Abstract
Purpose:
A possible association between metformin use and the development of prostate cancer (PCa) has been reported. However, there is limited information on the impact of long-term metformin use on serum prostate-specific antigen (PSA) levels. We investigated the association between exposure to metformin and PSA levels among diabetic patients who were not previously diagnosed with PCa.
Methods:
The analytic sample consisted of 1363 US men aged above 40 in the National Health and Nutrition Examination Survey 2007 to 2008 cycle. Men who had previous diagnoses of PCa or prostatitis and men exposed to manipulations that might have affected PSA levels were excluded. Multivariate logistic regression analyses were used to evaluate the association between PSA levels and metformin use by adjusting for potential confounders.
Results:
The mean PSA level of the overall population was 1.8 (standard deviation = 3.1) ng/mL. There were no differences in PSA levels according to the presence of diabetes (P = .517). Among patients with diabetes, metformin users exhibited significantly lower PSA levels compared with nonmetformin users (odds ratio = 0.790; 95% confidence interval 0.666–0.938; P = .007). However, no significant difference was found in PSA levels among men over duration of metformin use when levels were stratified by either 1 year or 5 years by Pearson's coefficient.
Conclusion:
A negative association between serum PSA levels and metformin use was observed in patients with diabetes. Duration of metformin use did not influence PSA levels. Further studies are warranted to elucidate whether the reduction in PSA level with metformin truly reflects reduced risk of PCa development and progression.
Keywords: diabetes mellitus, metformin, prostate cancer, prostate-specific antigen
1. Introduction
Prostate cancer (PCa) is the most commonly diagnosed noncutaneous malignancy and the second leading cause of cancer death among men in the United States.[1] Diabetes mellitus has been suggested to increase the risk of several malignancies. However, decreased risk of PCa has been seen in patients with diabetes, presumably secondary to a hyperinsulinemic state, which decreases testosterone levels.[2–4] Another feasible explanation is lower serum prostate-specific antigen (PSA) levels among diabetic men, as this would lead to less PCa detected by PSA elevation-driven biopsies and ultimately delayed diagnosis.
Biguanide metformin, the first-line therapy of choice for diabetes, has been suggested to be useful for preventing and treating various cancer types through direct and indirect mechanisms.[5–6] Metformin, an activator of adenosine monophosphate-activated protein kinase, exerts its antineoplastic effects by inhibiting the mammalian target of rapamycin and, thus, decreasing cell growth.[5–6] In PCa, metformin reduces the hyperinsulinemic state, which may indirectly reduce the risk of PCa development and progression.[7]
Existing studies have evaluated the role of metformin in PCa.[8] However, results from epidemiological studies are conflicting, and there is no definite conclusion on the exact pharmacological role of metformin underlying PCa development. Several studies have reported a negative association between PCa and serum PSA, the most widely used biomarker for PCa risk and progression.[9] However, it is still not clear whether diabetes exerts a protective role against PCa or whether long-term use of metformin itself is related to decreased PSA levels.
We investigated serum PSA levels according to the presence of diabetes, metformin use, and the duration of metformin use in patients with diabetes who have not been previously diagnosed with PCa. To our knowledge, this is the first study to utilize the National Health and Nutrition Examination Survey (NHANES) 2007 to 2008, a large, cross-sectional dataset that is representative of the US population.
2. Materials and methods
2.1. Study population
NHANES is a cross-sectional observational study that collects health-related information using a multistage probability design representative of the general noninstitutionalized US population. The institutional review board of the National Center for Health Statistics approved the protocol for NHANES, and informed consent was obtained. This study included data from male participants aged 40 and older in the 2007 to 2008 cycle.
2.2. Measurement and classification of variables
Serum PSA concentration was measured in men 40 years of age and older who had never been diagnosed with PCa or prostatitis. Patients who received prostate manipulation, such as prostate biopsy, surgery, or cystoscopy within 1 month, and rectal examination within 1 week, were excluded from analysis. Serum PSA level was measured using the Hybritech method on Beckman Access (Fullerton, CA). Serum total cholesterol (TC) concentration was measured enzymatically. All data were retrieved and utilized from NHANES website (https://wwwn.cdc.gov/nchs/nhanes).
Data for body mass index (BMI) and blood pressure (BP) were obtained from the examination component of the NHANES. BMI was calculated as weight in kilograms divided by height in meters squared. BP was measured using a mercury sphygmomanometer.[10]
Information on the prescribed medication was collected using self-reported household interviews. Metformin use and duration of use were obtained using standardized generic prescription drug codes.
2.3. Statistical analysis
Data are reported as means (standard deviation) for continuous variables and as percentages for categorical variables. For univariate analysis, the t-test was used to compare continuous variables. Multivariate analysis used multivariate models of logistic regression including all risk factors that were significantly associated in the univariate analysis. Determination for the presence of statistically significant correlative relationships was conducted by using Pearson's coefficients (R).
SPSS software version 23.0 (SPSS Inc., Chicago, IL) was used for statistical analyses, and all statistical tests were 2-tailed. A P value of <.05 was considered statistically significant.
3. Results
3.1. Characteristics of the study population
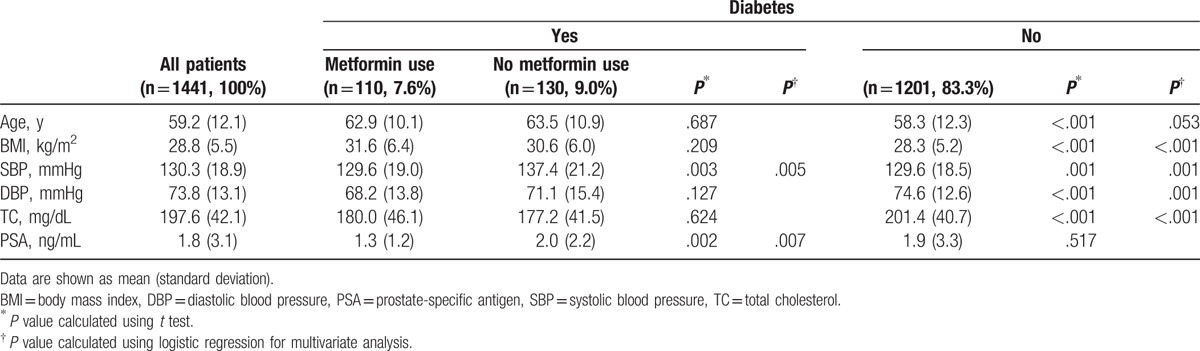
Baseline characteristics of the study population are shown in Table 1. Among 1441 participants, 240 (16.7%) patients had diabetes. Among these patients, 110 (45.8%) patients were on metformin. BMI, systolic blood pressure (SBP), and TC were significantly higher in patients with diabetes both in univariate and multivariate analyses. However, diastolic blood pressure (DBP) was significantly higher in the nondiabetic group.
Table 1.
Characteristics of the study population.
3.2. Serum PSA levels
There were no significant differences in serum PSA levels between the diabetic group and the nondiabetic group (P = .517). Among patients with diabetes, patients on metformin had significantly lower PSA levels (P = .007) and SBP (P = .005) than those who were not on metformin (Table 1). The PSA level of patients with diabetes on metformin was on average 34% lower than that of patients with diabetes and not on metformin.
PSA levels were also compared according to the duration of metformin use. There were no differences in PSA levels by duration of metformin use when stratified by either 1 year (Table 2) or 5 years (Table 3). Two-way scatter plot evaluation for PSA levels and duration of metformin use showed weak negative relationship (Fig. 1), with Pearson's correlation coefficient of −0.119 which was not significant (P = .217).
Table 2.
Characteristics of patients with diabetes according to duration of metformin use by 1 year.
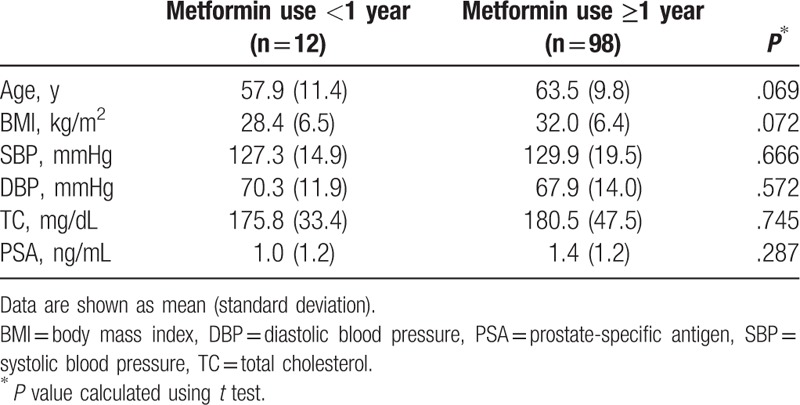
Table 3.
Characteristics of patients with diabetes according to duration of metformin use by 5 years.
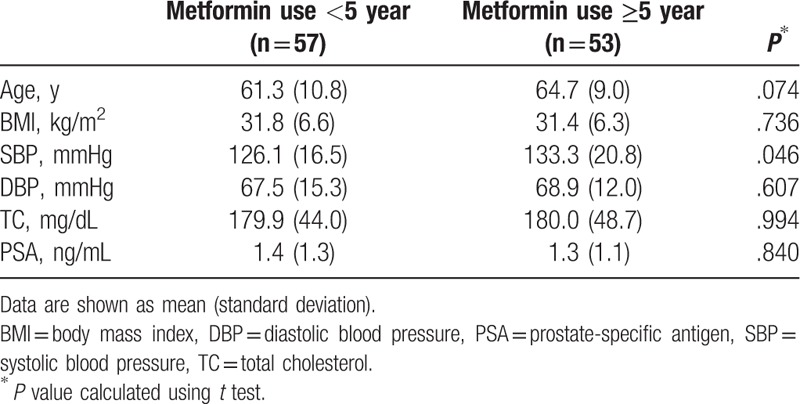
Figure 1.
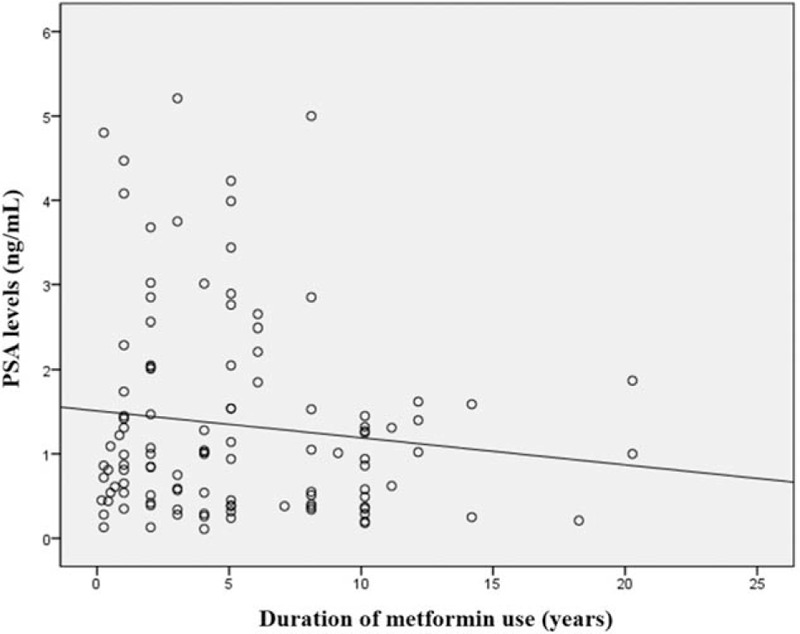
Pearson correlation scatter plot of PSA levels and duration of metformin use. PSA = prostate-specific antigen.
4. Discussion
It is unclear whether diabetes itself exerts a protective role against PCa or whether the use of metformin is related to decreased PSA levels. After adjusting for confounders, PSA level was significantly and independently associated with metformin use in diabetic men, and diabetes itself was not a factor for lower serum PSA levels. Moreover, the duration of metformin use did not influence PSA levels. To the best of our knowledge, this is the first study to utilize the NHANES registry to compare differences in PSA levels according to metformin use and duration of use in patients who have not been previously diagnosed with PCa.
Our results compare favorably with several studies in the literature that compared serum PSA levels in diabetic men. Haring et al evaluated PCa incidence among users of metformin and other antidiabetic drugs in the Finnish Randomized Study of Screening for PCa.[11] In their study, patients on antidiabetic drugs exhibited lower PSA levels compared to nonusers.[11] However, the effect of metformin on PSA levels was not separately evaluated. Moreover, patients on drugs that may affect PSA levels, namely 5α-reductase inhibitors or nonsteroidal or anti-inflammatory drugs were not excluded from analysis. Jayalath et al[9] reported a relationship between metformin use and serum PSA levels in which mean PSA levels were 30% lower in metformin users, similar to our observation. In addition, an inverse dose–response relationship was observed. In vitro studies have reported that metformin treatment reduces PSA gene expression and cancer cell viability, a possible explanation of our results.[12–15] To date, no existing literature has reported that metformin increases PSA levels, which would contradict our observation.
A noteworthy finding in our study was that there was no significant difference in PSA levels between patients with and without diabetes. On the contrary, several studies have reported an inverse relationship between diabetes and overall risk of PCa.[16–17] A general hypothesis is that the hyperinsulinemic state of diabetes decreases testosterone levels, which may reduce the risk of PCa.[2–4] However, not all study results are consistent, and studies have reported a high prevalence of high-grade cancers in diabetic men.[18–19] Although the pathophysiology is incompletely understood, a possible explanation is that insufficient testosterone affects the prostate proliferation cycle and promotes abnormal epithelial differentiation, stimulating cancer cells to dedifferentiate to more aggressive tumors.[20]
In contrast, studies have reported that diabetes may increase the risks of several cancers such as pancreatic cancer, liver cancer, breast cancer, and colorectal cancer.[21] This result could be explained by reduced insulin sensitivity with compensatory hyperinsulinemia and elevated levels of insulin-like growth factors, which may in turn stimulate cell proliferation in the liver, pancreas, colon, ovary, breast, and prostate.[21] These 2 hypotheses are opposing, and evidence is sparse and inconsistent. Existing epidemiological studies are also controversial. Bansal et al[22] reported that diabetes is associated with a reduced risk of PCa. Conversely, multiple studies including a meta-analysis strongly support that diabetes is associated with an increased risk of PCa in Asian men.[23,24] The retrospective nature of these studies and the immortal time bias along with incomplete adjustments for confounders support the need of a larger, prospective study to arrive at a definite conclusion.
There has been considerable debate regarding whether the use of metformin use is significantly associated with reduced risk of PCa development. Several meta-analyses demonstrated that metformin use is associated with decreased incidence of PCa.[25–26] Although our study did not evaluate the incidence of PCa due to a limited number of events, we inferred that low PSA levels may potentially lead to less PSA-driven prostate biopsies, eventually leading to a lower rate of PCa diagnosis. This detection bias may have influenced the results of other studies that reported a lower incidence of PCa in metformin use groups.
Obesity induces a state of chronic inflammation and insulin resistance, which may finally lead to diabetes.[27] Our results confirmed that BMI is significantly higher in patients with diabetes. Furthermore, our results showed that patients with diabetes had significantly higher SBP but lower DBP compared with patients without diabetes. It is generally reported that patients with diabetes have a greater incidence of cardiovascular disease, cerebrovascular disease, and renal disease than the general population.[28] Contrary to common findings, our results showed that DBP was significantly lower in patients with diabetes. Further study on the difference between SBP and DBP in patients with cardiovascular disease would be illuminating.
Several studies reported that increased metformin use duration was associated with decreased incidence of PCa. Preston et al reported that metformin use of less than 1.5 years was not associated with a risk reduction, but metformin use of more than or equal to 3 years was associated with a risk reduction compared with nonusers.[29] Although our study did not evaluate the association between PCa incidence and duration of metformin use, we presumed that use of metformin for at least 1 year may have decreased PSA levels to a minimum level. Future studies should evaluate whether the prolonged effect of low PSA level might eventually decrease the incidence of PCa.
This study has several limitations. First, due to the limitations of its cross-sectional design, a causal relationship cannot be established. Second, our study endpoint was confined to serum PSA level, which is an imprecise proxy for PCa risk. Moreover, the association between metformin use and diagnosis of PCa was not evaluated due to a limited number of events in our population. Third, type I and type II diabetes were not distinguished and separately analyzed. Some studies reported that type I diabetes, which accounts for 5% to 10% of all diagnosed diabetes,[30] was not associated with increased risk of cancer.[31–32] Therefore, the association between PSA levels in patients with and without diabetes might have been underestimated.
Our study showed a significant association between metformin use and PSA levels in patients with diabetes that had not been previously diagnosed with PCa. Since duration of metformin use did not significantly influence PSA levels, we infer that after a minimal use of 1 year, PSA levels do not significantly decrease with metformin use. Moreover, we observed no difference in PSA levels between patients with and without diabetes. Future studies confirming the exact role of metformin in the prostate microenvironment are warranted to confirm the feasibility of metformin use for preventing and treating PCa.
5. Conclusion
A negative association between serum PSA levels and metformin use was observed in patients with diabetes. Metformin use of more than 1 year in duration did not influence PSA levels. Further studies are warranted to elucidate whether the reduced PSA level with metformin truly reflects reduced risk of disease development and progression.
Footnotes
Abbreviations: BMI = body mass index, BP = blood pressure, DBP = diastolic blood pressure, NHANES = National Health and Nutrition Examination Survey, PCa = prostate cancer, PSA = prostate-specific antigen, SBP = systolic blood pressure, TC = total cholesterol.
Compliance with ethical standards.
Ethical approval: all procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
This study was supported by the Young Researcher Program Grant from the National Research Foundation of Korea (2017R1C1B5017513).
The authors have no conflicts of interest to disclose.
References
- [1].Center MM, Jemal A, Lortet-Tieulent J, et al. International variation in prostate cancer incidence and mortality rates. Eur Urol 2012;61:1079–92. [DOI] [PubMed] [Google Scholar]
- [2].Tsilidis KK, Kasimis JC, Lopez DS, et al. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ 2015;350:g7607. [DOI] [PubMed] [Google Scholar]
- [3].Waters KM, Henderson BE, Stram DO, et al. Association of diabetes with prostate cancer risk in the multiethnic cohort. Am J Epidemiol 2009;169:937–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rastmanesh R, Hejazi J, Marotta F, et al. Type 2 diabetes: a protective factor for prostate cancer? An overview of proposed mechanisms. Clin Genitourin Cancer 2014;12:143–8. [DOI] [PubMed] [Google Scholar]
- [5].Brunmair B, Staniek K, Gras F, et al. Thiazolidinediones, like metformin, inhibit respiratory complex I: a common mechanism contributing to their antidiabetic actions? Diabetes 2004;53:1052–9. [DOI] [PubMed] [Google Scholar]
- [6].Zakikhani M, Dowling R, Fantus IG, et al. Metformin is an AMP kinase-dependent growth inhibitor for breast cancer cells. Cancer Res 2006;66:10269–73. [DOI] [PubMed] [Google Scholar]
- [7].Clements A, Gao B, Yeap SH, et al. Metformin in prostate cancer: two for the price of one. Ann Oncol 2011;22:2556–60. [DOI] [PubMed] [Google Scholar]
- [8].Hankinson SJ, Mina F, Patel NN. A review for clinicians: prostate cancer and the antineoplastic properties of metformin. Urol Oncol 2017;35:21–9. [DOI] [PubMed] [Google Scholar]
- [9].Jayalath VH, Ireland C, Fleshner NE, et al. The relationship between metformin and serum prostate-specific antigen levels. Prostate 2016;76:1445–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].National Center of Health Statistics. National Health and Nutrition Examination Survey examination files. 2002. Available from: http://www.cdc.gov/nchs/nhanes/nhanes2001-2002/current_nhanes_01_02.htm [Accessed on April 7, 2017]. [Google Scholar]
- [11].Haring A, Murtola TJ, Talala K, et al. Antidiabetic drug use and prostate cancer risk in the Finnish randomized study of screening for prostate cancer. Scand J Urol 2017;51:5–12. [DOI] [PubMed] [Google Scholar]
- [12].Besla R, Venier N, Coloquhoun A, et al. Dutasteride and metformin reduce the growth of LNCaP cells and alter the SREBP-1 pathway. Open Prostate Cancer J 2013;6:10–5. [Google Scholar]
- [13].Colquhoun AJ, Venier NA, Vandersluis AD, et al. Metformin enhances the antiproliferative and apoptotic effect of bicalutamide in prostate cancer. Prostate Cancer Prostatic Dis 2012;15:346–52. [DOI] [PubMed] [Google Scholar]
- [14].Lee SY, Song CH, Xie YB, et al. SMILE upregulated by metformin inhibits the function of androgen receptor in prostate cancer cells. Cancer Lett 2014;354:390–7. [DOI] [PubMed] [Google Scholar]
- [15].Wang Y, Liu G, Tong D, et al. Metformin represses androgen-dependent and androgen-independent prostate cancers by targeting androgen receptor. Prostate 2015;75:1187–96. [DOI] [PubMed] [Google Scholar]
- [16].Bonovas S, Filioussi K, Tsantes A. Diabetes mellitus and risk of prostate cancer: a meta-analysis. Diabetologia 2004;47:1071–8. [DOI] [PubMed] [Google Scholar]
- [17].Kasper JS, Giovannucci E. A meta-analysis of diabetes mellitus and the risk of prostate cancer. Cancer Epidemiol Biomarkers Prev 2006;15:2056–62. [DOI] [PubMed] [Google Scholar]
- [18].Moreira DM, Anderson T, Gerber L, et al. The association of diabetes mellitus and high-grade prostate cancer in a multiethnic biopsy series. Cancer Causes Control 2011;22:977–83. [DOI] [PubMed] [Google Scholar]
- [19].Li Q, Kuriyama S, Kakizaki M, et al. History of diabetes mellitus and the risk of prostate cancer: the Ohsaki Cohort Study. Cancer Causes Control 2010;21:1025–32. [DOI] [PubMed] [Google Scholar]
- [20].García-Cruz E, Piqueras M, Huguet J, et al. Low testosterone levels are related to poor prognosis factors in men with prostate cancer prior to treatment. BJU Int 2012;110:E541–6. [DOI] [PubMed] [Google Scholar]
- [21].Klil-Drori AJ, Azoulay L, Pollak MN. Cancer, obesity, diabetes, and antidiabetic drugs: is the fog clearing? Nat Rev Clin Oncol 2017;14:85–99. [DOI] [PubMed] [Google Scholar]
- [22].Bansal D, Bhansali A, Kapil G, et al. Type 2 diabetes and risk of prostate cancer: a meta-analysis of observational studies. Prostate Cancer Prostat Dis 2013;16:151–8. [DOI] [PubMed] [Google Scholar]
- [23].Tseng CH. Diabetes and risk of prostate cancer: a study using the National Health Insurance. Diabetes Care 2011;34:616–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Long XJ, Lin S, Sun YN, et al. Diabetes mellitus and prostate cancer risk in Asian countries: a meta-analysis. Asian Pac J Cancer Prev 2012;13:4097–100. [DOI] [PubMed] [Google Scholar]
- [25].Yu H, Yin L, Jiang X, et al. Effect of metformin on cancer risk and treatment outcome of prostate cancer: a meta-analysis of epidemiological observational studies. PLoS One 2014;9:e116327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Deng D, Yang Y, Tang X, et al. Association between metformin therapy and incidence, recurrence and mortality of prostate cancer: evidence from a meta-analysis. Diabetes Metab Res Rev 2015;31:595–602. [DOI] [PubMed] [Google Scholar]
- [27].Bapat SP, Myoung Suh J, Fang S, et al. Depletion of fat-resident Treg cells prevents age-associated insulin resistance. Nature 2015;528:137–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Adler AI, Stratton IM, Neil HA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ 2000;321:412–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Preston MA, Riis AH, Ehrenstein V, et al. Metformin use and prostate cancer risk. Eur Urol 2014;66:1012–20. [DOI] [PubMed] [Google Scholar]
- [30].Engelgau MM, Geiss LS, Saaddine JB, et al. The evolving diabetes burden in the United States. Ann Intern Med 2004;140:945–50. [DOI] [PubMed] [Google Scholar]
- [31].Wideroff L, Gridley G, Mellemkjaer L, et al. Cancer incidence in a population-based cohort of patients hospitalized with diabetes mellitus in Denmark. J Natl Cancer Inst 1997;89:1360–5. [DOI] [PubMed] [Google Scholar]
- [32].Zendehdel K, Nyren O, Ostenson CG, et al. Cancer incidence in patients with type 1 diabetes mellitus: a population-based cohort study in Sweden. J Natl Cancer Inst 2003;95:1797–800. [DOI] [PubMed] [Google Scholar]


