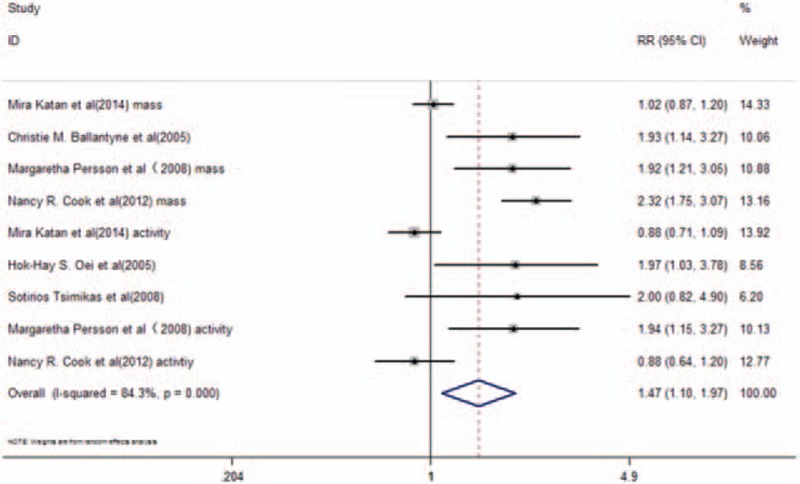
RR and 95% CI of Lp-PLA2 levels and the risk of stroke in the general population in a random-effects model.
Figure 5.
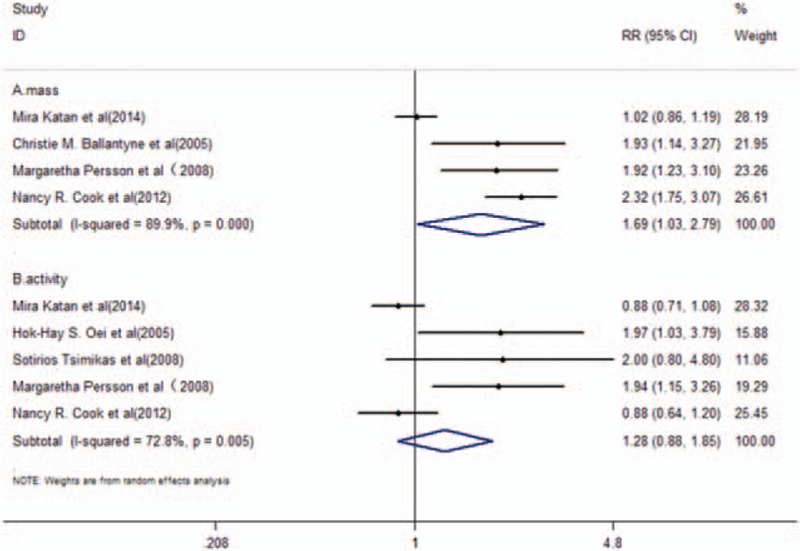
RR and 95% CI of Lp-PLA2 mass (A) or activity (B) levels and the risk of stroke in the general population in a random-effects model.
3.5. Sensitivity analyses and publication bias assessment
Significant heterogeneity was observed with a random-effects model. Sensitivity analysis was conducted by sequential omission of one study. The results of the sensitivity analyses demonstrated influences in the quantitative pooled estimates of RR and its 95% CI, and heterogeneity between the different studies examined. The heterogeneity decreased considerably (I2 = 0%, P = .934) for patients with TIA and/or stroke and the pooled adjusted RR was increased from 2.24 (95% CI, 1.33–3.78, P = .002) to 2.97 (95% CI, 1.86–4.75, P < .001) provided that one study[23] was excluded. The heterogeneity of patients from the general population was considerably altered (I2 = 0%, P = .714) between the studies that used the Lp-PLA2 mass assay determination after omission of one study.[24] Furthermore, the pooled adjusted RR changed from 1.69 (95% CI, 1.03–2.79, P = .039) to 2.15 (95% CI, 1.73–2.68, P < .001), whereas a minor change was noted between the studies that used the Lp-PLA2 activity assay determination.
No significant publication bias was noted within included studies of participants from the general population, as indicated by both Begg (P = .917) and Egger (P = .077) tests (Figs. 6 and 7).
Figure 6.
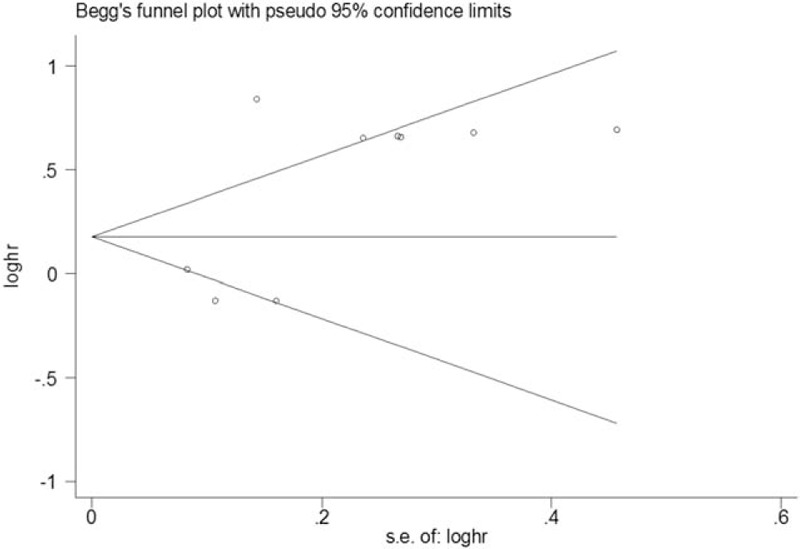
Begg funnel plot of studies in the general population.
Figure 7.
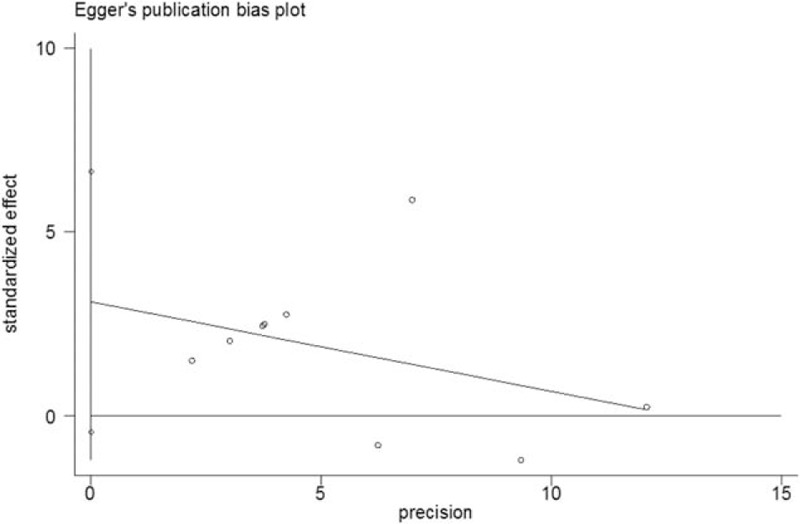
Egger publication bias of studies in the general population.
4. Discussion
The results of the present meta-analysis indicated that elevated blood levels of Lp-PLA2 activity were associated with increased risk of TIA/primary ischemic stroke-related recurrent vascular events. Higher blood mass levels of Lp-PLA2 were associated with the risk of stroke in the general population, whereas the association of blood Lp-PLA2 activity levels with stroke was less evident compared with that noted regarding Lp-PLA2 mass and the risk of stroke.
The findings of the present study indicated that patients with TIA and/or primary ischemic stroke who exhibited elevated blood levels of Lp-PLA2 activity were at higher risk of developing recurrent vascular events. A specific study[23] included in the analysis may be responsible for the heterogeneity in this group. This study was a prospective study focused on Asian subjects that measured Lp-PLA2 activity with an automated enzyme assay system on a Hitachi 7600 analyzer. The differences in the study design, race, and Lp-PLA2 activity measurement may directly influence the results. Moreover, Stafforini et al demonstrated that loss-of-function mutations in the PLA2G7 gene are common in East-Asian populations, which could effectively abolish Lp-PLA2 activity and/or largely reduce the activity levels in heterozygotes.[34] Subgroup analysis indicated that Lp-PLA2 activity levels in patients with TIA were associated with a higher risk of recurrent vascular events compared with patients with stroke. A study revealed higher expression of Lp-PLA2 and its products in carotid plaques from symptomatic patients with TIA and ischemic stroke compared with asymptomatic patients. It is interesting to note that in the symptomatic group TIA patients seemed to be responsible for the observed differences and showed the highest expression of Lp-PLA2.[35] The present study was notably based on a small number of studies. Consequently, the possibility of coincidence and the contribution of other confounding factors cannot be excluded and more studies are required to confirm the current findings.
The results of the current meta-analysis indicated that elevated blood levels of Lp-PLA2 mass were associated with the risk of stroke in the general population. A limited number of systematic reviews and meta-analyses supported the relationship between circulatory Lp-PLA2 levels and the risk of CVD. The majority of these studies included stroke in the list of combined endpoints.[16–18] A total of 2 main assays were used in the included studies to measure Lp-PLA2 levels, one corresponding to the determination of the mass levels and the other to the measurement of the activity levels. The correlations of these 2 assays varied in different studies, ranging from r = 0.36 in the PROVEIT trial[36] to r = 0.89 in a smaller study that included solely men,[37] which could possibly influence the results. Subgroup analysis was carried out to compare the different assays of Lp-PLA2 levels with the risk of stroke in the general population.
The relationship between elevated blood levels of Lp-PLA2 mass and the risk of stroke was observed in the present meta-analysis with evidence of heterogeneity. The results of the sensitivity analyses demonstrated that in the case of omitting one of the included studies, the heterogeneity would disappear. One possible explanation for this finding was the ethnic difference that influenced the results. The majority of the participants in the present study[24] were of Hispanic origin, whereas the populations examined in the majority of the other studies in this group were of white origin. Although Katan et al demonstrated that Lp-PLA2 mass levels were not associated with overall ischemic stroke, it was suggested that the corresponding levels were associated with the risk of atherosclerotic stroke among non-Hispanic white participants,[24] which partly supported the results of our findings.
Contrary to Lp-PLA2 mass levels, the elevated levels of Lp-PLA2 activity seemed not to significantly affect the incidence of stroke after further adjustment for risk factors. The present study supported the results of a former collaborative analysis,[16] which documented the association of Lp-PLA2 protein and activity levels with the levels of proatherogenic lipids after adjustment for conventional risk factors (RRs) that were 1.14 (95% CI, 1.02–1.27) and 1.08 (95% CI, 0.97–1.20) for ischemic stroke, respectively. Certain studies indicated that Lp-PLA2 activity levels exhibited a higher association with the expression of multiple lipid markers, namely, high-density lipoproteins (HDL) and LDL cholesterol compared with Lp-PLA2 protein levels.[25,37–39] This finding could indicate differences after various adjustments and differences in the measurement precision. Furthermore, the unreliable correction for regression dilution during long-term follow-up in prospective studies may underestimate risk associations.[40] In addition, in case of adjusted analyses, the specific information regarding certain potential confounding factors, such as medication for vascular diseases, was not uniformly obtained from included studies.[16] This discrepancy further affected the results obtained in the present analysis.
The majority of the included studies focused mainly on the relationship between Lp-PLA2 mass and/or activity levels and ischemic stroke. Clinical data suggested that the elevated levels of Lp-PLA2 mass and the higher levels of the corresponding activity were associated with the progression of atherosclerotic disease.[41] Certain human- and animal-based studies further highlighted an increase in the expression of Lp-PLA2 in atherosclerotic lesions and plasma, which were related to accelerated atherogenesis.[35,42,43] Although the most common cause of ischemic stroke is atherosclerosis, the causes of ischemic stroke are more heterogeneous compared with those noted in atherosclerotic heart disease. Subsequently, the effects of particular risk factors may be underestimated considering all types of ischemic stroke as one. One included study indicated that Lp-PLA2 mass levels were associated with the risk of atherosclerotic stroke among non-Hispanic white participants,[24] whereas other studies have shown that Lp-PLA2 activity levels were increased significantly when considering a large-artery atherosclerotic (LAA) etiology as the most likely mechanism for the TIA incidence.[26] It is tempting to speculate that due to the limited outcomes recorded, there is insufficient specific evidence regarding the relationship between Lp-PLA2 levels and the etiological classifications of ischemic stroke. Thus, further studies are required to provide more information on this topic.
The potential advantages and limitations of the current systematic review should be taken into consideration. Instead of mainly focusing on Lp-PLA2 levels and CVD as shown by previous meta-analyses,[16,17] we emphasized on the relationship between Lp-PLA2 mass and/or activity levels and the incidence of recurrent vascular events and/or primary stroke events, respectively. However, the limited number of included studies was not sufficient to eliminate a part of the heterogeneity observed. In addition, the restricted access to the full spectrum of data required for the study analysis was a significant limitation of the present study. Second, most of the Lp-PLA2 levels were measured at a single time point rather than conducting serial measurements, which could not correct for the regression dilution.[40] Third, studies included were not based on random controlled trails (RCTs), the power of the results might be decreased to some extent, and certain potential confounding factors could not uniformly be adjusted in individual studies, which might lead to an overestimation and/or underestimation of the risk. In addition, the study was based on published data and the publication bias might have a significant effect on the results interpretation. Consequently, unpublished data should also be included to reduce the effect of selective reporting. The aforementioned limitations should be addressed by larger well-designed studies focusing on the relationship between serial measurements of Lp-PLA2 levels and the risk of stroke subtypes. In addition, well-designed studies are required to be conducted that will explore the association between Lp-PLA2 levels and stroke with regard to the ethnic origin of the sample population, notably between Asian and European subjects.
The present meta-analysis suggested that blood Lp-PLA2 activity levels could potentially be used as a predictor of recurrent vascular events in patients with TIA or first ischemic stroke. Furthermore, Lp-PLA2 mass levels could be used for stroke risk stratification in the general population. Lp-PLA2 can be regarded as a therapeutic target in the prevention of stroke and random trails of potent pharmacological inhibitors should be conducted.
Elevated blood levels of Lp-PLA2 activity were associated with increased risk of recurrent vascular events in patients with TIA or primary ischemic stroke, whereas elevated Lp-PLA2 mass levels were related to the risk of stroke in the general population. The relationship between Lp-PLA2 activity levels and stroke was less profound compared with the relationship noted between the Lp-PLA2 mass levels and the risk of stroke. Further well-designed studies are required to update and confirm the findings presented in the current meta-analysis.
Footnotes
Abbreviations: Lp-PLA2 = lipoprotein-associated phospholipase A2, TIA = transient ischemic attack, LDL = low-density lipoproteins, HDL = high-density lipoproteins, CVD = cardiovascular diseases, PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-analyses, CBMdisc = China Biology Medical Disc, HR = hazard ratio, RR = risk ratio, CI = corresponding 95% confidence intervals, MRI = magnetic resonance imaging, NOS = Newcastle-Ottawa Scale, LAA = large-artery atherosclerotic, RCTs = random controlled trails.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Strong K, Mathers C, Bonita R. Preventing stroke: saving lives around the world. Lancet Neurol 2007;6:182–7. [DOI] [PubMed] [Google Scholar]
- [2].Bembenek JP, Karlinski M, Kurkowska-Jastrzebska I, et al. Changes in pre-hospital management of vascular risk factors among patients admitted due to recurrent stroke in Poland from 1995 to 2013. Arch Med Sci 2016;12:754–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Swarowska M, Burkot J, Janowska A, et al. Improvement of survival in Polish stroke patients is related to reduced stroke severity and better control of risk factors: the Krakow Stroke Database. Arch Med Sci 2016;12:552–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zanchetti A, Liu L, Mancia G, et al. Blood pressure and low-density lipoprotein-cholesterol lowering for prevention of strokes and cognitive decline: a review of available trial evidence. J Hypertens 2014;32:1741–50. [DOI] [PubMed] [Google Scholar]
- [5].Glasser SP, Mosher A, Howard G, et al. What is the association of lipid levels and incident stroke? Int J Cardiol 2016;220:890–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pawelczyk M, Kaczorowska B, Baj Z. The impact of hyperglycemia and hyperlipidemia on plasma P-selectin and platelet markers after ischemic stroke. Arch Med Sci 2017;13:1049–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ferretti G, Bacchetti T, Johnston TP, et al. Lipoprotein(a): a missing culprit in the management of athero-thrombosis? J Cell Physiol 2017;Jun 13. doi: 10.1002/jcp.26050. [DOI] [PubMed] [Google Scholar]
- [8].Shiber JR, Fontane E, Adewale A. Stroke registry: hemorrhagic vs ischemic strokes. Am J Emerg Med 2010;28:331–3. [DOI] [PubMed] [Google Scholar]
- [9].Corrado E, Rizzo M, Coppola G, et al. An update on the role of markers of inflammation in atherosclerosis. J Atheroscler Thromb 2010;17:1–1. [DOI] [PubMed] [Google Scholar]
- [10].Stafforini DM, Tjoelker LW, McCormick SP, et al. Molecular basis of the interaction between plasma platelet-activating factor acetylhydrolase and low density lipoprotein. J Biol Chem 1999;274:7018–24. [DOI] [PubMed] [Google Scholar]
- [11].Wilensky RL, Shi Y, Mohler ER, 3rd, et al. Inhibition of lipoprotein-associated phospholipase A2 reduces complex coronary atherosclerotic plaque development. Nat Med 2008;14:1059–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].MacPhee CH, Moores KE, Boyd HF, et al. Lipoprotein-associated phospholipase A2, platelet-activating factor acetylhydrolase, generates two bioactive products during the oxidation of low-density lipoprotein: use of a novel inhibitor. Biochem J 1999;338(Pt 2):479–87. [PMC free article] [PubMed] [Google Scholar]
- [13].Carpenter KL, Dennis IF, Challis IR, et al. Inhibition of lipoprotein-associated phospholipase A2 diminishes the death-inducing effects of oxidised LDL on human monocyte-macrophages. FEBS Lett 2001;505:357–63. [DOI] [PubMed] [Google Scholar]
- [14].Rosenson RS. Future role for selective phospholipase A2 inhibitors in the prevention of atherosclerotic cardiovascular disease. Cardiovasc Drugs Ther 2009;23:93–101. [DOI] [PubMed] [Google Scholar]
- [15].Gorelick PB. Lipoprotein-associated phospholipase A2 and risk of stroke. Am J Cardiol 2008;101(12a):34f–40f. [DOI] [PubMed] [Google Scholar]
- [16].Thompson A, Gao P, Orfei L, et al. Lipoprotein-associated phospholipase A(2) and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet 2010;375:1536–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Garza CA, Montori VM, McConnell JP, et al. Association between lipoprotein-associated phospholipase A2 and cardiovascular disease: a systematic review. Mayo Clin Proc 2007;82:159–65. [DOI] [PubMed] [Google Scholar]
- [18].Madjid M, Ali M, Willerson JT. Lipoprotein-associated phospholipase A2 as a novel risk marker for cardiovascular disease: a systematic review of the literature. Tex Heart Inst J 2010;37:25–39. [PMC free article] [PubMed] [Google Scholar]
- [19].Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. [DOI] [PubMed] [Google Scholar]
- [20].Iso H, Rexrode K, Hennekens CH, et al. Application of computer tomography-oriented criteria for stroke subtype classification in a prospective study. Ann Epidemiol 2000;10:81–7. [DOI] [PubMed] [Google Scholar]
- [21].Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [accessed February 27, 2017]. [Google Scholar]
- [22].Higgins JP, Thompson SG, Deeks JJ, et al. Measuring inconsistency in meta-analyses. BMJ 2003;327:557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lin J, Zheng H, Cucchiara BL, et al. Association of Lp-PLA2-A and early recurrence of vascular events after TIA and minor stroke. Neurology 2015;85:1585–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Katan M, Moon YP, Paik MC, et al. Lipoprotein-associated phospholipase A2 is associated with atherosclerotic stroke risk: the Northern Manhattan Study. PLoS One 2014;9:e83393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Cook NR, Paynter NP, Manson JE, et al. Clinical utility of lipoprotein-associated phospholipase A(2) for cardiovascular disease prediction in a multiethnic cohort of women. Clin Chem 2012;58:1352–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Delgado P, Chacon P, Penalba A, et al. Lipoprotein-associated phospholipase A(2) activity is associated with large-artery atherosclerotic etiology and recurrent stroke in TIA patients. Cerebrovasc Dis 2012;33:150–8. [DOI] [PubMed] [Google Scholar]
- [27].Elkind MS, Tai W, Coates K, et al. Lipoprotein-associated phospholipase A2 activity and risk of recurrent stroke. Cerebrovasc Dis 2009;27:42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Massot A, Pelegri D, Penalba A, et al. Lipoprotein-associated phospholipase A2 testing usefulness among patients with symptomatic intracranial atherosclerotic disease. Atherosclerosis 2011;218:181–7. [DOI] [PubMed] [Google Scholar]
- [29].Cucchiara BL, Messe SR, Sansing L, et al. Lipoprotein-associated phospholipase A2 and C-reactive protein for risk-stratification of patients with TIA. Stroke 2009;40:2332–6. [DOI] [PubMed] [Google Scholar]
- [30].Ballantyne CM, Hoogeveen RC, Bang H, et al. Lipoprotein-associated phospholipase A2, high-sensitivity C-reactive protein, and risk for incident ischemic stroke in middle-aged men and women in the Atherosclerosis Risk in Communities (ARIC) study. Arch Intern Med 2005;165:2479–84. [DOI] [PubMed] [Google Scholar]
- [31].Oei HH, van der Meer IM, Hofman A, et al. Lipoprotein-associated phospholipase A2 activity is associated with risk of coronary heart disease and ischemic stroke: the Rotterdam Study. Circulation 2005;111:570–5. [DOI] [PubMed] [Google Scholar]
- [32].Tsimikas S, Willeit J, Knoflach M, et al. Lipoprotein-associated phospholipase A2 activity, ferritin levels, metabolic syndrome, and 10-year cardiovascular and non-cardiovascular mortality: results from the Bruneck study. Eur Heart J 2009;30:107–15. [DOI] [PubMed] [Google Scholar]
- [33].Persson M, Berglund G, Nelson JJ, et al. Lp-PLA2 activity and mass are associated with increased incidence of ischemic stroke: a population-based cohort study from Malmo, Sweden. Atherosclerosis 2008;200:191–8. [DOI] [PubMed] [Google Scholar]
- [34].Stafforini DM, Satoh K, Atkinson DL, et al. Platelet-activating factor acetylhydrolase deficiency. A missense mutation near the active site of an anti-inflammatory phospholipase. J Clin Invest 1996;97:2784–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Mannheim D, Herrmann J, Versari D, et al. Enhanced expression of Lp-PLA2 and lysophosphatidylcholine in symptomatic carotid atherosclerotic plaques. Stroke 2008;39:1448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].O’Donoghue M, Morrow DA, Sabatine MS, et al. Lipoprotein-associated phospholipase A2 and its association with cardiovascular outcomes in patients with acute coronary syndromes in the PROVE IT-TIMI 22 (PRavastatin Or atorVastatin Evaluation and Infection Therapy-Thrombolysis In Myocardial Infarction) trial. Circulation 2006;113:1745–52. [DOI] [PubMed] [Google Scholar]
- [37].Caslake MJ, Packard CJ, Suckling KE, et al. Lipoprotein-associated phospholipase A(2), platelet-activating factor acetylhydrolase: a potential new risk factor for coronary artery disease. Atherosclerosis 2000;150:413–9. [DOI] [PubMed] [Google Scholar]
- [38].Khuseyinova N, Imhof A, Rothenbacher D, et al. Association between Lp-PLA2 and coronary artery disease: focus on its relationship with lipoproteins and markers of inflammation and hemostasis. Atherosclerosis 2005;182:181–8. [DOI] [PubMed] [Google Scholar]
- [39].Gazi I, Lourida ES, Filippatos T, et al. Lipoprotein-associated phospholipase A2 activity is a marker of small, dense LDL particles in human plasma. Clin Chem 2005;51:2264–73. [DOI] [PubMed] [Google Scholar]
- [40].Clarke R, Shipley M, Lewington S, et al. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol 1999;150:341–53. [DOI] [PubMed] [Google Scholar]
- [41].Serruys PW, Garcia-Garcia HM, Buszman P, et al. Effects of the direct lipoprotein-associated phospholipase A(2) inhibitor darapladib on human coronary atherosclerotic plaque. Circulation 2008;118:1172–82. [DOI] [PubMed] [Google Scholar]
- [42].Hakkinen T, Luoma JS, Hiltunen MO, et al. Lipoprotein-associated phospholipase A(2), platelet-activating factor acetylhydrolase, is expressed by macrophages in human and rabbit atherosclerotic lesions. Arterioscler Thromb Vasc Biol 1999;19:2909–17. [DOI] [PubMed] [Google Scholar]
- [43].Singh U, Zhong S, Xiong M, et al. Increased plasma non-esterified fatty acids and platelet-activating factor acetylhydrolase are associated with susceptibility to atherosclerosis in mice. Clin Sci (Lond) 2004;106:421–32. [DOI] [PubMed] [Google Scholar]


