Abstract
Invasive pulmonary aspergillosis (IPA) is a common fungal infection with high mortality rates in immunocompromised patients. Early diagnosis of IPA is still challenging because of its nonspecific clinical symptoms and radiological presentations.
To evaluate the clinical value of a commercial Aspergillus fumigates-specific IgM antibody assay in diagnosis of IPA, a multicenter prospective study was performed in 12 hospitals in Zhejiang Province, China, from January 1 to December 31, 2016.
A total of 59 patients were enrolled in this study, including 30 IPA and 29 non-IPA patients. The sensitivities of IgM assay were 30.0%, 26.7%, 23.3%, and 20.0%, and the specificities were 79.3%, 86.2%, 86.2%, and 96.6% at the cutoff values of 50, 60, 70 and 80 AU/mL, respectively. The area under the curve of the IgM assay revealed by the receiver-operating characteristic analysis was 0.511 in the IPA cases. This study is the first to evaluate the clinical performance of a commercial A. fumigatus-specific IgM antibody assay that uses envelopes galactomannan extracted from A. fumigatus as the sole antigen in diagnosis of IPA.
In conclusion, the A. fumigatus-specific IgM antibody assay has limited value and should not be a prior recommendation for IPA diagnosis.
Keywords: invasive pulmonary aspergillosis, IPA, serum Aspergillus fumigatus-specific IgM antibody assay
1. Introduction
Aspergillus spp. are ubiquitous in the environment. Aspergillus fumigatus (A. fumigatus) is the most common pathogen among Aspergillus spp. and may cause infections in multiple organ systems, including lung, paranasal sinuses, central nervous system, ears, and skin.[1] Invasive pulmonary aspergillosis (IPA) is considered to be a life-threatening infection in immunocompromised patients. Patients with prolonged neutropenia, allogeneic hematopoietic stem cell transplants, solid organ transplants, inherited or acquired immunodeficiency, and corticosteroid treatment are in the highest risk for IPA.[2] Despite the application of new antifungal drugs, the mortality rates of IPA remain high.[3] Early diagnosis of IPA is difficult because of nonspecific clinical symptoms and chest computed tomography (CT) scan, which is one of the major causes of high mortality rates.
Sterile material for histologic, direct microscopic examination or culture is often obtained by needle aspiration or biopsy, which can be challenging in debilitated patients, especially those who are immunocompromised. Respiratory sample culture is time-consuming and the sensitivity is usually low. The clinical utility of blood-based polymerase chain reaction (PCR) in diagnosing IPA remains controversial.[2] The use of serum and bronchoalveolar lavage fluid (BALF) galactomannan (GM) assay was recommended as an accurate marker for diagnosis of IPA in certain adult patient subpopulations (hematologic malignancy or hematopoietic stem cell transplantation).[2,4] Serum (1→3)-β-D-glucan (BDG) assay is recommended for diagnosing IPA in high-risk patients, but BDG is not specific marker for Aspergillus.[2] A novel and simple lateral-flow device (LFD) using monoclonal antibody JF5 for IPA diagnosis has been developed. It only requires minimum laboratory training and equipment, which considerably reduces the processing time.[5,6] However, the detection is qualitative. Thus, a culture-independent and quantitative approach with high diagnostic value for IPA is urgently needed.
Numerous and different antigens may be produced by Aspergillus during the growth cycle, and their corresponding antibodies would be produced after interacting with the immune system.[7] The IgM antibody is noticeably produced in the early stages of infection.[8] Recently, a new commercial A. fumigatus-specific IgM antibody assay that uses GM extracted from A. fumigatus as the sole antigen became available in China. We wondered whether this commercial Aspergillus-specific IgM antibody assay could play a role in diagnosis of IPA. The purpose of this study was to evaluate the clinical value of the IgM antibody assay in IPA diagnosis.
2. Patients and methods
This prospective study was performed in 12 hospitals in Zhejiang Province, Eastern China from January to December, 2016. The core institute for the study was the First Affiliated Hospital, School of Medicine, Zhejiang University, a 2000-bed referral hospital in Hangzhou. The institutional review board of Clinical Research of the First Affiliated Hospital, School of Medicine, Zhejiang University approved the study protocol (Number: 2015443), and all methods were performed in accordance with the approved guidelines and regulations. Written informed consent was obtained from all of the patients. Laboratory technicians who were responsible for testing the samples could not identify individual participants or access clinical data, whereas other authors had access to information that could identify individual participants during or after data collection.
Adult inpatients with severe immunocompromised conditions, clinical symptoms, or radiological findings <1 month, and an infiltration of chest CT were included. Patients who had received treatment for Aspergillus for >3 days in the last 3 months, patients who had malignant hematological diseases, or patients refusing enrollment were excluded from the study. Flow of participants was showed in Figure 1. CT data were examined and agreed by 2 experienced radiologists. IPA diagnosis was determined according to revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group.[9]
Figure 1.
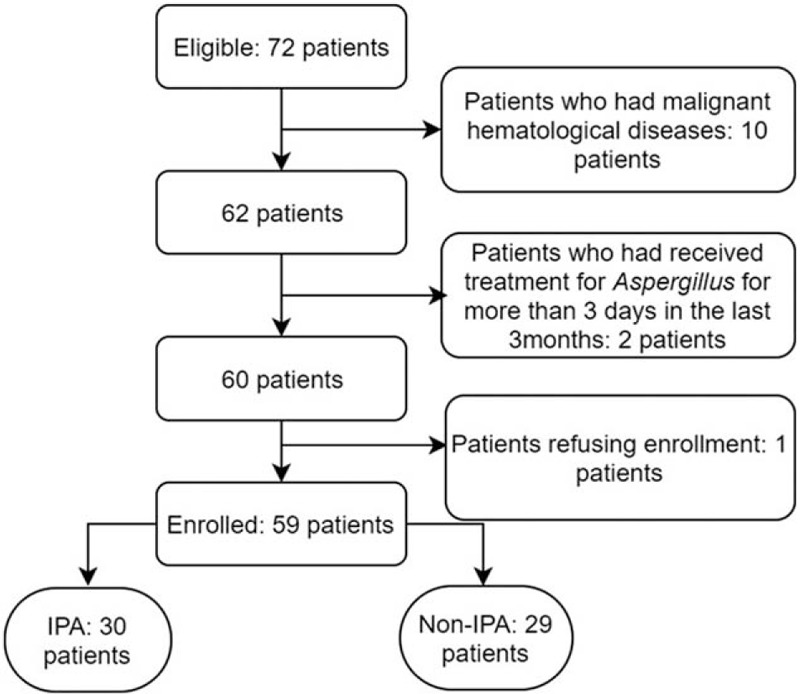
Flow diagram showing the inclusion of patients in the study. IPA = invasive pulmonary aspergillosis.
All of the enrolled patients received examinations including at least 1 sputum culture/smear and 1 serum GM test. Alternative examinations included bronchoscopy (BALF culture/smear/GM, biopsied tissue culture/pathology) and percutaneous CT-guided lung biopsy (biopsied tissue culture/pathology). Enrolled patients were classified as IPA (proven and probable) and non-IPA patients according to revised definitions of invasive fungal disease from the EORTC/MSG Consensus Group.[9] Clinical data including disease history, clinical manifestations, diagnostic results, and treatment were also collected for every patient and reported to the core unit for further confirmation. The enrollment and definitive diagnosis were determined by 2 experienced respiratory physicians in the core unit.
2.1. Serological analysis
The serum A. fumigatus-specific IgM antibody level was detected on all of the samples by using Dynamiker A. fumigatus IgM assay (Dynamiker, Tianjin, China). Plate enzyme-linked immunosorbent assays (ELISAs) were performed on all of the samples in accordance with the manufacturers’ instructions. The manufacturer-recommended positive cutoff value for the A. fumigatus-specific IgM kit is 60 arbitrary units (AU)/mL; the antibody level <50 AU/mL is defined as negative, and the antibody level between 50 and 60 AU/mL is defined as intermediate.
The GM antigen was detected using EIA kit (Bio-Rad, Marnes-la-Coquette, France), according to the manufacturer's instructions. Positive and negative controls were included in each assay. A result with index values of >0.5 (serum)[10] or >0.8 (BALF) in duplicate tests[11] was considered positive.
2.2. Statistical analysis
The mean and standard deviation were calculated for continuous variables with a normal distribution. To assess the differences, the χ2 or Fisher exact test was used for independent binomial variables according to the number of observations. Sensitivity, specificity, Youden index, positive predictive value (PPV), and negative predictive value (NPV) were calculated. The receiver-operating characteristic curve (ROC) were constructed for the IgM assay to acquire the optimal cutoff indices.[12] Statistics were performed with the Statistical Package for Social Science (IBM SPSS, version 19, Chicago, IL). A P value of <0.05 was considered as statistically significant.
3. Results
3.1. General clinical characteristics
A total of 59 patients were enrolled in our study including 30 IPA (1 proven and 29 probable) and 29 non-IPA patients. Of all the enrolled patients, 69.5% were males, and the mean age was 60.1 years (ranging from 23 to 87 years). The most common clinical respiratory symptoms were productive cough (67.8%) and fever (57.6%). In IPA patients, prolonged use of corticosteroids was the most frequent underlying condition (50.0%), followed by treatment for malignant diseases (20.0%) and use of T cell immunosuppressants (16.7%). In non-IPA patients, the most frequent underlying conditions were prolonged use of corticosteroids (34.5%) and use of T cell immunosuppressants (31.0%).
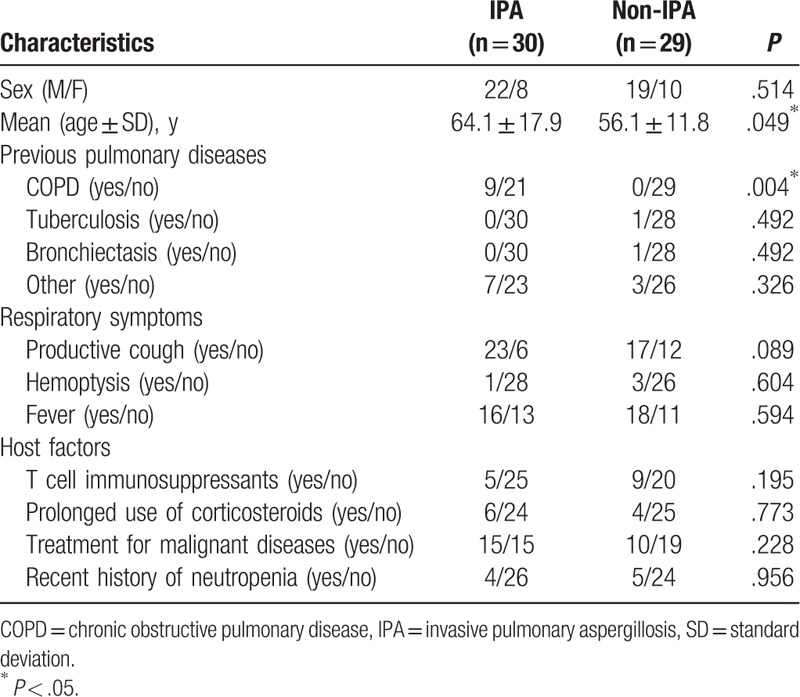
As shown in Table 1, IPA patients were older (Mean age: 64.1 VS 56.1, P = .049) and had higher frequencies of chronic obstructive pulmonary disease (COPD) history (P = .004) when compared to non-IPA patients. There was no difference in sex, previous pulmonary disease history other than COPD, respiratory symptoms, and host factors between IPA and non-IPA patients.
Table 1.
Clinical characteristics of the enrolled patients.
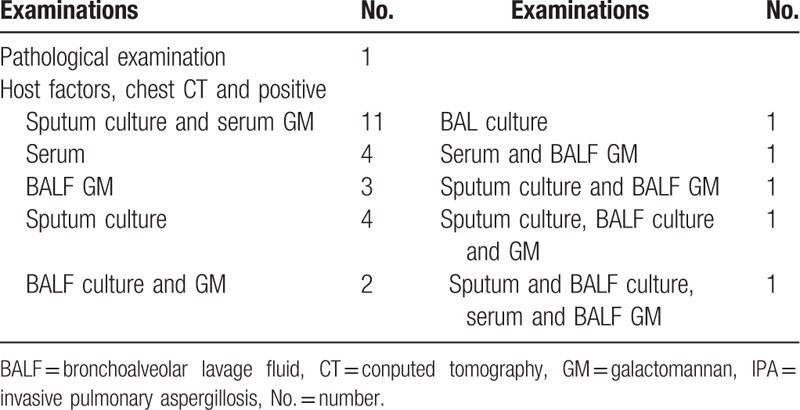
Proven IPA was diagnosed by the histopathology of a lung biopsy. The detailed diagnostic information of 29 probable IPA patients is listed in Table 2. In the IPA patients, a culture positive with Aspergillus spp. was seen in 18 sputum and 5 BALF specimens, and the positive rate of serum GM was 56.7%. A total of 13 IPA patients received the BALF GM assay, and the positive rate was 69.2%.
Table 2.
Microbiological diagnostic methods of IPA patients.
3.2. Clinical performance of the serum A. fumigatus-specific IgM antibody assay
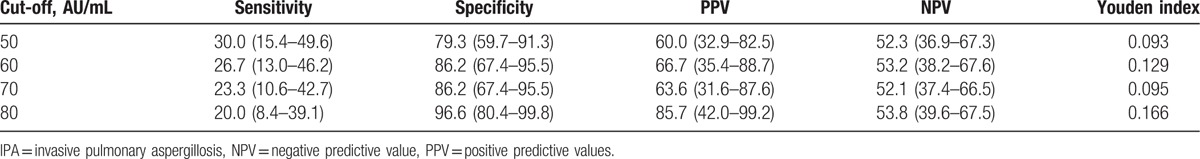
The clinical performance of the serum A. fumigatus-specific IgM antibody assay in IPA patients was assessed. The sensitivity, specificity, Youden index, PPV and NPV, and their 95% confidence intervals (CIs) in different diagnostic cut-offs are shown in Table 3. The sensitivities were 30.0%, 26.7%, 23.3%, and 20.0%, and specificities were 79.3%, 86.2%, 86.2%, and 96.6% at a cutoff value of 50, 60, 70, and 80 AU/mL, respectively. The area under the curve (AUC) of the IgM assay revealed by the ROC analysis was 0.511 (0.360–0.662) in the IPA cases. The optimal cutoff value revealed by ROC analysis was 94.2 AU/mL, and the sensitivity and specificity were 16.7% and 100.0%, respectively.
Table 3.
Serum Aspergillus fumigatus–specific IgM antibody sensitivity and specificity in IPA patients.
4. Discussion
In the present study, we evaluated the clinical performance of a commercial A. fumigatus IgM antibody assay in diagnosis of IPA. Our results suggest that serum A. fumigatus-specific IgM antibody assay can offer little assistance to current diagnostic methods in IPA.
Compared with the non-IPA patients, the IPA patients had a higher frequency of COPD history. The possible reason may be the increased incidence of IPA in patients with COPD in recent years.[10] Taccone et al studied the epidemiology of invasive aspergillosis in critically ill patients and showed that the most common comorbidity condition was COPD.[13] The IPA and non-IPA patients had no significant difference in respiratory symptoms, and the reason could be that IPA patients presented nonspecific clinical symptoms. Our study enrolled suspected IPA patients as the control rather than the healthy patients, which resulted in the similar host factors between IPA and non-IPA patients.
For the A. fumigatus-specific-IgM assay, the sensitivities were 20.0% to 30.0%, and the specificities were 79.3% to 96.6% at diagnostic cutoffs of 50 to 80 AU/mL. The ROC analysis showed the A. fumigatus specific-IgM assay had limited clinical value in diagnosis of IPA, which was in consistent with results from other existing commercial IgM kits.[14] IgM antibody production may not occur during the course of IPA, as most of IPA patients were in immunocompromised conditions.[15] Detection of Aspergillus-specific IgM antibodies is not accepted as the widely used IPA diagnostic criteria.[2] Kappe et al[16] studied IgM antibody levels in IPA patients, and only 2 of 26 patients showed positive IgM antibodies, which agreed with the poor sensitivities shown in our current study. The sensitivities of the A. fumigatus–specific IgM assay on all of the above cut-offs was not competitive compared with other widely used diagnostic methods. The sensitivity of GM assay ranges from 36.4% to 97.0% in BALF sample and 11.6% to 90.9% in serum samples.[4,11,12,17–19] The sensitivity of PCR-based assays ranges from 66.7% to 95.5%[5,20,21] and 77% to 81.8% for Aspergillus-specific LFD tests.[5,19] In regard to the specificity of the A. fumigatus-specific IgM assay, the overall result is acceptable at 80 AU/mL (96.6%) when comparing it with the GM assay (at least 85%),[12,18,20] PCR assays (92.5%–98.7%)[5,20,21] and Aspergillus-specific LFD tests (92%–98.0%).[8,21]
To our best of knowledge, this is the first study to evaluate the clinical performance of a commercial A. fumigatus IgM antibody kit that uses GM extracted from A. fumigatus as sole antigen in the diagnosis of IPA. In conclusion, this serum A. fumigatus-specific IgM antibody assay has a limited value and should not be recommended for IPA diagnosis.
Acknowledgments
The authors offer genuine appreciation to all involved respiratory physicians in the all 12 hospitals for their contribution in specimen and data retrieval.
Footnotes
Abbreviations: A. fumigatus = Aspergillus fumigatus, AU = arbitrary units, AUC = area under the curve, BALF = bronchoalveolar lavage fluid, BDG = (1→3)-β-D-glucan, CI = confidence interval, COPD = chronic obstructive pulmonary disease, CT = computed tomography, ELISA = enzyme-linked immunosorbent assay, EORTC/MSG = The European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group, GM = galactomannan, IPA = invasive pulmonary aspergillosis, LFD = lateral-flow device, NPV = negative predictive value, PCR = polymerase chain reaction, PPV = positive predictive value, ROC = receiver-operating characteristic curve.
YY and HZ contributed equally to this work.
This work was supported by research grants from the Natural Science Foundation of Zhejiang Province (LY16H190004 and LQ13H190001), grants from Health and family planning commission of Zhejiang Province (2015RCA009, 2016KYA075, and 2016ZDA005).
The authors report no conflicts of interest.
References
- [1].Prasad A, Agarwal K, Deepak D, et al. Pulmonary aspergillosis: what CT can offer before it is too late!. J Clin Diagn Res 2016;10:TE01–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Patterson TF, Thompson GR, 3rd, Denning DW, et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis 2016;63:e1–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Brown GD, Denning DW, Gow NA, et al. Hidden killers: human fungal infections. Sci Transl Med 2012;4:165rv113. [DOI] [PubMed] [Google Scholar]
- [4].Pfeiffer CD, Fine JP, Safdar N. Diagnosis of invasive aspergillosis using a galactomannan assay: a meta-analysis. Clin Infect Dis 2006;42:1417–27. [DOI] [PubMed] [Google Scholar]
- [5].White PL, Parr C, Thornton C, et al. Evaluation of real-time PCR, galactomannan enzyme-linked immunosorbent assay (ELISA), and a novel lateral-flow device for diagnosis of invasive aspergillosis. J Clin Microbiol 2013;51:1510–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Thornton CR. Development of an immunochromatographic lateral-flow device for rapid serodiagnosis of invasive aspergillosis. Clin Vaccine Immunol 2008;15:1095–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Bozza S, Clavaud C, Giovannini G, et al. Immune sensing of Aspergillus fumigatus proteins, glycolipids, and polysaccharides and the impact on Th immunity and vaccination. J Immunol 2009;183:2407–14. [DOI] [PubMed] [Google Scholar]
- [8].Page ID, Richardson M, Denning DW. Antibody testing in aspergillosis–quo vadis? Med Mycol 2015;53:417–39. [DOI] [PubMed] [Google Scholar]
- [9].De Pauw B, Walsh TJ, Donnelly JP, et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis 2008;46:1813–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Zhang S, Wang S, Wan Z, et al. The diagnosis of invasive and noninvasive pulmonary aspergillosis by serum and bronchoalveolar lavage fluid galactomannan assay. Biomed Res Int 2015;2015:943691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].D’Haese J, Theunissen K, Vermeulen E, et al. Detection of galactomannan in bronchoalveolar lavage fluid samples of patients at risk for invasive pulmonary aspergillosis: analytical and clinical validity. J Clin Microbiol 2012;50:1258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Zhang XB, Chen GP, Lin QC, et al. Bronchoalveolar lavage fluid galactomannan detection for diagnosis of invasive pulmonary aspergillosis in chronic obstructive pulmonary disease. Med Mycol 2013;51:688–95. [DOI] [PubMed] [Google Scholar]
- [13].Taccone FS, Van den Abeele AM, Bulpa P, et al. Epidemiology of invasive aspergillosis in critically ill patients: clinical presentation, underlying conditions, and outcomes. Crit Care 2015;19:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Kappe R, Schulze-Berge A, Sonntag HG. Evaluation of eight antibody tests and one antigen test for the diagnosis of invasive aspergillosis. Mycoses 1996;39:13–23. [DOI] [PubMed] [Google Scholar]
- [15].Young RC, Bennett JE. Invasive aspergillosis. Absence of detectable antibody response. Am Rev Respir Dis 1971;104:710–6. [DOI] [PubMed] [Google Scholar]
- [16].Kappe R, Rimek D. [Antibody detection in patients with invasive aspergillosis]. Mycoses 2004;47(Suppl 1):55–9. [DOI] [PubMed] [Google Scholar]
- [17].Zou M, Tang L, Zhao S, et al. Systematic review and meta-analysis of detecting galactomannan in bronchoalveolar lavage fluid for diagnosing invasive aspergillosis. PloS One 2012;7:e43347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Fortun J, Martin-Davila P, Gomez Garcia de la Pedrosa E, et al. Galactomannan in bronchoalveolar lavage fluid for diagnosis of invasive aspergillosis in non-hematological patients. J Infect 2016;72:738–44. [DOI] [PubMed] [Google Scholar]
- [19].Prattes J, Flick H, Pruller F, et al. Novel tests for diagnosis of invasive aspergillosis in patients with underlying respiratory diseases. Am J Respir Crit Care Med 2014;190:922–9. [DOI] [PubMed] [Google Scholar]
- [20].Zhang S, Wang S, Wan Z, et al. Quantitative real-time PCR and platelia galactomannan assay for the diagnosis of invasive pulmonary aspergillosis: bronchoalveolar lavage fluid performs better than serum in non-neutropaenic patients. Mycopathologia 2016;181:625–9. [DOI] [PubMed] [Google Scholar]
- [21].Imbert S, Gauthier L, Joly I, et al. Aspergillus PCR in serum for the diagnosis, follow-up and prognosis of invasive aspergillosis in neutropenic and nonneutropenic patients. Clin Microbiol Infect 2016;22:562.e561–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


