Abstract
Background:
The multidrug resistance gene 1(MDR1) C3435T polymorphism has been reported to be associated with colorectal cancer (CRC) risk in Asians, however the results were inconsistent. Thus, we performed a meta-analysis to generate large-scale evidence on the association between C3435T polymorphism and CRC risk in Asian populations.
Methods:
The PubMed, Web of Science, Embase, CNKI, and Chinese Biomedicine databases were searched up to January 15, 2017. The odd ratios (ORs) and 95% confidence intervals (95% CIs) were calculated by a fixed-effects or random-effects model. Sensitivity and cumulative meta-analysis were also performed.
Results:
A total of 7 studies involving 4818 individuals were included in this pooled-analysis. The results suggested that persons carrying a T allele at the C3435T polymorphism had a significantly decreased risk of CRC in Asian population (T vs C: OR = 0.897, 95%CI = 0.826–0.975, P = .01), and the significant association was also observed in another 2 genetic models (TT vs CC: OR = 0.721, 95%CI = 0.605–0.861, P < .001; TT vs TC+CC: OR = 0.679, 95%CI = 0.579–0.795, P < .001). Moreover, the results of sensitivity and cumulative meta-analysis indicated the stable of our results. Finally, funnel plot and Egger's test showed no evidence of publication bias.
Conclusions:
In summary, this meta-analysis provided evidence that MDR1 C3435T polymorphism is associated with a decreased risk of CRC in Asian population.
Keywords: colorectal cancer, MDR1, meta-analysis, polymorphism
1. Introduction
Colorectal cancer (CRC) is one of the most common cancer in worldwide.[1,2] In 2015, colorectal cancer leaded to 753,000 deaths worldwide and was the fourth leading cause of cancer mortality.[2] Previous epidemiological studies have shown that genetic susceptibility factors together with environmental factors—in particular, diet, cigarette smoke, drugs, and bacterial toxins—might increase the risk of colorectal cancer.[3–6] However, the exact mechanisms underlying the development of this malignant digestive tumor remain unclear.
The multidrug resistance gene 1 (MDR1, also named as ATP-binding cassette sub-family B member 1, ABCB1) encodes a 170Kd ATP transmembrane glycoprotein, P-glycoprotein (P-gp).[7] Recent studies indicated MDR1 gene seemed to play an important role in tumor progression, especially in the carcinogenesis of colorectal.[8–10] In 2005, Kurzawski et al[11] reported the first study that demonstrated carriers of MDR1 3435TT genotype were at 2.7-fold higher risk of the colon cancer development in Polish. Following the first report of the association, a growing number of studies focused on the association between MDR1 C3435T polymorphism and colorectal cancer risk in Asian population, however, these genetic studies have produced inconclusive results.[12–14] These contradictions might be due the small sample sizes and inadequate statistical power.
A meta-analysis is a proper method to overcome the problem of small sample sizes and inadequate statistical power in different genetic studies. A recent meta-analysis suggested that there were no significant associations between MDR1 C3435T and colorectal cancer risk in Caucasians.[15] However, to date, there was no meta-analysis which investigated the association between MDR1 C3435T polymorphism and colorectal cancer risk in Asians. Thus, we performed a meta-analysis to clarify the effect of C3435T polymorphism on susceptibility to colorectal cancer, by systematically summarizing all eligible data in Asian population.
2. Methods
2.1. Search strategy
A comprehensive electronic search in PubMed, Embase, Medline, Web of Science database was performed to identify the association between MDR1 C3435T variant and colorectal cancer by using the following search terms: “multidrug resistance 1 gene” or “ABCB1” or “MDR1” or “rs1045642,” “polymorphism” or “variant,” and “colorectal cancer” (the last update was January 15, 2017). Moreover, the references of all retrieved articles were checked by hand-search for additional potential studies.
2.2. Inclusion and exclusion criteria
The following inclusion criteria were used to select eligible studies for the meta-analysis: case-control studies; population of ethnic descent was Asians; reporting the association between MDR1 C3435T polymorphism and colorectal cancer risk. Exclusion criteria for studies were as follows: no control population; incomplete genotype data; duplicate publications; comments, review articles, or articles only with an abstract.
2.3. Data extraction
Two independent reviewers extracted the following data from each eligibly study: name of first author, country of origin, publication year, sex ratio, and mean age in individuals, and number of genotypes or allele frequency in cases and controls. Finally, any disagreement was resolved by discussion or through a third investigator. Moreover, this study was based on previously published studies; thus, no ethical approval was required.
2.4. Statistical methods
The odds ratio (OR), and its 95% confidence interval (CI) was estimated for assess the strength of the association between MDR1 C3435T and colorectal cancer risk. The significance of the pooled OR was determined by the Z-test; a P value of .05 was considered significant. We examined pooled OR for T versus C, TT versus CC, TC versus CC, the dominant model (TT+TC vs CC), as well as the recessive model (TT vs TC+CC). Moreover, data in the control group of each study were used to assess the Hardy–Weinberg equilibrium (HWE), and a P < .05 was considered as disequilibrium.
The heterogeneity between studies was evaluated by chi-squared based Q test and I2 test.[16] When a P value was <.5, obvious heterogeneity exists and a random effects model was used. Otherwise, a fixed effects model was used to calculate pooled effect estimates.[17,18]I2 takes values between 0% and 100% with higher values denoting a greater degree of heterogeneity.[19]
Cumulative meta-analysis was carried out to evaluate the trend and the stability of the genetic risk effect as evidence accumulating over time. Additionally, we also performed sensitivity analysis by sequential removal of each study to assess the stability of the results.[20] Publication bias was assessed by the symmetry of the funnel plot, which was further evaluated by Egger linear regression test.[21] A P value of <.05 from the Egger test was considered significant publication bias. All statistical analyses were performed by using STATA software, version 12 (StataCorp LP, College Station, TX).
3. Results
3.1. Characteristics of the eligible studies
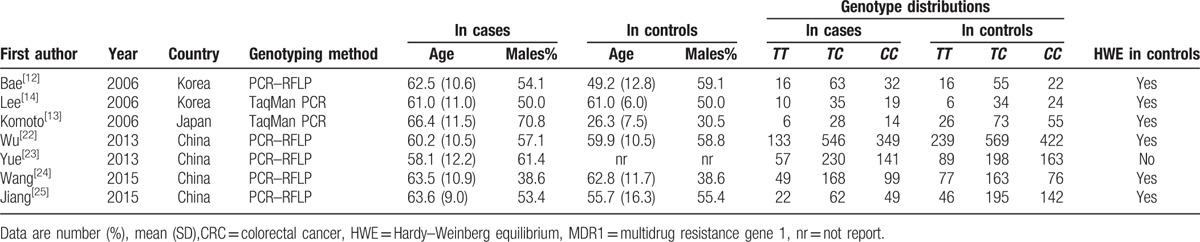
A total of 99 studies involving the relationship between the MDR1 C3435T polymorphism and colorectal cancer risk met the search criteria. After a preliminary screening, full texts of 32 studies were reviewed in detail. Of which, 14 reported in Caucasian population, 6 were reviews, 2 were about cell studies, 2 investigated the prognosis of CRC, and 1 reported other disease. Finally, a total of 7 articles comprising 4818 individuals (2128 patients and 2690 controls) were included in this meta-analysis.[12–14,22–25] The distribution of the genotypes in control group of all included studies was in HWE except for one.[23] The flow chart for the process of study selection is shown in Fig. 1, and the detailed characteristics of all eligible studies are shown in Table 1.
Figure 1.
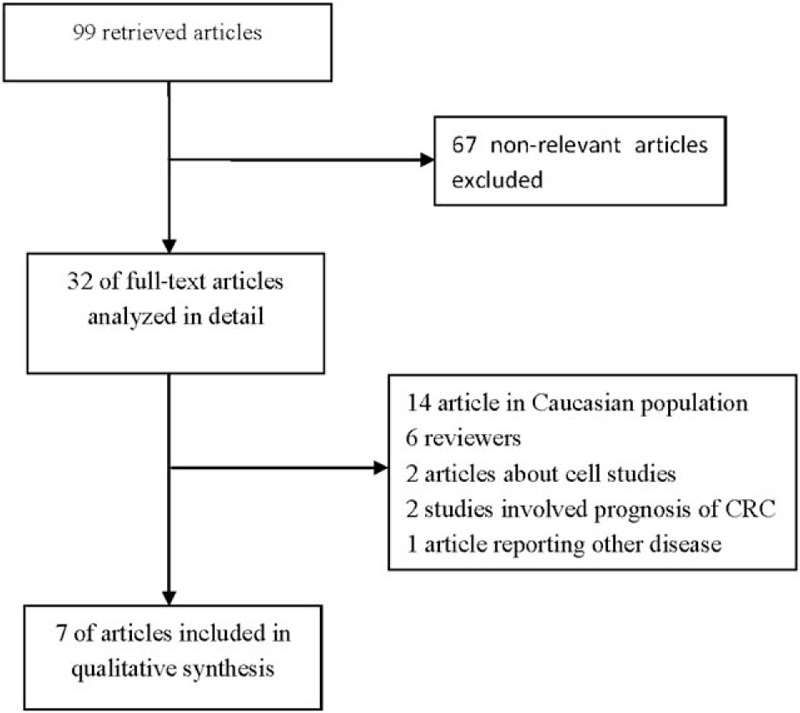
Flowchart of search strategy for the meta-analysis.
Table 1.
Characteristics of eligible studies in a meta-analysis of the MDR1 C3435T polymorphism and CRC risk.
3.2. Quantitative synthesis
The summary of meta-analysis for MDR1 C3435T polymorphism and colorectal cancer susceptibility is shown in Table 2 and Fig. 2. The results of pooled analysis demonstrated a significant association of MDR1 C3435T variant with colorectal cancer risk (T vs C: OR = 0.897, 95%CI = 0.826–0.975, P = .01; TT vs CC: OR = 0.721, 95%CI = 0.605–0.861, P < .001; TT vs TC+CC: OR = 0.679, 95%CI = 0.579–0.795, P < .001). Non-significant association was observed in the other genetic models. (TC vs CC: OR = 1.111, 95%CI = 0.976–1.264, P = .111; TT+TC vs CC: OR = 1.001, 95%CI = 0.885–1.131, P = .989). Overall, there was no significant between-study heterogeneity was found in all genetic models.
Table 2.
Pooled analysis for the associations between the MDR1 C3435T polymorphism and the risk of CRC.

Figure 2.

Forest plot of CRC risk associated with the MDR1 C3435T polymorphism in Asians (T vs C). CI = confidence interval, CRC = colorectal cancer, OR = odds ratio.
3.3. Sensitivity analysis and cumulative meta-analysis
We performed sensitivity analysis to assess the stability of the results by sequential omission of each eligible study. The results showed that no single study qualitatively changed the pooled ORs, indicating the results were highly stable (Fig. 3). Moreover, sensitivity analysis limited to studies in HWE also showed significant association between MDR1 C3435T polymorphism and colorectal cancer risk. In the cumulative meta-analysis, the results demonstrated that the pooled OR tended stable and significant with accumulation of more data over time (Fig. 4).
Figure 3.
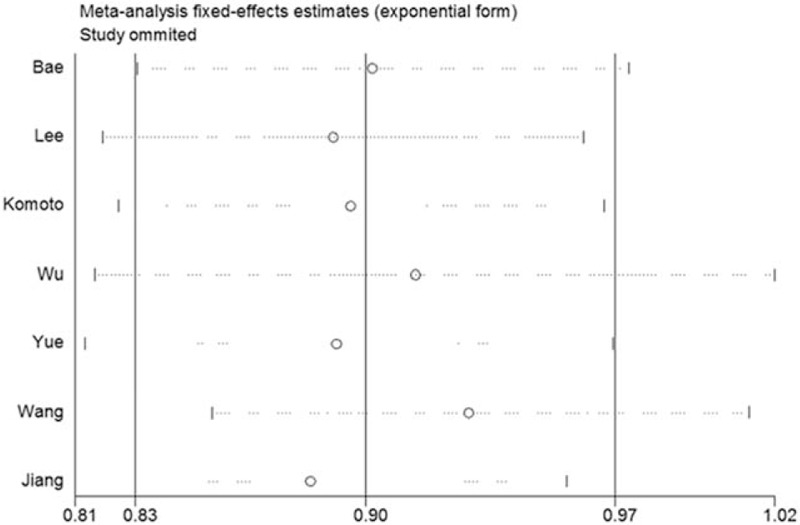
Sensitivity analysis on the associations between MDR1 C3435T polymorphism and CRC risk. Results were computed by omitting each study (left column) in turn. Bars = 95% confidence interval, CRC = colorectal cancer.
Figure 4.

Odds ratio (OR) estimates for the results of cumulative meta-analysis of the association between MDR1 C3435T polymorphism and CRC risk. Bars represent 95% confidence intervals (CIs); CRC = colorectal cancer.
3.4. Publication bias
The potential publication bias of eligible literatures was assessed by funnel plot and Egger test. As shown in Fig. 5, the shapes of the funnel plots did not indicate any evidence of obvious asymmetry. In addition, the Egger test, which was used to provide statistical evidence of funnel plot symmetry, also did not show any significant publication bias. (T vs C: P = .322; TT vs CC: P = .227; TC vs CC: P = .642; TT+TC vs CC: P = .947; TT vs TC+CC: P = .124)
Figure 5.

Funnel plots of the association between MDR1 C3435T polymorphism and CRC risk. CRC = colorectal cancer; Logor = natural logarithm of the OR, SE of logor = standard error of the logor.
4. Discussion
Colorectal cancer (CRC), occurring in the colon and the rectum, is a multifactorial disease resulting from genetic, environmental, socioeconomic, and lifestyle factors.[26] Vogelstein et al[27] initially described the model of colorectal tumorigenesis, which showed the genetic alterations involved in the development of colorectal cancer. Recently, an accumulating number of studies provided evidence that genetic factors played an important role in the pathogenesis of CRC development. For example, methionine synthase reductase (MTRR) A66G polymorphism and rs12970291 on chromosome 18q22 were significantly associated with decreased CRC risk,[28,29] whereas, GSTM1 null polymorphism, CASC8 rs7837328 variant, and TLR4 Asp299Gly were proved to confer the susceptibility to CRC.[30–32]
The MDR1 gene, located on chromosomal region 7q21, encoded an ATP-dependent efflux transporter (P-gp) that protected the body from environmental toxins and xenobiotics.[33] To date, >50 single nucleotide polymorphisms (SNPs) have been identified for MDR1 gene. Recent study indicated that C3435T mutation, one of the most researched polymorphisms in MDR1, was associated with an altered structure of substrate and inhibitor interaction sites of P-gp.[34] In Asian population, studies that investigated the association of MDR1 C3435T polymorphism with CRC risk produced inconclusive results. For instance, some studies suggested a significant association between MDR1 C3435T and susceptibility to CRC[22,24], which, whereas, could not be confirmed in several studies.[12–14,23] Thus, the possible role of MDR1 polymorphism in carcinogenesis of CRC remains unclear. Here, we aimed to perform a meta-analysis to explore the correlation between MDR1 C3435T polymorphism and CRC susceptibility.
The present study involved 4818 individuals is the first meta-analysis to investigate the relationship between MDR1 C3435T polymorphism and CRC susceptibility in Asian population. The results of our meta-analysis suggested that persons carrying T allele had a significantly decreased CRC risk, which was also observed in homozygote comparison (TT vs CC) and recessive model (TT vs TC+CC). A previous meta-analysis indicated that there were no significant associations of MDR1 C3435T polymorphism with colorectal cancer in Caucasian population, which included 10 studies of Caucasians.[15] It is widely accepted that the relative contribution of genetic markers in predisposition to CRC may vary across different ethnic groups. For instance, MDM2 SNP309 and x-ray repair cross-complementing group 1 (XRCC1) Arg399Gln polymorphisms were significantly associated with CRC risk in Asians but not among European populations.[35,36] Moreover, for MDR1 C3435T polymorphism, a recent study showed a significant difference in the prevalence of the MDR1 3435TT allele among healthy individuals of Asians (27.8%) and Caucasians (49.4%).[37] Taken together, these results suggested that the relative contribution of MDR1 C3435T polymorphism might vary across different populations.
Non between-study heterogeneity was observed in most genetic models. When excluding the study departed from HWE, the pooled ORs were not materially altered. The sensitivity analysis also showed that no single study qualitatively changed the pooled ORs. Also, cumulative meta-analysis suggested that the pooled ORs tended stable and significant as evidence accumulating over time. In addition, to assess the publication bias, we also performed funnel plot and Egger test, which did not show any evidence of publication bias. Thus, these results indicated that the results of this meta-analysis are highly stable.
Several limitations should be acknowledged for interpretation of our results. First, CRC is a complex disease and different environments existed among different countries, whereas, in this meta-analysis, the subgroup analysis stratified by countries was not performed due to the insufficient data. Second, our study was designed to analyze single polymorphism, however, a haplotype analysis may be more powerful to find a significant association between MDR1 polymorphisms and CRC risk. Finally, our meta-analysis was based on published articles, and there was no sufficient data for adjustment for individual level factors including sex, obesity, and smoking, which might affect the genetic effect.[4,38]
In conclusion, despite these limitations, our results were still significant. The present meta-analysis involved 4818 individuals demonstrated that MDR1 C3435T polymorphism might be significantly associated with decreased risk of CRC in Asian population. Moreover, further studies with larger sample size are required to clarify exact role of MDR1 C3435T polymorphism in the pathogenesis of CRC, especially the gene–gene and gene–environment interactions
Footnotes
Abbreviations: ABCB1 = ATP-binding cassette sub-family B member 1, CI = confidence interval, CRC = colorectal cancer, HWE = Hardy–Weinberg equilibrium, MDR1 = multidrug resistance gene 1, MTRR = methionine synthase reductase, OR = odds ratio, P-gp = P-glycoprotein, SNPs = single nucleotide polymorphisms, XRCC1 = x-ray repair cross-complementing group 1.
Conflict of Interest: No competing financial interests exist.
References
- [1].Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893–917. [DOI] [PubMed] [Google Scholar]
- [2].Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359–86. [DOI] [PubMed] [Google Scholar]
- [3].Veronese N, Nottegar A, Pea A, et al. Prognostic impact and implications of extracapsular lymph node involvement in colorectal cancer: a systematic review with meta-analysis. Ann Oncol 2016;27:42–8. [DOI] [PubMed] [Google Scholar]
- [4].Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet 2014;383:1490–502. [DOI] [PubMed] [Google Scholar]
- [5].Singh S, Singh H, Singh PP, et al. Antidiabetic medications and the risk of colorectal cancer in patients with diabetes mellitus: a systematic review and meta-analysis. Cancer Epidemiol Biomarkers Prev 2013;22:2258–68. [DOI] [PubMed] [Google Scholar]
- [6].Leake I. Colorectal cancer: Metabolic signature of CRC revealed by spectroscopic profiling. Nat Rev Gastroenterol Hepatol 2013;10:503. [DOI] [PubMed] [Google Scholar]
- [7].Loscher W, Potschka H. Role of multidrug transporters in pharmacoresistance to antiepileptic drugs. J Pharmacol Exp Ther 2002;301:7–14. [DOI] [PubMed] [Google Scholar]
- [8].Linn SC, Giaccone G. MDR1/P-glycoprotein expression in colorectal cancer. Eur J Cancer 1995;31A:1291–4. [DOI] [PubMed] [Google Scholar]
- [9].de Kant E, Heide I, Thiede C, et al. MDR1 expression correlates with mutant p53 expression in colorectal cancer metastases. J Cancer Res Clin Oncol 1996;122:671–5. [DOI] [PubMed] [Google Scholar]
- [10].Mitsuhashi J, Tsukahara S, Suzuki R, et al. Retroviral integration site analysis and the fate of transduced clones in an MDR1 gene therapy protocol targeting metastatic breast cancer. Hum Gene Ther 2007;18:895–906. [DOI] [PubMed] [Google Scholar]
- [11].Kurzawski M, Drozdzik M, Suchy J, et al. Polymorphism in the P-glycoprotein drug transporter MDR1 gene in colon cancer patients. Eur J Clin Pharmacol 2005;61:389–94. [DOI] [PubMed] [Google Scholar]
- [12].Bae SY, Choi SK, Kim KR, et al. Effects of genetic polymorphisms of MDR1, FMO3 and CYP1A2 on susceptibility to colorectal cancer in Koreans. Cancer Sci 2006;97:774–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Komoto C, Nakamura T, Sakaeda T, et al. MDR1 haplotype frequencies in Japanese and Caucasian, and in Japanese patients with colorectal cancer and esophageal cancer. Drug Metab Pharmacokinet 2006;21:126–32. [DOI] [PubMed] [Google Scholar]
- [14].Lee BI, Choi KY, Lee KM, et al. [Is C3435T polymorphism of MDR1 related to inflammatory bowel disease or colorectal cancer in Korean?]. Korean J Gastroenterol 2006;47:22–9. [PubMed] [Google Scholar]
- [15].Zhao L, Li K, Li W, et al. Association between the C3435T polymorphism of ABCB1/MDR1 gene (rs1045642) and colorectal cancer susceptibility: a meta-analysis based on 11,339 subjects. Tumour Biol 2013;34:1949–57. [DOI] [PubMed] [Google Scholar]
- [16].Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- [17].DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177–88. [DOI] [PubMed] [Google Scholar]
- [18].Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst 1959;22:719–48. [PubMed] [Google Scholar]
- [19].Zintzaras E, Lau J. Synthesis of genetic association studies for pertinent gene-disease associations requires appropriate methodological and statistical approaches. J Clin Epidemiol 2008;61:634–45. [DOI] [PubMed] [Google Scholar]
- [20].Thakkinstian A, McElduff P, D’Este C, et al. A method for meta-analysis of molecular association studies. Stat Med 2005;24:1291–306. [DOI] [PubMed] [Google Scholar]
- [21].Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Wu H, Kang H, Liu Y, et al. Association of ABCB1 genetic polymorphisms with susceptibility to colorectal cancer and therapeutic prognosis. Pharmacogenomics 2013;14:897–911. [DOI] [PubMed] [Google Scholar]
- [23].Yue AM, Xie ZB, Zhao HF, et al. Associations of ABCB1 and XPC genetic polymorphisms with susceptibility to colorectal cancer and therapeutic prognosis in a Chinese population. Asian Pac J Cancer Prev 2013;14:3085–91. [DOI] [PubMed] [Google Scholar]
- [24].Wang F, Huang Z, Zheng K, et al. Two SNPs of ATP-binding cassette B1 gene on the risk and prognosis of colorectal cancer. Int J Clin Exp Pathol 2015;8:3083–9. [PMC free article] [PubMed] [Google Scholar]
- [25].Jiang L, Zhou R, Zhou F, et al. Association between multidrug resistance 1 gene polymorphisms and genetic susceptibility to sporadic colorectal adenocarcinoma. Chin J Exp Surg 2014;31:2573–6. [Google Scholar]
- [26].Aran V, Victorino AP, Thuler LC, et al. Colorectal cancer: epidemiology, disease mechanisms and interventions to reduce onset and mortality. Clin Colorectal Cancer 2016;15:195–203. [DOI] [PubMed] [Google Scholar]
- [27].Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal-tumor development. N Engl J Med 1988;319:525–32. [DOI] [PubMed] [Google Scholar]
- [28].Cheng TH, Thompson D, Painter J, et al. Meta-analysis of genome-wide association studies identifies common susceptibility polymorphisms for colorectal and endometrial cancer near SH2B3 and TSHZ1. Sci Rep 2015;5:17369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Pabalan N, Singian E, Tabangay L, et al. Associations of the A66G methionine synthase reductase polymorphism in colorectal cancer: a systematic review and meta-analysis. Biomark Cancer 2015;7(Suppl):21–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Li J, Xu W, Liu F, et al. GSTM1 polymorphism contribute to colorectal cancer in Asian populations: a prospective meta-analysis. Sci Rep 2015;5:12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Yao K, Hua L, Wei L, et al. Correlation between CASC8, SMAD7 polymorphisms and the susceptibility to colorectal cancer: an updated meta-analysis based on GWAS results. Medicine (Baltimore) 2015;94:e1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Sheng WY, Yong Z, Yun Z, et al. Toll-like receptor 4 gene polymorphisms and susceptibility to colorectal cancer: a meta-analysis and review. Arch Med Sci 2015;11:699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bodor M, Kelly EJ, Ho RJ. Characterization of the human MDR1 gene. AAPS J 2005;7:E1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kimchi-Sarfaty C, Oh JM, Kim IW, et al. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science 2007;315:525–8. [DOI] [PubMed] [Google Scholar]
- [35].Fu Q, Zhang G, Chen H, et al. Current evidence on the relationship between SNP309 polymorphism in the MDM2 gene and colorectal cancer risk. Tumour Biol 2013;34:3721–9. [DOI] [PubMed] [Google Scholar]
- [36].Zeng FR, Ling Y, Yang J, et al. X-ray repair cross-complementing group 1 Arg399Gln gene polymorphism and susceptibility to colorectal cancer:a meta-analysis. Tumour Biol 2013;34:555–63. [DOI] [PubMed] [Google Scholar]
- [37].Wang J, Wang B, Bi J, et al. MDR1 gene C3435T polymorphism and cancer risk: a meta-analysis of 34 case-control studies. J Cancer Res Clin Oncol 2012;138:979–89. [DOI] [PubMed] [Google Scholar]
- [38].Liang PS, Chen TY, Giovannucci E. Cigarette smoking and colorectal cancer incidence and mortality: systematic review and meta-analysis. Int J Cancer 2009;124:2406–15. [DOI] [PubMed] [Google Scholar]


