Abstract
Rationale:
Keytruda (pembrolizumab) is an inhibitor of programmed cell death receptor-1 (PD-1), which was approved to treat advanced melanoma and nonsmall cell lung cancer patients who do not respond to other treatment. However, its efficacy and security in the treatment of advanced liver cancer is still under investigation.
Patient concerns:
A 60-year-old man was diagnosed with pulmonary metastatic liver cancer who accepted pembrolizumab treatment after the failure of sorafenib. When injected pembrolizumab, in spite of pulmonary metastatic lesion shrink, the patient experienced severe liver dysfunction.
Diagnoses:
Based on the features of the clinical signs and laboratory examination,
the patient was diagnosed with pembrolizumab-induced immune-related hepatitis by excluding other etiologies and drug-induced side effects.
Interventions:
The patient received glucocorticoid and artificial liver (plasma exchange) therapy after failed conservative liver protection treatment.
Outcomes:
The patient's liver dysfunction continuously progressed and he finally died of liver failure and its complications during his hospitalization.
Lessons:
Pembrolizumab showed efficacy in an advanced hepatocellular carcinoma patient with lung metastases. However, it can generate immune-related adverse events such as immune-related hepatitis which can be lethal.
Keywords: immune-related hepatitis, liver cancer, liver failure, pembrolizumab, side effects
1. Introduction
Keytruda (pembrolizumab) is an inhibitor of programmed cell death receptor-1 (PD-1), which was approved by the US Food and Drug Administration (FDA) on September 4, 2014 to treat advanced melanoma and nonsmall cell lung cancer patients who do not respond to other treatment. In previous studies, PD-1 receptor inhibitors achieved remarkable efficacy not only in advanced melanoma and nonsmall cell lung cancer but also in renal cell carcinoma[1] and Hodgkin's lymphoma.[2] However, pembrolizumab's efficacy in the treatment of advanced liver cancer is still under investigation. Here, we report a case of a 60-year-old male metastatic liver cancer patient who presented with fatal immune-related hepatitis after receiving pembrolizumab treatment.
2. Case report
A 60-year-old male patient, who presented with yellow urine and eyes, fatigue, and anorexia for 7 days, was admitted to our hospital on December 23, 2016. The patient had been diagnosed with liver cancer (tumor size of about 10.0 cm × 12.0 cm, no distant metastases) in May 2012 and underwent right hepatectomy at Shanghai Oriental Hepatobiliary Hospital. Postoperative pathology indicated hepatocellular carcinoma with negative margin. The patient did not take any anticancer drugs after the surgery and he showed no signs of recurrence in the follow-up visits until December 2015 when the patient was admitted to the hospital due to hemoptysis. Computed tomography (CT) scans showed multiple pulmonary nodules, with the largest one being 0.8 × 1.2 cm and no abnormality was found in the liver. Considering his history, he was first diagnosed with hepatocellular carcinoma with bilateral pulmonary metastases.
The patient then received sorafenib therapy (400 mg, PO bid) at Shanghai Oriental Hepatobiliary Hospital again, but CT scans revealed that the pulmonary nodules had enlarged, with a maximum nodal size of 2.9 × 1.4 cm (Fig. 1) 9 months later (October 2016). With disease progression and failure of sorafenib, the patient discontinued sorafenib on October 10, 2016. He was given 2 options that included one for palliative care and the other one for pembrolizumab therapy on a compassionate use basis, and he finally chose the latter option. Before the beginning of the pembrolizumab treatment, his laboratory data showed normal liver function, and then the PD-1 antibody pembrolizumab (2 mg/kg, every 3 weeks) was given to the patient starting on November 3, 2016. Before the beginning of the second pembrolizumab treatment cycle (after pembrolizumab treatment for 3 weeks), his laboratory data showed liver dysfunction and therefore the patient received hepatic protectants. As the liver damage became further aggravated, he was transferred to our department and the pembrolizumab treatment was delayed.
Figure 1.
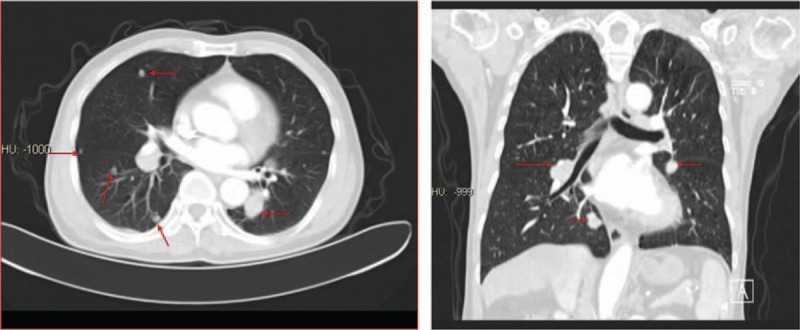
CT before treatment with pembrolizumab. After sorafenib treatment for 9 months, CT scan revealed that pulmonary nodules were enlarged, with a maximum nodal size of 2.9× 1.4 cm. CT = computed tomography.
After admission to our department, we found that the patient had a history of gastric ulcers due to subtotal gastrectomy and he denied a history of alcohol intake and intake of Chinese herbs. Clinical examination revealed skin and sclera jaundice. The laboratory data (November 23, 2016) showed that alanine aminotransferase (ALT) was increased at 1269 U/L, aspartate aminotransferase (AST) at 1193 U/L, total bilirubin (TBil) at 49.8 μmol/L, and direct bilirubin (DBil) at 31.7 μmol/L. The patient was negative for markers of hepatitis virus (hepatitis A, hepatitis B, hepatitis C, and hepatitis E). Antibodies to cytomegalovirus and Epstein–Barr virus were negative. Ceruloplasmin and serum copper were normal, and no Kayser–Fleischer ring was observed upon examination by an experienced ophthalmologist. A qualitative urinary porphyrin test was negative. Autoimmune antibodies including antimitochondrial antibody, antinuclear antibodies, and antineutrophil cytoplasmic antibody were all negative, and serum α-1-antitrypsin concentration, thyroid function, coagulation function, and other laboratory investigation results were normal including alpha-fetoprotein (AFP). Pulmonary CT scan revealed that metastatic lesions in his lungs were reduced. The maximum lesion size was 1.2 cm × 0.7 cm (Fig. 2), suggesting that pembrolizumab led to a dramatic response in this patient.
Figure 2.
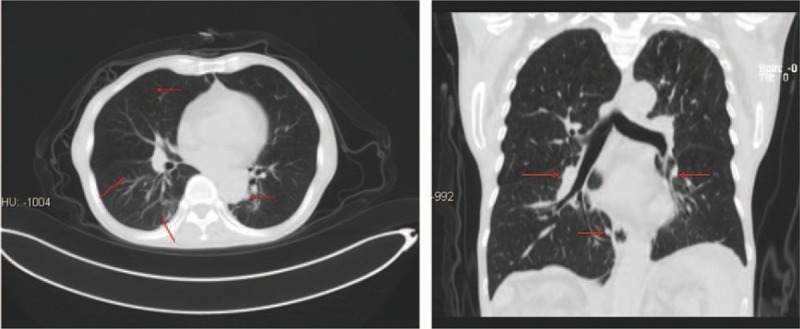
CT after treatment with pembrolizumab for 3 weeks. After injection of pembrolizumab for 3 weeks, CT scan revealed that metastatic lesions in his lungs were reduced. The maximum lesion size was 1.2 cm × 0.7 cm. CT = computed tomography.
A skin rash was detected 4 days after his admission and the skin rash regressed after local use of corticosteroid cream for 4 days (Fig. 3). Liver function test showed ALT 256 U/L, AST 466 U/L, TBil 214.7 μmol/L, and DBil 169.3umol/L after liver protection therapy on December 6, 2016. Coagulation function showed a prothrombin time (PT) of 14.3 seconds, activated partial thromboplastin time (APTT) of 42.5 seconds, and prothrombin activity (PTA) at 57.6%. Before this liver injury, aside from the pembrolizumab, the patient also received sorafenib. However, in the 9 months of sorafenib therapy, the patient did not show any signs of liver injury during long-term follow-up. We considered that after the 3-week administration of pembrolizumab, severe liver injury was caused by immune-related hepatitis after excluding viral hepatitis, autoimmune diseases, metabolic liver diseases, and other drug-induced liver injury. Therefore, prednisone (2.0 mg/kg/d) was the treatment prescribed on the basis of treatment for pembrolizumab side effects except for the liver protection therapy on December 8, 2016.
Figure 3.
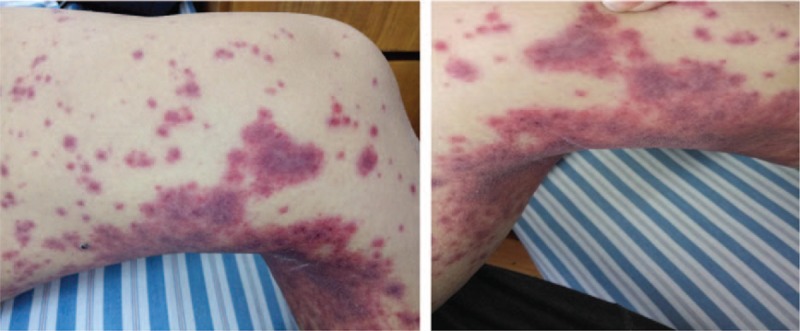
After treatment with pembrolizumab, the patient experienced a skin rash. Skin rash was detected 4 days after his admission, mainly in the lower limbs.
After 7 days of glucocorticoid treatment, the liver function test showed ALT 269 U/L, AST 236 U/L, TBil significantly increased at 381.3 μmol/L, and DBil 259.8 μmol/L. Coagulation function revealed the PT increased at 16.0 seconds, APTT at 37.7 seconds, and PTA at 49.7%. Liver protection and prednisone therapy failed to reverse the liver injury, and his liver dysfunction progressed to liver failure. In spite of artificial liver (plasma exchange) treatment, his liver function did not improve either. The patient died of acute liver failure, hepatic encephalopathy, on January 22, 2017.
3. Discussion
Hepatocellular carcinoma is one of the most common malignant tumors in the world. The global incidence rate has increased to >626,000 per year and mortality is close to 600,000 per year.[3] Surgical resection is the main curative option. However, treatment of unresectable advanced hepatocellular carcinoma is extremely limited. Sorafenib is the first choice for hepatocellular carcinoma patients to improve the survival of patients. However, the response rate for sorafenib therapy is low.[4] Remarkable tumor responses have been seen in the treatment of melanoma and nonsmall cell lung cancer with anti-PD-1/programmed cell death ligand-1 (PD-L1) immunotherapy. Immunotherapy provides a new and effective treatment for advanced hepatocellular carcinoma patients who do not respond to sorafenib therapy.
Keytruda (pembrolizumab) is the first PD-1 inhibitor approved for clinical treatment of malignant tumors. It has demonstrated efficacy in melanoma and nonsmall cell lung cancers.[5,6] Furthermore, it is currently being assessed in advanced liver cancer in clinical trials.
Pembrolizumab is a human monoclonal antibody which inhibits the PD-1/PD-L1 signaling pathway. PD-1 is mainly expressed in T cells, which inhibit immune cell activation upon ligation to PD-L1 and PD-L2.[7] PD-1 can also be expressed in B cells, natural killer (NK) cells, dendritic cells, and activated monocytes. PD-1 ligands include PD-L1 and PD-L2, which are mainly expressed on macrophages, monocytes, and other inflammatory cells in various inflammatory environments.[8] A large number of studies have shown that the expression of PD-L1 on tumor cells is upregulated, while the expression of PD-1 on tumor-infiltrating lymphocytes (TILs) is upregulated. PD-L1 binding to PD-1 induces apoptosis of T cells and protects tumor cells from immune system attack.[9] By blocking the PD-L1 pathway using pembrolizumab, T cells can be activated and attack tumor cells.[5]
We present a 60-year-old patient who experienced severe liver dysfunction 3 weeks after the first injection of pembrolizumab. He was diagnosed with pembrolizumab-induced immune-related hepatitis by excluding other etiologies and drug-induced side effects (assessed by the Naranjo drug-induced side effects assessment (Table 1).[10] The main adverse reactions of pembrolizumab in the treatment of melanoma and nonsmall cell lung cancer were fatigue, diarrhea, rash, etc. About 0.7% patients show immune-mediated hepatitis. However, severe liver damage is rare.[11] In spite of glucocorticoid and artificial liver (plasma exchange) therapy, the patient's liver dysfunction continuously progressed, and he finally died of liver failure and its complications. However, it is noteworthy that the lung lesions of the patient significantly shrank after the first injection of pembrolizumab.
Table 1.
The Naranjo ADR probability scale.

PD-1 inhibitors cause severe immune-related hepatitis, the mechanism of which has not yet been fully elucidated. Studies have revealed that in normal physiological conditions, the liver immune state remains in tolerance. Hepatic sinusoidal endothelial cells express high levels of PD- L1 to prevent overimmune response and protect normal liver tissue from immune damage through the activation of the PD-1/PD-L1 signaling pathway.[12] Pembrolizumab blocks the PD-1/PD-L1 signaling pathway and reverses the depletion of T-cell function. Therefore, it unbalanced the hepatic immune system by decreasing inhibitory regulation of the autoimmune response and strengthening the immune response, which caused significant liver dysfunction. Clarifying its pathogenesis will provide guidance for correct and effective treatment.
In conclusion, we found that pembrolizumab showed efficacy in an advanced hepatocellular carcinoma patient with lung metastases. However, it can generate immune-related adverse events such as immune-related hepatitis which can be lethal. A variety of clinical trials of pembrolizumab in advanced liver cancer are underway (ClinicalTrials.gov identifiers NCT02658019, NCT02702414, and NCT02940496). Clinicians should be alert to its immune-related toxicities. Further exploration is still needed to identify how to screen the right patients and how to minimize the side effects of pembrolizumab.
Footnotes
Abbreviations: AFP = alpha-fetoprotein, CT = computed tomography, PD-1 = programmed cell death receptor-1, PD-L1 = programmed cell death ligand-1, TILs = tumor-infiltrating lymphocytes.
WZ and LL contributed equally to this paper.
Ethical approval and patient consent: The study was approved by the Institutional Review Boards of the first affiliated hospital of Nanchang University, Nanchang, China. The patient's family signed informed consent for the publication of this case report and any associated images.
The authors declare that they have no conflict of interest.
References
- [1].Boussiotis VA, Chatterjee P, Li L. Biochemical signaling of PD-1 on T cells and its functional implications. Cancer J 2014;20:265–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 2012;12:252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: A phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25–34. [DOI] [PubMed] [Google Scholar]
- [4].Harding JJ, El Dika I, Abou-Alfa GK. Immunotherapy in hepatocellular carcinoma: primed to make a difference? Cancer 2016;122:367–77. [DOI] [PubMed] [Google Scholar]
- [5].Bruix J, Sherman M. American Association for the Study of Liver D. Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Naranjo CA, Busto U, Sellers EM, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther 1981;30:239–45. [DOI] [PubMed] [Google Scholar]
- [7].Li Y, Li F, Jiang F, et al. A mini-review for cancer immunotherapy: molecular understanding of PD-1/PD-L1 pathway and translational blockade of immune checkpoints. Int J Mol Sci 2016;17:pii: E1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ansell SM, Lesokhin AM, Borrello I, et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin's lymphoma. N Engl J Med 2015;372:311–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 2016;387:1540–50. [DOI] [PubMed] [Google Scholar]
- [10].Deeks ED. Pembrolizumab: a review in advanced melanoma. Drugs 2016;76:375–86. [DOI] [PubMed] [Google Scholar]
- [11].McDermott DF, Drake CG, Sznol M, et al. Survival, durable response, and long-term safety in patients with previously treated advanced renal cell carcinoma receiving nivolumab. J Clin Oncol 2015;33:2013–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Naidoo J, Page DB, Li BT, et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann Oncol 2015;26:2375–91. [DOI] [PMC free article] [PubMed] [Google Scholar]


