Abstract
Rationale:
Blastic plasmacytoid dendritic cell neoplasm (BPDCN), derived from precursors of plasmacytoid dendritic cells, is a rare and aggressive malignancy with frequent cutaneous involvement. Although cutaneous lesions are often chemosensitive, BPDCN portends a poor prognosis as most patients relapse after developing drug resistance.
Patient concerns:
We report a case of a 65-year-old man who presented with a rapidly enlarging hyperpigmented plaque on his shoulder with subsequent similarly appearing macules and plaques on his chest, back, and neck.
Diagnosis:
Skin biopsy revealed a dense adnexocentric dermal infiltrate of immature blastoid cells without epidermal involvement. The infiltrate was immunoreactive for CD4, CD56, CD123, and Bcl-2, but negative for CD3, CD8, CD30, MPO, EBER, and ISH. The patient was diagnosed with BPDCN based on these cell markers.
Intervention:
Bone marrow biopsy and radiologic work-up showed no evidence of extracutaneous involvement. The patient attained partial remission after undergoing 2 rounds of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP regimen) before autologous stem cell transplantation, however, he quickly relapsed and developed new cutaneous lesions.
Outcomes:
The patient was treated with venetoclax, a Bcl-2 inhibitor, and exhibits complete resolution of prior skin findings and continues to remain free of new cutaneous lesions 10 months posttreatment initiation with venetoclax.
Lessons:
Herein, we present a case that supports the use of venetoclax, a Bcl-2 inhibitor, in the off-label treatment of BPDCN with Bcl-2 overexpression. Only 1 prior case has reported the off-label use of venetoclax for the treatment of BPDCN. This case highlights a novel therapeutic option for BPDCN patients unresponsive to traditional treatment.
Keywords: Bcl-2, blastic plasmacytoid dendritic cell neoplasm treatment, hyperpigmented lesions, venetoclax
1. Introduction
Blastic plasmacytoid dendritic cell neoplasm (BPDCN), which has previously been termed blastic natural killer (NK) cell lymphoma or CD4+/CD56+ hematodermic neoplasm, is a rare and aggressive malignancy derived from plasmacytoid dendritic cells (pDCs), which are of myeloid origin.[1] This immature hematopoietic malignancy is most often reported in elderly male adults and is commonly associated with skin, bone marrow, lymph node, and peripheral blood involvement.[2] Patients initially present with numerous erythematous and violaceous papules, nodules, and plaques that resemble purpura or vasculitis.[1] In most cases, cutaneous involvement is the only recognizable extramedullary presentation of this malignancy.[3] Diagnosis of BPDCN relies on immunophenotypic expression of markers CD4, CD56, CD123, CD303, and TCL1 and concomitant absence of CD3, CD11c, MPO, and CD79 on histopathological examination.[2] Skin lesions are fairly responsive to chemotherapy, focal radiation therapy, and systemic glucocorticoids. However, these treatment modalities do not alter the poor prognosis of BPDCN.[2] Due to the lack of prospective data regarding this disease, most patients are treated with chemotherapeutic regimens used for leukemia and lymphomas. These patients tend to have high initial response rates yet tend to relapse relatively quickly. Few studies discuss successful treatment of BPDCN. Herein, we present a case of BPDCN expressing Bcl-2 positivity undergoing novel treatment with an anti-Bcl2 agent, venetoclax.
2. Clinical Data
A 65-year-old Caucasian retired male, previously employed as a firefighter, presented to the Dermatology service after developing an asymptomatic, 1.5 cm pigmented lesion on his right shoulder and scattered erythematous plaques on the trunk and back (Fig. 1A and B). The lesions were enlarging at a rate of 1 mm per week, with about 5 new lesions forming every week over a period of 1 month.
Figure 1.
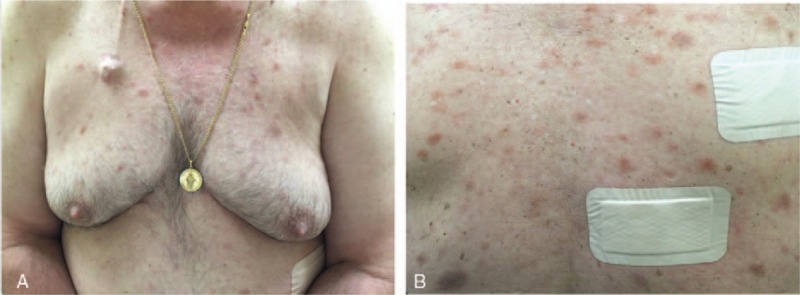
(A and B) Scattered erythematous macules and plaques on the patient's chest and back (color).
The patient had no prior history of skin disease, and past medical history was significant for hypertension, diabetes mellitus type 2, benign lung nodules, and kidney cysts. Family history was significant for a maternal grandmother with leukemia. Physical examination demonstrated a nonspecific 3 cm × 1.5 cm hyperpigmented plaque with mild hyperkeratosis on his right shoulder, with scattered erythematous plaques on the trunk and back. The patient reported frequent chills but denied other associated constitutional symptoms. No lymphadenopathy was identified. The patient was not exposed to any factors that may have correlated with his cutaneous findings.
Histopathological analysis of the lesion demonstrated a dense dermal adnexocentric infiltrate of medium to large cells with irregular angulated nuclei and scant cytoplasm, which spared the epidermis (Fig. 2A and B). Additionally, there was an associated inflammatory infiltrate with scattered B and T-cells. On immunohistochemistry, cells were positive for CD4, CD56, CD123, and LCA (Fig. 3A–C) and negative for CD3, MPO, and in situ hybridization for Epstein-Barr Virus (Table 1). Based on these findings, a diagnosis of BPDCN was rendered.
Figure 2.
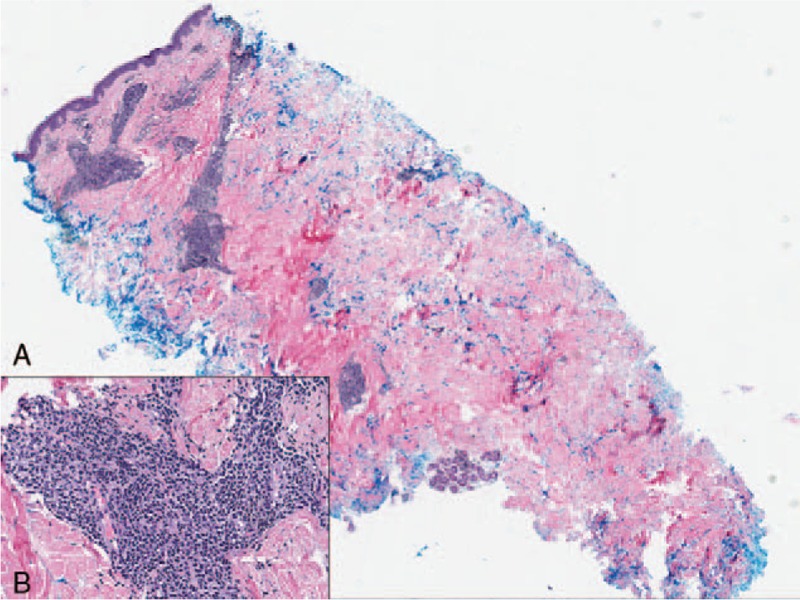
(A and B) Hematoxylin and eosin stain showing dermal adnexocentric infiltrate of medium to large cells with irregular angulated nuclei and scant cytoplasm; 40× magnification of malignant cells (color).
Figure 3.
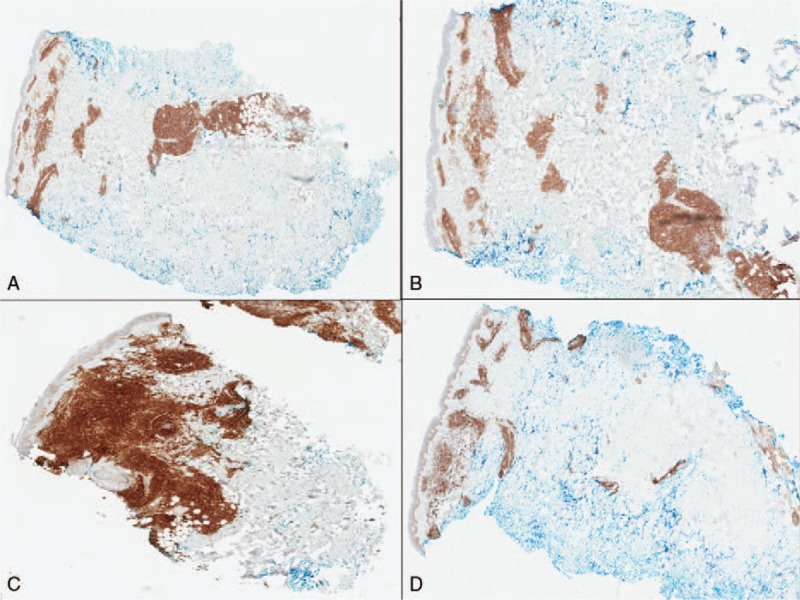
(A) Positive immunohistochemical (IHC) staining with CD4 marker, (B) positive IHC staining with CD56 marker, (C) positive IHC staining with CD123 marker, (D) positive IHC staining with Bcl-2 marker (color). IHC = immunohistochemical.
Table 1.
Immunohistochemical markers present in first skin biopsy.

The results of routine laboratory tests including complete blood count with differential, lactate dehydrogenase, renal and liver function were within normal limits. A bone marrow biopsy and whole body positron emission tomography and computed tomography (PET-CT) scans were performed which demonstrated no evidence of extracutaneous involvement. Two months later, a similar lesion appeared on the patient's left shoulder. Biopsy also revealed BPDCN. The presence of multiple lesions eliminated radiotherapy as a treatment option. The patient was treated systemically with 2 rounds of chemotherapy in July and August of 2015, and achieved complete remission and resolution of his skin lesions. Except for mild hyperglycemia, the patient did not experience adverse effects with CHOP therapy.
Due to the patient's comorbidities, he was not a candidate for allogeneic stem cell transplant and instead underwent autologous stem cell transplant. Eleven months after his transplant, the patient returned to clinic with a new eruption of scattered urticarial poorly marginized plaques on his chest, back, and bilateral shoulders with extension to his neck. Biopsy of 2 representative lesions demonstrated a dense perivascular, perifollicular, and interstitial cellular infiltrate extending into the deep reticular dermis and subcutaneous tissue. The infiltrate was comprised of medium to large blasts with irregular nuclei, finely dispersed chromatin, small nuclei, and a scant cytoplasm that was slightly basophilic. On immunohistochemical staining, the neoplastic cells were positive for CD4, CD56, and Bcl-2 (Fig. 3D), and negative for CD3, CD20, CD8, CD23, CD5, Bcl-6, and MUM-1 (Table 2). BDCA-2/CD303 and BPDCA-4 have also been reported as specific markers for a proportion of pDCs derived tumors.[4] Staining for these markers was not performed in our study. However, positive staining with CD4, CD56, and Bcl-2 in our biopsy was consistent with a diagnosis of relapsed BPDCN.[4,5] No extracutaneous involvement was evident on PET or CT scans. Due to newly positive staining of tumor cells for Bcl-2, treatment was initiated with daily oral administration of venetoclax 400 mg, a Bcl-2 inhibitor normally used to treat chronic lymphocytic leukemia (CLL). Two weeks after initiation of venetoclax, the patient reported onset of infrequent chills and mild diarrhea that did not prompt medical intervention or an infectious work-up (Grade 1—Common Terminology Criteria for Adverse Events (CTCAE)). He denied nausea, vomiting, or a change in appetite. The patient did not experience major adverse effects specific to this medication, such as neutropenia, thrombocytopenia, anemia, or upper respiratory tract infection.[6] After 5 months of treatment with venetoclax, the patient had complete resolution of all cutaneous lesions, including the chest and arms (Fig. 4A and B). Most recent PET/CT scans showed no evidence of abnormal uptake that would suggest neoplastic disease. The patient continues to do well at 10 month follow up, with no new cutaneous lesions or evidence of recurrence.
Table 2.
Immunohistochemical markers present in second skin biopsy.

Figure 4.
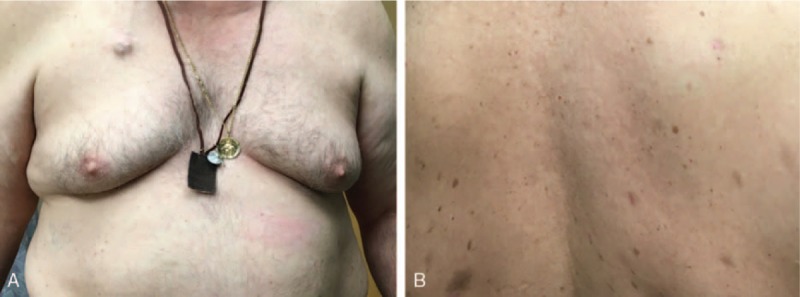
(A) Complete resolution of macules and plaques on patient's chest 5 months after treatment with venetoclax (color). (B) Complete resolution of macules and plaques on patient's back 5 months after treatment with venetoclax (color).
3. Discussion
BPDCN is a rare hematodermic malignancy that has been renamed frequently since it was first reported by Adachi et al in 1994.[5] Initially, cutaneous BPDCN was classified as a T-cell lymphoma based on its CD4+ and CD56+ expression, 2 prominent antigens belonging to the T cell lineage.[3] Subsequently, the lack of other conventional T-cell antigens such as CD3 designated this neoplasm a primary cutaneous NK/T cell lymphoma.[7] In 2008, researchers discovered a strong presence of interleukin-3 receptor alpha chain (CD123) in this neoplasm.[1] Since CD123 is commonly expressed in pDCs, BPDCN was reclassified as a unique myeloid derived pDC neoplasm.[1,3] pDCs are mononuclear cells that are produced in the bone marrow, accumulate in lymph nodes when the immune system is activated, and secrete type-1 interferon in response to nucleic acids.[5,8] These unusual cells, which account for less than 1% of mononuclear cells, also present antigens to T cells, bridging the innate and adaptive immune system.[1,8]
BPDCN typically affects elderly adult males, with reported mean age at diagnosis ranging from 60 to 67 years old (>60 years old, 64%).[1,7] However, several cases have also been reported in childhood and infancy.[1,7] The male to female ratio of this disease is 3.5:1.[9] There are 2 main patterns of evolution of BPDCN. The majority of cases (90%) are characterized by the early presence of multiple skin nodules, followed by rapid tumor dissemination. The second pattern is much less common (10%) and may initially present as a leukemic phase without any skin involvement. Skin lesions most commonly involve the trunk and limbs, often sparing the maxillofacial region.[10] The lesions can appear as patches, plaques, or nodules and are typically brown to violet in color, resembling bruises.[11] Early recognition of this neoplasm is crucial, as up to 15% to 20% of BPDCN cases progress to myelomonocytic leukemia or acute myeloid leukemia.[3,9]
Histologically, BPDCN shows a diffuse, monomorphic infiltrate of medium-sized immature blastoid cells with round nuclei, finely dispersed chromatin, and absent or indistinct nucleoli.[12] Typically, these cells involve the dermis and the subcutaneous adipose tissue, but spare the epidermis.[1,10] Cota et al[12] studied 44 biopsies from cutaneous BPDCN and reported a wide variety of morphological and phenotypic features, with the typical monomorphic blastoid cells appearing in only 44% of their samples. Histopathologic diagnosis can be challenging, contributing to the high rate of misdiagnosis of BPDCN.
Immunohistochemical analysis is the most reliable method to confirm diagnosis and guide treatment. Typically, a diagnosis of BPDCN requires positivity for CD4, CD56, CD123, and TCL-1 without any lineage-specific markers of T cells or B cells.[1–3,7,12,13] Although 1 or 2 of the markers may not stain positive in certain cases, it is uncommon for all 4 to be negative.[12] Cytogenic analysis can also aid in diagnosis. Leroux et al performed cytogenic analysis on 21 cases of BPDCN and reported chromosomal abnormalities in 66% of patients. Specifically, chromosomal losses on 5q, 6q, 13q, 12p, and chromosomes 9 and 15 were frequently encountered.[3,8] This methodology may serve as an effective way to exclude other hematopoietic malignancies such as myeloid sarcoma, acute myeloid leukemia, myelodysplastic syndrome, T cell lymphoblastic leukemia, and cutaneous peripheral T-cell lymphoma.[14] Researchers have also discovered a high frequency of MYB rearrangements in BPDCN, which may expedite diagnosis in patients presenting without a leukemic phase for whom cytogenic analysis would be difficult.[15] A variation in MYB gene fusion induces constitutive MYB activity, resulting in significantly upregulated cell surface molecule-encoding genes such as CD56, CD68, CD363, and CXCR4.[15] As a result, MYB gene translocations have the potential to serve as molecular targets for anticancer therapy as well as diagnostic tools.[15] In addition to MYB gene rearrangement, researchers have utilized gene expression assays to detect levels of antiapoptotic gene Bcl-2 in BPDCN.[16] Sapienza et al[16] found a higher level of Bcl-2 expression in BPDCN compared to normal pDCs, suggesting BPDCN may be a result of aberrant Bcl-2 function. However, there is no research describing the unique phenomenon of Bcl-2 plasticity in BPDCN as was demonstrated in our patient's first (Bcl-2 negative) and second biopsy (Bcl-2 positive).
Although over 90% of patients diagnosed with BPDCN present with cutaneous lesions, this malignancy tends to spread quickly throughout the peripheral blood and bone marrow. From the time of diagnosis, patients typically have a median survival of 9 to 13 months, regardless of their original presentation.[5,14,17] Patients with BPDCN have an extremely poor prognosis, as most patients are older and there are no effective targeted therapies. Studies report that patients presenting with the leukemic form of BPDCN have a shorter median survival rate than patients with cutaneous form, however, retrospective reports needed to confirm this prognostic marker are lacking.[2]
Currently, there is no standardized treatment for this neoplasm and therapy varies based on institutional preference. Initially, cutaneous lesions can be treated with focal radiation, oral glucocorticoids, or chemotherapy.[1,2] Although skin-limited disease has been reported to have a better prognosis than extracutaneous disease, it should be treated aggressively since leukemic dissemination is inevitable.[1,12] Patients with BPDCN are usually treated with regimens used for other hematopoietic malignancies such as non-Hodgkin lymphoma (CHOP) and acute myeloid leukemia and lymphoma (cyclophosphamide/vincristine/doxorubicin/dexamethasone alternating with methotrexate and cytarabine [hyper-CVAD]).[2,14] Initially, patients commonly respond well to chemotherapy with complete resolution of skin lesions, but frequently relapse as chemotherapeutic drug resistance develops.[14]
Research regarding alternative targeted therapies to treat BPDCN is currently underway. In a phase 1 to 2 study, Frankel et al reported a high rate of successful remission in patients treated with SL-401, an IL-3 receptor-targeted drug. Monoclonal antibodies targeting CD123, BDCA2, or BDCA4 may also be successful in the management of BPDCN.[18] Although some cases of BPDCN have been reported with positivity for Bcl-2, only one study has reported the effect of targeted therapy aimed at this protein.[12] A study by Montero et al[19] demonstrated that BPDCN was dependent on the antiapoptotic gene Bcl-2 and was significantly sensitive to Bcl-2 inhibition with venetoclax. They reported 2 patients with relapsed chemotherapy refractory disease who experienced decreased cutaneous involvement and improved lymphadenopathy following treatment with venetoclax.[19] In fact, they found the BPDCN response to venetoclax to be similar to that of CLL.[19]
Due to the aggressive nature of this malignancy, allogeneic bone marrow transplantation is one of the best therapeutic strategies to effectively treat BPDCN.[14] Dalle et al evaluated 47 patients and compared their mean survival after treatment with bone marrow transplant (BMT) or non-BMT. Mean survival time for a total of 10 patients that received BMT was 31.3 months compared with 12.8 months in non-BMT patients.[18] BMT should be considered first line therapy in eligible patients. However, advanced age and comorbidities in many patients with BPDCN limit the use of allogeneic BMT. Studies demonstrate that the best long-term prognosis is in younger patients who undergo allogeneic BMT after chemotherapy-induced remission.[1]
4. Conclusion
We present a case of BPDCN that was treated with 2 rounds of CHOP regimen, autologous stem cell transplant, and an anti-Bcl2 agent, venetoclax. At 10 months posttreatment, the patient exhibits complete resolution of previous skin findings and remains free of new cutaneous lesions (Fig. 4A and B). The patient responded extremely well to treatment with venetoclax and we hope to continue to see positive improvement. Though few reports have described the off-label use of venetoclax for the treatment for BPDCN, this case unveils novel therapeutic possibilities for BPDCN patients unresponsive to traditional treatment modalities.
Footnotes
Abbreviations: BMT = bone marrow transplant, BPDCN = blastic plasmacytoid dendritic cell neoplasm, CHOP regimen = cyclophosphamide, doxorubicin, vincristine, and prednisone, CLL = chronic lymphocytic leukemia, IHC = immunohistochemical, NK = natural killer, pDCs = plasmacytoid dendritic cells, PET-CT = positron emission tomography and computed tomography.
Informed patient consent was obtained to publish the photographic data and clinical history, all in accordance with Declaration of Helsinki.
The authors have no conflicts of interest to disclose.
References
- [1].Gera S, Dekmezian MS, Duvic M, et al. Blastic plasmacytoid dendritic cell neoplasm: evolving insights in an aggressive hematopoietic malignancy with a predilection of skin involvement. Am J Dermatopathol 2014;36:244–51. [DOI] [PubMed] [Google Scholar]
- [2].Riaz W, Zhang L, Horna P, et al. Blastic plasmacytoid dendritic cell neoplasm: update on molecular biology, diagnosis, and therapy. Cancer Control 2014;21:279–89. [DOI] [PubMed] [Google Scholar]
- [3].Kaune KM, Baumgart M, Bertsch HP, et al. Solitary cutaneous nodule of blastic plasmacytoid dendritic cell neoplasm progressing to overt leukemia cutis after chemotherapy: immunohistology and FISH analysis confirmed the diagnosis. Am J Dermatopathol 2009;31:695–701. [DOI] [PubMed] [Google Scholar]
- [4].Marafioti T, Paterson JC, Ballabio E, et al. Novel markers of normal and neoplastic human plasmacytoid dendritic cells. Blood 2008;111:3778–92. [DOI] [PubMed] [Google Scholar]
- [5].Adachi M, Maeda K, Takekawa M, et al. High expression of CD56 (N-CAM) in a patient with cutaneous CD4-positive lymphoma. Am J Hematol 1994;47:278–82. [DOI] [PubMed] [Google Scholar]
- [6].Roberts AW, Davids MS, Pagel JM, et al. Targeting BCL2 with venetoclax in relapsed chronic lymphocytic leukemia. N Engl J Med 2016;374:311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Su O, Onsun N, Demirkesen C, et al. A case of CD4/CD56 hematodermic neoplasm (plasmacytoid dendritic cell neoplasm). Dermatol Online J 2010;16:8. [PubMed] [Google Scholar]
- [8].Leroux D, Mugneret F, Callanan M, et al. CD4(+), CD56(+) DC2 acute leukemia is characterized by recurrent clonal chromosomal changes affecting 6 major targets: a study of 21 cases by the Groupe Français de Cytogénétique Hématologique. Blood 2002;99:4154–9. [DOI] [PubMed] [Google Scholar]
- [9].Facchetti F, Ungari M, Marocolo D, et al. Blastic plasmacytoid dendritic cell neoplasm. Hematology Meeting Reports (formerly Haematologica Reports) 2009;3: [Google Scholar]
- [10].Huang Y, Liu Y, Li K, et al. A woman with rare blastic plasmacytoid dendritic cell neoplasm on the face. Oral Surg Oral Med Oral Pathol Oral Radiol 2016;121:e16–8. [DOI] [PubMed] [Google Scholar]
- [11].Julia F, Petrella T, Beylot-Barry M, et al. Blastic plasmacytoid dendritic cell neoplasm: clinical features in 90 patients. Br J Dermatol 2013;169:579–86. [DOI] [PubMed] [Google Scholar]
- [12].Cota C, Vale E, Viana I, et al. Cutaneous manifestations of blastic plasmacytoid dendritic cell neoplasm-morphologic and phenotypic variability in a series of 33 patients. Am J Surg Pathol 2010;34:75–87. [DOI] [PubMed] [Google Scholar]
- [13].Laribi K, Denizon N, Besançon A, et al. Blastic plasmacytoid dendritic cell neoplasm: from origin of the cell to targeted therapies. Biol Blood Marrow Transplant 2016;22:1357–67. [DOI] [PubMed] [Google Scholar]
- [14].Xin X, Tian M, Huang L, et al. Case report cutaneous lesions from a blastic plasmacytoid dendritic cell neoplasm: a case report and literature review. Int J Clin Exp Med 2016;9:5017–22. [Google Scholar]
- [15].Suzuki K, Suzuki Y, Hama A, et al. Recurrent MYB rearrangement in blastic plasmacytoid dendritic cell neoplasm. Leukemia 2017;31:1629–33. [DOI] [PubMed] [Google Scholar]
- [16].Sapienza M, Fuligni F, Agostinelli C, et al. Molecular profiling of blastic plasmacytoid dendritic cell neoplasm reveals a unique pattern and suggests selective sensitivity to NF-kB pathway inhibition. Leukemia 2014;28:1606–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Frankel AE, Woo JH, Ahn C, et al. Activity of SL-401, a targeted therapy directed to interleukin-3 receptor, in blastic plasmacytoid dendritic cell neoplasm patients. Blood 2014;124:385–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dalle S, Beylot-Barry M, Bagot M, et al. Blastic plasmacytoid dendritic cell neoplasm: is transplantation the treatment of choice? Br J Dermatol 2010;162:74–9. [DOI] [PubMed] [Google Scholar]
- [19].Montero J, Stephansky J, Cai T, et al. Blastic plasmacytoid dendritic cell neoplasm is dependent on BCL2 and sensitive to venetoclax. Cancer Discov 2017;7:156–64. [DOI] [PMC free article] [PubMed] [Google Scholar]


