Supplemental Digital Content is available in the text
Keywords: elderly, Klebsiella pneumoniae, liver abscess, rhabdomyolysis
Abstract
Rationale:
Rhabdomyolysis is a well-known syndrome in clinical practice, although rhabdomyolysis caused by a liver abscess is rarely reported and the patient may lack symptoms that are associated with a primary site of infection. Early recognition of this possibility is needed to avoid diagnostic delay and facilitate treatment. We report the case of a 71-year-old woman with a Klebsiella pneumoniae (KP) pyogenic liver abscess who presented with myasthenia and tea-colored urine and also review the 77 reported cases of bacterial rhabdomyolysis.
Patient concerns:
The patient was 71 years old and presenting with a 7-day history of myasthenia and a 3-day history of tea-colored urine, but without fever or abdominal pain.
Diagnoses:
Laboratory testing in our case revealed rhabdomyolysis, and blood culture revealed KP. Abdominal ultrasonography revealed a hypoechoic enclosed mass, and computed tomography (CT) revealed an enclosed low-density mass (8.3 × 6.6 × 6.1 cm). The main diagnoses were a pyogenic liver abscess with rhabdomyolysis.
Interventions:
Empirically intravenous piperacillin-sulbactam and intravenous potassium treatment, as well as fluid infusions and other supportive treatments were provided after admission. After the diagnosis was confirmed and susceptibility test results were available, we adjusted the antibiotics to cefoperazone and sulbactam, which were maintained for 6 weeks.
Outcomes:
The patient's symptoms relieved and the abnormal laboratory parameters corrected. Follow-up abdominal ultrasonography at 24 months after her discharge revealed that the abscess had disappeared.
Lessons:
Early recognition and careful consideration of the underlying cause of rhabdomyolysis are critical to improving the patient's prognosis. Thus, physicians should carefully consider the underlying cause in elderly patients who present with rhabdomyolysis, as they may lack symptoms of a primary infection.
1. Case report
The patient approved the publication of this report. A 71-year-old woman with a 7-day history of myasthenia and a 3-day history of tea-colored urine, but without fever or abdominal pain, was admitted to our ward on December 1, 2014. She had hypertension, diabetes mellitus, and hyperlipidemia, which had been treated using nifedipine, repaglinide, metformin, and atorvastatin. No herbal supplements or herbal teas were used. The patient did not have a history of strenuous exercise. A physical examination at the admission revealed a temperature of 38.2°C, which increased to 39.5°C during the same day, as well as percussion-related pain in the hepatic region and muscle weakness in both legs.
Laboratory testing revealed leukocytosis (white blood cell (WBC) was 12.97 × 109/L, 94.33% neutrophils), newly emerged severe thrombocytopenia (platelet (PLT) of 27.2 × 109/L), and the urine blood test (dry-chemistry method) was strongly positive without complete red blood cells or WBCs. The serum potassium level was 3.12 mmol/L, the serum creatine kinase (CK) level was significantly elevated (6020 IU/L), and the blood and urine were positive for myohemoglobin (2396.3 and 275.4 ng/mL, respectively). Her C-reactive protein level was 195 mg/L, her procalcitonin level was 3.34 ng/mL, and her glycosylated hemoglobin level was 7.1%. A blood culture from the admission revealed KP. Her serum creatinine level was normal and no findings were observed during screening for cerebrovascular disease, autoimmune disease, hemolytic disease, or altered thyroid function. Negative results were observed from testing for influenza A and B viruses, as well as for antibodies to Mycoplasma and Chlamydia.
Abdominal ultrasonography revealed a hypoechoic mass in the S6 segment of the liver, and abdominal CT (Fig. 1A and B) revealed an enclosed low-density mass in the liver (8.3 cm × 6.6 cm × 6.1 cm), which suggested a liver abscess. Based on these findings, the diagnoses were a pyogenic liver abscess with hypokalemia and rhabdomyolysis. After admission, we empirically provided intravenous piperacillin–sulbactam (2.5 g every 8 hours) for 6 days before the blood culture results were available. Intravenous potassium treatment (9 g/day) was also administered for 4 days before the serum potassium level returned to normal, as well as fluid infusions (2.5–3 L/day) and other supportive treatments. After the diagnosis was confirmed and susceptibility test results were available, we adjusted the antibiotics to cefoperazone and sulbactam (3 g twice per day), which were maintained for 6 weeks. This was because the abscess was large and the patient and her family refused puncture and placement of a drainage tube. The patient's body temperature, CK level, and myohemoglobin levels in the urine and blood subsequently returned to normal (Supplementary Fig. 1). Abdominal ultrasonography at 24 months after discharge revealed that the abscess had disappeared (Fig. 1C).
Figure 1.
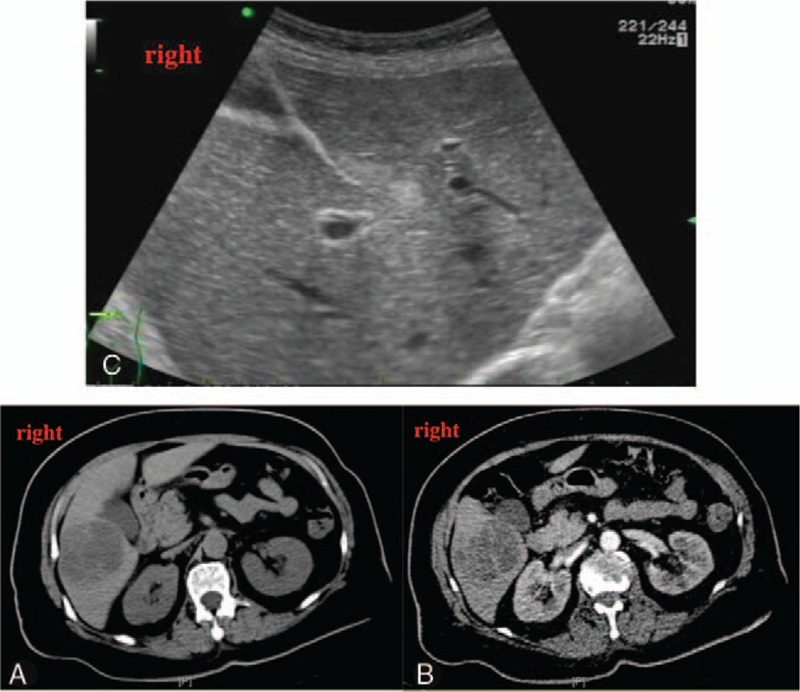
Plain (A) and enhanced (B) abdominal computed tomography revealed an enclosed plurilocular mass. The walls and compartment of the mass exhibited enhancement, although the contents did not. Follow-up ultrasonography at 24 months revealed that the abscess had disappeared (C).
2. Discussion
Liver abscess is a common type of visceral abscess that is caused by a bacterium, fungus, or amoeba. The estimated annual incidence of pyogenic liver abscess is approximately 2.3 cases per 100,000 population, and the incidence is higher among male patients.[1] In China, pyogenic liver abscesses are predominantly caused by KP (77.1% of all cases),[2] although liver abscesses presenting with myasthenia and tea-colored urine (indicative of rhabdomyolysis) are rare. To the best of our knowledge, there is only one English report describing a case of liver abscess-induced rhabdomyolysis.
Rhabdomyolysis is a syndrome that results from separation of striated muscle fibers, which allows muscle enzymes, myoglobin, potassium, calcium, and other molecules to enter the circulation. Two previous studies have indicated that infectious rhabdomyolysis accounts for 5% to 31% of all cases.[3,4] Our review of the 77 reported cases of bacterial rhabdomyolysis indicate that the most common organisms are Legionella spp., Streptococcus spp., and Salmonella spp. (Table 1),[5–32] and that cases most frequently involve patients who are 40 to 50 years old (Fig. 2). The most common site of primary infection leading to rhabdomyolysis is the respiratory system (38–46.1% of all analyzed cases, Fig. 3),[3,5–32] and the liver is rarely involved, with only one case of rhabdomyolysis caused by a liver abscess described in the English literature. That case involved Pantoea agglomerans (bolded in Table 1).[14] Compared to noninfectious rhabdomyolysis, cases of infectious rhabdomyolysis tend to involve older patients (70.81 years old vs 55.31 years old) and more female patients (31.3% vs 9.3%), as well as significantly lower CK levels (3710.1 IU/L vs 19785.4 IU/L).[3]
Table 1.
The 77 reported cases of bacterial rhabdomyolysis.
Figure 2.
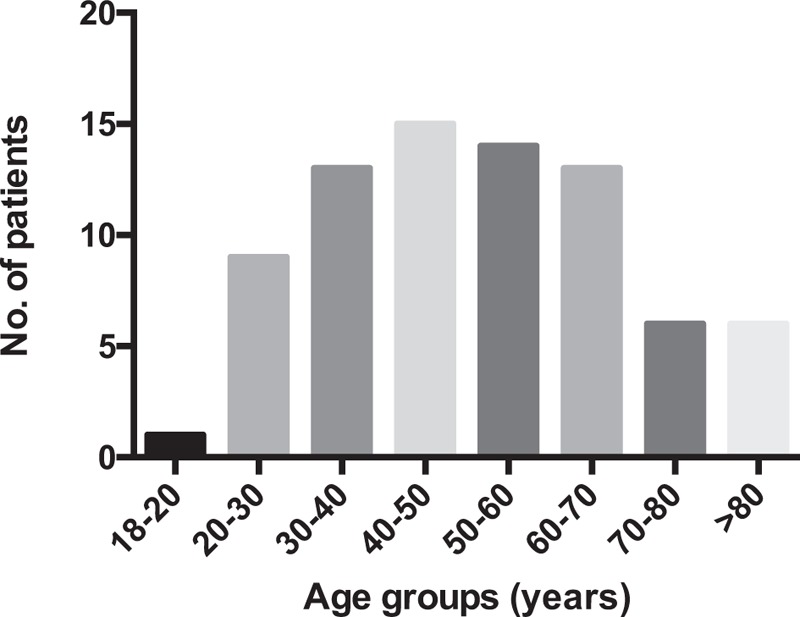
Age distribution for the 77 patients with bacterial rhabdomyolysis.
Figure 3.
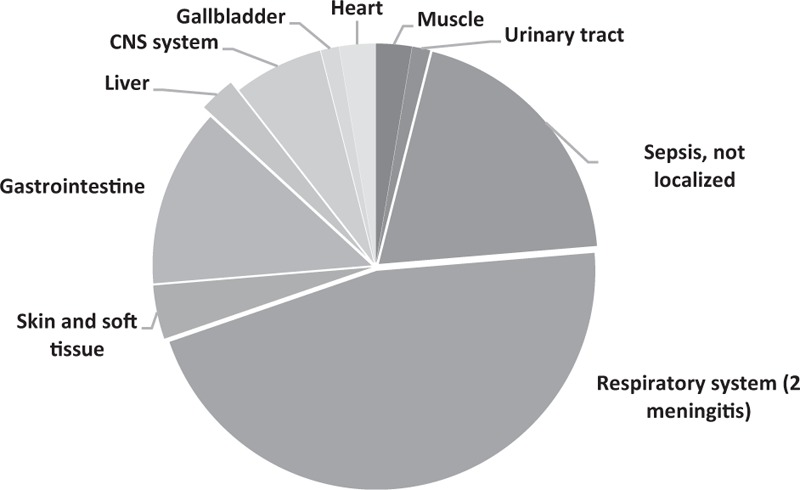
Distribution of the primary site of infection among 76 patients with bacterial rhabdomyolysis. Information regarding the primary site of infection was not available for 1 patient.
Although complete CK data were not available for all reported cases, the CK level in the present case was relatively low (as a product of the rhabdomyolysis), which agrees with the previously reported data. In addition, our patient exhibited concurrent hypokalemia, which likely reflects an even lower serum potassium level before the rhabdomyolysis occurred. Serious hypokalemia can also cause rhabdomyolysis, although the patient did not have any related medical condition and responded well to potassium supplementation after the infection was controlled. Furthermore, studies have revealed a hypokalemia prevalence of 23% among hospitalized patients with infectious disease.[33] Thus, we speculate that the hypokalemia might have been caused by infection and associated multiple organ dysfunction, which can be inferred from the Sequential Organ Failure Assessment score of 6 points at the admission. Moreover, her serum potassium levels normalized after treatment, and the hypokalemia might have contributed to the rhabdomyolysis.
As medications are common causes of rhabdomyolysis, we investigated the patient's medications and observed that she had received nifedipine, repaglinide, metformin, and atorvastatin. A search of the literature (“nifedipine, rhabdomyolysis” and “repaglinide, rhabdomyolysis”) revealed no related cases or data. Although there are cases of metformin-induced rhabdomyolysis, they are typically associated with overdose.[34,35] Interestingly, atorvastatin is the second most prescribed statin (24.4–36%),[36,37] and the incidence of atorvastatin-induced myopathy is approximately 14.9%.[38] However, the patient had discontinued atorvastatin for 1 month before the onset of rhabdomyolysis, and we are not aware of any delayed cases of atorvastatin-induced rhabdomyolysis. Thus, there is no evidence to indicate that our patient had drug-induced rhabdomyolysis.
This case was diagnosed during the influenza season, and influenza can also present with myasthenia and fever, as well as be complicated by myositis or rhabdomyolysis.[39] Thus, this possibility should be considered in similar cases. However, the patient denied being exposed to individuals with a fever, and no influenza A or B viruses were detected after her admission. Therefore, there is no evidence to indicate that influenza virus infection was involved in this case.
In conclusion, elderly patients with a liver abscess may lack symptoms that are associated with a primary site, which can make it difficult to diagnose. However, this condition can cause serious complications, such as rhabdomyolysis. Thus, it is important to investigate the underlying cause in elderly patients with rhabdomyolysis, and to consider the possibility of a liver abscess.
Supplementary Material
Footnotes
Abbreviations: ARF = acute renal failure, CK = creatine kinase, CNS = central nervous system, CT = computed tomography, KP = Klebsiella pneumoniae, PLT = platelet, WBC = white blood cell.
The authors have no conflicts of interest to disclose.
Supplemental Digital Content is available for this article.
References
- [1].Kaplan GG, Gregson DB, Laupland KB. Population-based study of the epidemiology of and the risk factors for pyogenic liver abscess. Clin Gastroenterol Hepatol 2004;2:1032–8. [DOI] [PubMed] [Google Scholar]
- [2].Wang J, Yan Y, Xue X, et al. Comparison of pyogenic liver abscesses caused by hypermucoviscous Klebsiella pneumoniae and non-Klebsiella pneumoniae pathogens in Beijing: a retrospective analysis. J Int Med Res 2013;41:1088–97. [DOI] [PubMed] [Google Scholar]
- [3].Blanco JR, Zabalza M, Salcedo J, et al. Rhabdomyolysis of infectious and noninfectious causes. South Med J 2002;95:542–4. [PubMed] [Google Scholar]
- [4].Byrd RP, Jr, Roy TM. Rhabdomyolysis of infectious and noninfectious causes. South Med J 2002;95:1356–7. [DOI] [PubMed] [Google Scholar]
- [5].Aouina H, Aissa I, Baccar MA, et al. [Acute rhabdomyolysis during pneumococcal pneumonia: two cases]. Rev Pneumol Clin 2007;63:105–8. [DOI] [PubMed] [Google Scholar]
- [6].Boucree MC. Legionnaire's disease and acute renal failure: a case report and literature review. J Natl Med Assoc 1988;80:1065–71. [PMC free article] [PubMed] [Google Scholar]
- [7].Brivet F, Pham Van T, Petitpretz P, et al. Rhabdomyolysis, acute renal failure and Legionnaires’ disease. Chest 1984;86:943–4. [DOI] [PubMed] [Google Scholar]
- [8].Brncic N, Viskovic I, Sasso A, et al. Salmonella infection-associated acute rhabdomyolysis. Some pathogenic considerations. Arch Med Res 2002;33:313–5. [DOI] [PubMed] [Google Scholar]
- [9].Campistol JM, Perez Villa F, Montoliu J, et al. Rhabdomyolysis and acute renal failure associated with Salmonella enteritidis infection. J Hosp Infect 1989;14:267–8. [DOI] [PubMed] [Google Scholar]
- [10].Chun CH, Raff MJ. Rhabdomyolysis associated with pneumococcal sepsis. Diagn Microbiol Infect Dis 1985;3:257–61. [DOI] [PubMed] [Google Scholar]
- [11].Clark P, Lough M, Whiting B. Rhabdomyolysis and listeria monocytogenes. Scott Med J 1989;34:503. [DOI] [PubMed] [Google Scholar]
- [12].Dhaene M, Thys JP, Askenasi R, et al. Pneumococcal cellulitis. Am J Emerg Med 1986;4:225–6. [DOI] [PubMed] [Google Scholar]
- [13].Erdogan H, Yilmaz A, Kal O, et al. Rhabdomyolysis-induced acute renal failure associated with legionnaires’ disease. Scand J Urol Nephrol 2006;40:345–6. [DOI] [PubMed] [Google Scholar]
- [14].Fullerton DG, Lwin AA, Lal S. Pantoea agglomerans liver abscess presenting with a painful thigh. Eur J Gastroenterol Hepatol 2007;19:433–5. [DOI] [PubMed] [Google Scholar]
- [15].Georgescu AM, Azamfirei L, Szalman K, et al. Fatal endocarditis with methicilin-sensible Staphylococcus aureus and major complications: rhabdomyolysis, pericarditis,;1; and intracerebral hematoma: a case report and review of the literature. Medicine (Baltimore) 2016;95:e5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Henrich WL, Prophet D, Knochel JP. Rhabdomyolysis associated with Escherichia coli septicemia. South Med J 1980;73:936–7. [DOI] [PubMed] [Google Scholar]
- [17].Hroncich ME, Rudinger AN. Rhabdomyolysis with pneumococcal pneumonia: a report of two cases. Am J Med 1989;86:467–8. [DOI] [PubMed] [Google Scholar]
- [18].Kaiser AB, Rieves D, Price AH, et al. Tularemia and rhabdomyolysis. JAMA 1985;253:241–3. [PubMed] [Google Scholar]
- [19].Kennedy DH, Love WC, Pinkerton IW. Rhabdomyolysis and systemic infection. Br Med J (Clin Res Ed) 1983;286:1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Khan FY, Al-Ani A, Ali HA. Typhoid rhabdomyolysis with acute renal failure and acute pancreatitis: a case report and review of the literature. Int J Infect Dis 2009;13:e282–5. [DOI] [PubMed] [Google Scholar]
- [21].Malvy D, Dessalles PH, Monseau Y, et al. Legionnaire's disease and rhabdomyolysis. Intensive Care Med 1992;18:132–3. [DOI] [PubMed] [Google Scholar]
- [22].Marino PL, Nahass GT, Novick W. Bacteremic pneumococcal pneumonia and myoglobinuric renal failure. Am J Med 1986;80:521–2. [DOI] [PubMed] [Google Scholar]
- [23].Naschitz JE, Yeshurun D, Shagrawi I. Rhabdomyolysis in pneumococcal sepsis. Am J Med 1989;87:479–80. [DOI] [PubMed] [Google Scholar]
- [24].Posner MR, Caudill MA, Brass R, et al. Legionnaires’ disease associated with rhabdomyolysis and myoglobinuria. Arch Intern Med 1980;140:848–50. [PubMed] [Google Scholar]
- [25].Ravry C, Fedou AL, Dubos M, et al. Severe rhabdomyolysis associated with staphylococcus aureus acute endocarditis requiring surgery. Surg Infect (Larchmt) 2015;16:840–2. [DOI] [PubMed] [Google Scholar]
- [26].Rodriguez EE, Woods KL, Deno RE, et al. Rhabdomyolysis-induced acute renal failure in Legionnaires’ disease. South Med J 1983;76:1328–9. [DOI] [PubMed] [Google Scholar]
- [27].Shimura C, Saraya T, Wada H, et al. Pathological evidence of rhabdomyolysis-induced acute tubulointerstitial nephritis accompanying Legionella pneumophila pneumonia. J Clin Pathol 2008;61:1062–3. [DOI] [PubMed] [Google Scholar]
- [28].Simcock D. Gastroenteritis, fever and myoglobinuric renal failure. J R Soc Med 2004;97:185–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Singh U, Scheld WM. Infectious etiologies of rhabdomyolysis: three case reports and review. Clin Infect Dis 1996;22:642–9. [DOI] [PubMed] [Google Scholar]
- [30].Spataro V, Marone C. Rhabdomyolysis associated with bacteremia due to Streptococcus pneumoniae: case report and review. Clin Infect Dis 1993;17:1063–4. [DOI] [PubMed] [Google Scholar]
- [31].Stano F, Brindicci G, Monno R, et al. Aeromonas sobria sepsis complicated by rhabdomyolysis in an HIV-positive patient: case report and evaluation of traits associated with bacterial virulence. Int J Infect Dis 2009;13:e113–8. [DOI] [PubMed] [Google Scholar]
- [32].Thomas F, Ravaud Y. Rhabdomyolysis and acute renal failure associated with Listeria meningitis. J Infect Dis 1988;158:492–3. [DOI] [PubMed] [Google Scholar]
- [33].Widodo D, Setiawan B, Chen K, et al. The prevalence of hypokalemia in hospitalized patients with infectious diseases problem at Cipto Mangunkusumo Hospital, Jakarta. Acta Med Indones 2006;38:202–5. [PubMed] [Google Scholar]
- [34].Ursini F, Succurro E, Grembiale A, et al. Acute rhabdomyolysis during treatment with amisulpride and metformin. Eur J Clin Pharmacol 2010;66:321–2. [DOI] [PubMed] [Google Scholar]
- [35].Galea M, Jelacin N, Bramham K, et al. Severe lactic acidosis and rhabdomyolysis following metformin and ramipril overdose. Br J Anaesth 2007;98:213–5. [DOI] [PubMed] [Google Scholar]
- [36].van Staa TP, Carr DF, O’Meara H, et al. Predictors and outcomes of increases in creatine phosphokinase concentrations or rhabdomyolysis risk during statin treatment. Br J Clin Pharmacol 2014;78:649–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Jiang L, Li J, Feng F, et al. Survey of statin usage in 4429 diabetic patients with atherosclerostic cardiovascular disease in China. Chin J Cardiovasc Med 2010;15:264–8. [Google Scholar]
- [38].Bruckert E, Hayem G, Dejager S, et al. Mild to moderate muscular symptoms with high-dosage statin therapy in hyperlipidemic patients—the PRIMO study. Cardiovasc Drugs Ther 2005;19:403–14. [DOI] [PubMed] [Google Scholar]
- [39].Sato E, Nakamura T, Koide H. Rhabdomyolysis induced by influenza A infection: case report and review of literature. Ther Apher Dial 2011;15:208–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


