Abstract
Rationale:
To date, the only treatment approved for acute ischemic strokes is thrombolysis. Whether intravenous thrombolysis may be safe in patients taking direct oral anticoagulants (DOACs) is currently a matter of debate.
Patient concerns:
A 74-year-old woman, who was on rivaroxaban 20 mg/d for nonvalvular atrial fibrillation, was admitted to our stroke unit with left-sided hemiparesis and aphasia. The onset of neurologic deficits had occurred 5 hours after the last rivaroxaban dose.
Diagnosis:
An acute ischemic stroke was diagnosed.
Interventions:
The patient was administered thrombolytic treatment with intravenous recombinant tissue plasminogen activator (r-TPA) 3 hours and 20 minutes after symptoms onset. Seven hours post-r-TPA treatment, the neurological deficit had worsened, and a type I intraparenchymal hematoma was detected on a computed tomography brain scan.
Outcomes:
The clinical/neuroradiological picture improved significantly in the following days. The patient was discharged to a rehabilitation facility after 3 weeks.
Lessons:
In this case, factor ten activated (Xa) inhibitor, rivaroxaban might have increased the risk of hemorrhagic transformation of the ischemic stroke. However, this risk was overweighed by the benefit of thrombolysis, as the patient's clinical condition had improved significantly in the following weeks. The current guidelines discourage the use of thrombolytic treatment in patients with DOACs administered within the last 24(48) hours. However, the case reported herein and other world experiences, even though limited, suggest that an ongoing DOAC medication could no longer be considered a barrier to r-TPA treatment which may be a reasonable and valuable option, at least in selected acute stroke patients taking factor Xa inhibitors.
Keywords: cerebral parenchymal hemorrhage, direct oral anticoagulants, hemorrhagic transformation, rivaroxaban, stroke, thrombolysis, tissue plasminogen activator
1. Introduction
To date, the only treatment approved for acute ischemic stroke is thrombolysis.[1] Whether intravenous thrombolysis may be safe in patients taking direct oral anticoagulants (DOACs) is currently a matter of debate. Indeed, despite the proven efficacy of DOACs in stroke prevention, approximately 1% to 2% of people with nonvalvular atrial fibrillation (NVAF) taking DOACs will experience an acute ischemic stroke.[1] Although the anticoagulant effect of dabigatran may be reversed by idarucizumab, making recombinant tissue plasminogen activator (r-TPA) a possible option for acute stroke therapy,[2] thrombolysis is not recommended in patients taking rivaroxaban and the other factor ten activated (Xa) inhibitors, because of the hemorrhagic risk, unless enough time (24–48 hours) has passed to allow for renal clearance of the drug or specific laboratory tests have demonstrated the absence of any anticoagulant effect.[3,4]
2. Case report
Herein, we report a case in which a 74-year-old Caucasian woman was admitted to our stroke unit owing to the acute onset of left-sided hemiparesis and aphasia, with a medical history of hypertension and NVAF, who had been on rivaroxaban at 20 mg/d for 1 year. Upon admission, the neurological examination showed expressive aphasia, left hemianopia and left-sided facio-brachio-crural hemiparesis (National Institutes of Health Stroke Scale, NIHSS = 14).[5] Her brain computed tomography (CT) scan was unremarkable and her platelet count (185,000; normal, 150,000–450,000) and activated partial thromboplastin time (28.3; normal, 20.0–29.6) were within the normal range; the international normalized ratio (INR) was 1.34 (normal, 0.90–1.30). Creatinine was 1.08 mg/mL (normal, 0.51–0.95) and the clearance was 52.84 mL/min. Her blood pressure was 150/80 mm Hg. An electrocardiography revealed atrial fibrillation. The onset of neurologic deficits occurred 5 hours after the last rivaroxaban dose. An acute ischemic stroke was diagnosed. Three hours and 20 minutes after symptom onset, a thrombolytic treatment with intravenous r-TPA was administered according to standard protocol and with patient's consent. Thrombolytic treatment was therefore administered 8 hours and 20 minutes after the last rivaroxaban intake. According to guidelines,[3,4] at least 24 hours should elapse between rivaroxaban intake and thrombolysis, but given the patient's clinical condition and the balance between the expected clinical benefit of r-TPA and the hemorrhagic risk, which seemed favorable to us, we took the decision to treat the patient regardless. After a temporary improvement of muscle strength, the clinical picture suddenly worsened. Seven hours after thrombolysis, the patient had developed left-sided hemiplegia and became unresponsive (NIHSS = 17; Glasgow Coma Scale = 14). A control CT brain scan (Fig. 1), performed 8 hours after r-TPA administration, revealed a hemorrhagic transformation of the ischemic area in the right basal ganglia, fulfilling the criteria for an intraparenchymal hematoma type I, according to the European Cooperative Acute Stroke Study I classification[6] (≤30% of the infarcted area covered, with some mild space-occupying effect). Subsequently, the patient's clinical and radiological picture improved significantly. After 3 weeks, she was discharged to a rehabilitation facility, with an NIHSS score of 8 (Fig. 2). (These data were published after informed consent was obtained from the patient).
Figure 1.
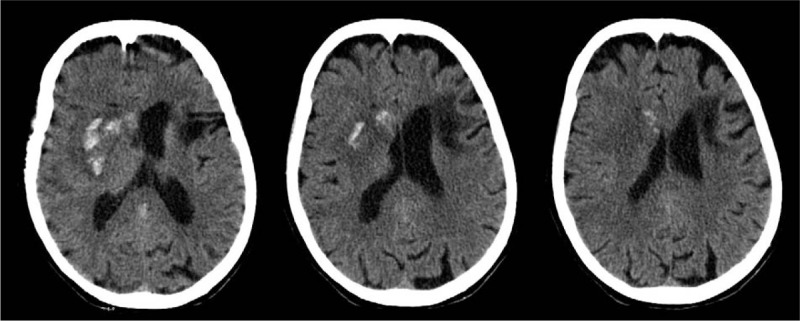
CT scan of the brain (8 h after r-TPA administration) showing a right intraparenchymal hematoma (3 axial scans at the basal ganglia) type 1 according to ECASS 1 classification. CT = computed tomography; ECASS = European Cooperative Acute Stroke Study; r-TPA = recombinant tissue plasminogen activator.
Figure 2.
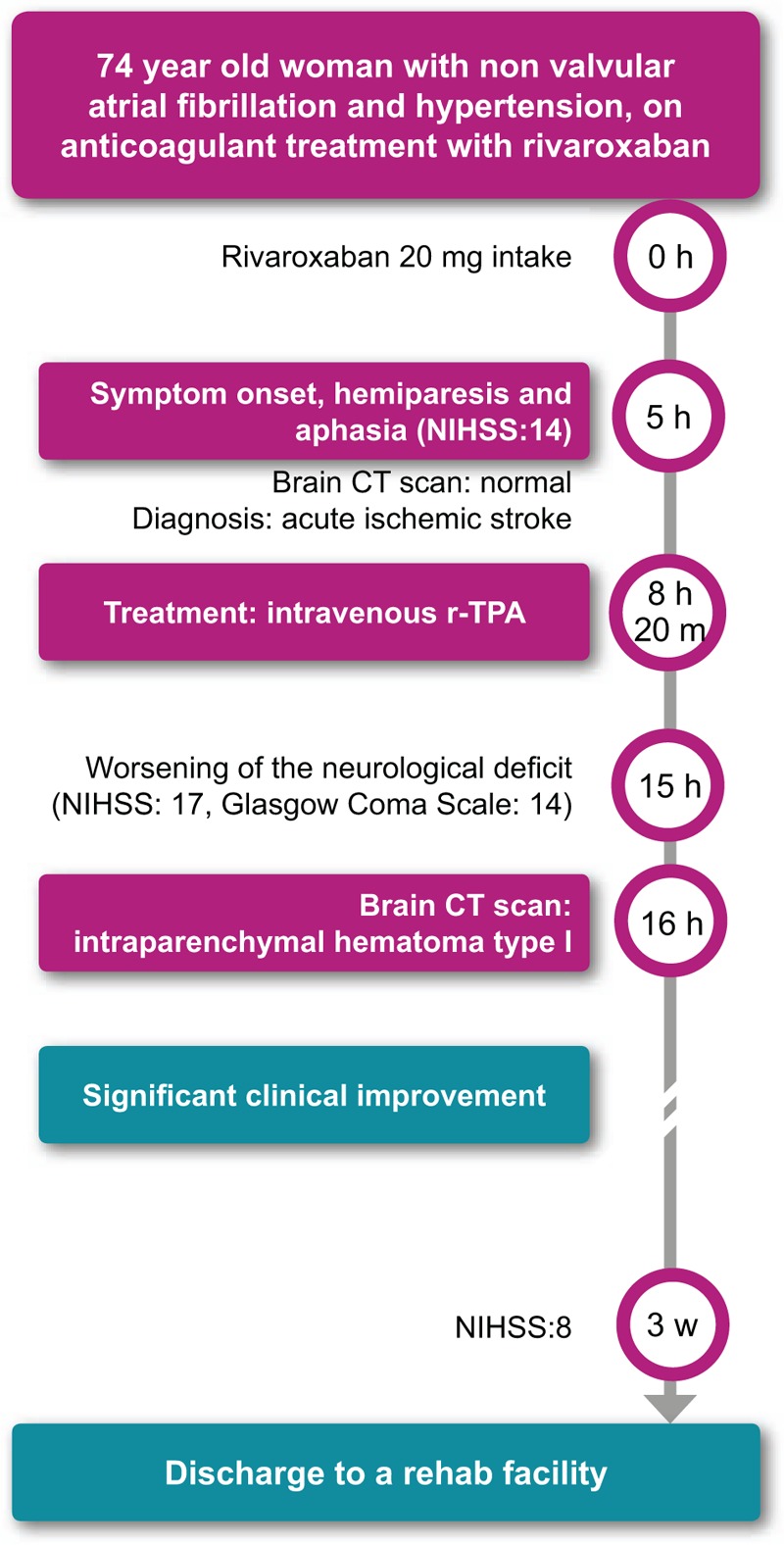
Timeline of the clinical evolution. CT = computed tomography; NIHSS = National Institutes of Health Stroke Scale, r-TPA = recombinant tissue plasminogen activator.
3. Discussion
Hemorrhagic transformation is a complication of acute ischemic stroke, which may occur spontaneously or secondarily to thrombolytic therapy. The main risk factors associated with hemorrhagic transformation include cardiogenic embolism, stroke severity (large ischemic lesions, mass effect, early hypodensity), high blood glucose levels, advanced age, white matter disease burden, aspirin, warfarin, and the thrombolytic treatment itself.[7,8] Despite an association with higher risk of gastrointestinal bleeding, particularly in women, DOACs, seem to be safer than warfarin if intracranial bleeding is considered,[9] with a 50% relative risk reduction of hemorrhagic stroke and intracranial hemorrhage (ICH).[10]
To date, limited data are available on the safety of intravenous r-TPA in stroke patients taking DOACs, especially factor Xa inhibitors, whose anticoagulant activity cannot be reversed. The question arises whether factor Xa inhibitors may increase the risk of hemorrhagic transformation in this context. Twelve cases have been reported, where thrombolytic treatment was performed without hemorrhagic complications in patients receiving rivaroxaban.[11–15] A recent study examined the outcomes of thrombolytic therapy in patients with ischemic stroke who received either DOACs (dabigatran 87, rivaroxaban 129, and apixaban 35), warfarin (INR <1.7), or did not receive an anticoagulant from 1289 hospital registers, between October 2012 and March 2015. There were no significant differences in the risk of life-threatening/serious systemic hemorrhages, any r-TPA complications, in-hospital mortality, or a modified Rankin Scale at discharge across the 3 groups.[16]
Our patient was treated with intravenous thrombolytics, despite being on rivaroxaban, because the expected clinical benefit seemed to overcome the hemorrhagic risk related to the treatment. The patient developed a parenchymal hematoma after intravenous thrombolysis. Rivaroxaban may have favored such a hemorrhagic transformation. R-TPA was administered 8 hours and 20 minutes after the last rivaroxaban intake, when the anticoagulant effect of the DOAC was presumed to still be at high levels, on the basis of the drug half-life and, as suggested by the abnormal INR value. An observational study found that INR values of ≤ 1.0 predicted rivaroxaban concentrations< 32 and < 100 ng/mL with a 90-96% specificity.[17,18] However, in the absence of specific laboratory tests, no conclusions could be drawn of a persistent anticoagulant effect of rivaroxaban in our patient. Moreover, the role of rivaroxaban in favoring the development of the hematoma in the case herein reported, could be debated, considering the high percentage of spontaneous hemorrhagic transformations occurring from ischemic strokes.[7,8]
It is noteworthy in addition that, despite the hemorrhagic transformation, the patient improved significantly, in the following days. This could be favored by the fact that the anticoagulant effect of DOACs does not persist over days unlike warfarin. Additionally, whether the same clinical improvement would have been possible without the thrombolytic therapy, is debatable.
Interestingly, a study conducted in Wistar rats, found that ICH volume was significantly lower in rats on rivaroxaban, compared with rats on warfarin, after an induced cerebral artery occlusion and reperfusion with r-TPA treatment.[19] The study also revealed an increased expression of protease-activated receptors (PAR)-1 and PAR-2 in the peri-ischemic lesions of the warfarin group, absent in the rivaroxaban group.[19] PAR-1 and PAR-2 are protease-activated receptors mediating the effects of thrombin, factor Xa, and TPA and are related to inflammation and neurodegeneration in stroke. If confirmed in studies on humans, a reduced activation of these receptors could help explain why rivaroxaban is associated with lower cerebral hemorrhagic complications compared with warfarin.
4. Conclusion
Although the current guidelines discourage the use of thrombolytics in patients like ours, with DOACs having been administered within the last 24(48) hours, and an uncertain anticoagulation status,[3,4] the case reported herein and other real-world experiences, even though limited, suggest that DOAC medication could no longer be considered a barrier to r-TPA treatment to improve clinical outcome in selected acute stroke patients taking factor Xa inhibitors such as rivaroxaban.[16] Future studies are needed to further assess the safety and efficacy of intravenous r-TPA in acute ischemic stroke patients who take DOACs, particularly factor Xa inhibitors.
Footnotes
Abbreviations: DOAC = direct oral anticoagulant, ECG = electrocardiography, ICH = intracranial hemorrhage, NIHSS = National Institutes of Health Stroke Scale, NVAF = nonvalvular atrial fibrillation, PAR = protease-activated receptors, r-TPA = recombinant tissue plasminogen activator, Xa = ten activated.
Ercules Comunicazioni provided editorial support in the preparation of this manuscript and funded the publication of the article with the support of Bayer (Italy).
The authors have no conflicts of interest to disclose
References
- [1].Powers WJ, Derdeyn CP, Biller J, et al. 2015 American Heart Association/American Stroke Association focused update of the 2013 guidelines for the early management of patients with acute ischemic stroke regarding endovascular treatment. Stroke 2015;46:3020–35. [DOI] [PubMed] [Google Scholar]
- [2].Diener H, Bernstein R, Butcher K, et al. Thrombolysis and thrombectomy in patients treated with dabigatran with acute ischemic stroke: expert opinion. Int J Stroke 2017;12:9–12. [DOI] [PubMed] [Google Scholar]
- [3].Heidbuchel H, Verhamme P, Alings M, et al. Updated European heart rhythm association practical guide on the use of non-vitamin-K antagonist anticoagulants in patients with non-valvular atrial fibrillation: executive summary. Eur Heart J 2017;38:2137–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Heidbuchel H, Verhamme P, Alings M, et al. Updated European heart rhythm association practical guide on the use of non-vitamin K antagonist anticoagulants in patients with non-valvular atrial fibrillation. Europace 2015;17:1467–507. [DOI] [PubMed] [Google Scholar]
- [5].Brott T, Adams HP, Olinger CP, et al. Measurements of acute cerebral infarction: a clinical examination scale. Stroke 1989;20:864–70. [DOI] [PubMed] [Google Scholar]
- [6].Fiorelli M, Bastianello S, von Kummer R, et al. Hemorrhagic transformation within 36 hours of a cerebral infarct: relationships with early clinical deterioration and 3-month outcome in the European Cooperative Acute Stroke Study I (ECASS I) cohort. Stroke 1999;30:2280–4. [DOI] [PubMed] [Google Scholar]
- [7].Paciaroni M, Agnelli G, Corea F, et al. Early hemorrhagic transformation of brain infarction: rate, predictive factors, and influence on clinical outcome: results of a prospective multicenter study. Stroke 2008;39:2249–56. [DOI] [PubMed] [Google Scholar]
- [8].Hiraga A. Prediction of hemorrhagic transformation in ischemic stroke. Neuroepidemiology 2009;33:266–7. [DOI] [PubMed] [Google Scholar]
- [9].Tawfik A, Bielecki JM, Krahn M, et al. Systematic review and network meta-analysis of stroke prevention treatments in patients with atrial fibrillation. Clin Pharmacol 2016;8:93–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet 2014;383:955–62. [DOI] [PubMed] [Google Scholar]
- [11].Ishihara H, Torii H, Imoto H, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator in a stroke patient treated with rivaroxaban. J Stroke Cerebrovasc Dis 2014;23:e457–9. [DOI] [PubMed] [Google Scholar]
- [12].Landais A, Ginoux C. Intravenous thrombolysis for acute ischemic stroke in a patient receiving rivaroxaban. J Stroke Cerebrovasc Dis 2015;24:e73–4. [DOI] [PubMed] [Google Scholar]
- [13].Cappellari M, Bovi P. Intravenous thrombolysis for stroke in patients taking non-VKA oral anticoagulants: an update. Thromb Haemost 2015;114:440–4. [DOI] [PubMed] [Google Scholar]
- [14].Nardetto L, Tonello S, Zuliani L, et al. Intravenous thrombolysis for acute stroke in a patient on treatment with rivaroxaban. Neurol Sci 2015;36:2291–2. [DOI] [PubMed] [Google Scholar]
- [15].Penge J, Hashi S, Simister R. Successful intravenous thrombolysis following full dose rivaroxaban 5 hours before ictus. Br J Hosp Med (Lond) 2015;76:244–5. [DOI] [PubMed] [Google Scholar]
- [16].Xian Y, Federspiel JJ, Hernandez AF, et al. Use of intravenous recombinant tissue plasminogen activator in patients with acute ischemic stroke who take non-vitamin K antagonist oral anticoagulants before stroke. Circulation 2017;135:1024–35. [DOI] [PubMed] [Google Scholar]
- [17].Ebner M, Peter A, Spencer C, et al. Point-of-care testing of coagulation in patients treated with non-vitamin K antagonist oral anticoagulants. Stroke 2015;46:2741–7. [DOI] [PubMed] [Google Scholar]
- [18].Ebner M, Birschmann I, Peter A, et al. Point-of-care testing for emergency assessment of coagulation in patients treated with direct oral anticoagulants. Crit Care 2017;21:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Morihara R, Yamashita T, Kono S, et al. Reduction of intracerebral hemorrhage by rivaroxaban after tPA thrombolysis is associated with downregulation of PAR-1 and PAR-2. J Neurosci Res 2017;95:1818–28. [DOI] [PubMed] [Google Scholar]


