Summary
Recent evidence has shown that microRNA‐126 (miR‐126) has been involved in the development and function of immune cells, which contributed to the pathogenesis of related clinical diseases. However, the potential role of miR‐126 in the development and function of CD4+ T cells remains largely unknown. Here we first found that the activation and proliferation, as well as the expression of interferon (IFN)‐γ, of CD4+T cells from miR‐126 knock‐down (KD) mice using the miRNA‐sponge technique were enhanced significantly in vitro, compared with those in CD4+ T cells from wild‐type (WT) mice. To monitor further the possible effect of miR‐126 deficiency on the function of CD4+ T cells in vivo, we used dextran sulphate sodium (DSS)‐induced murine model of acute autoimmune colitis and found that miR‐126 deficiency could elevate the pathology of colitis. Importantly, the proportion of CD4+ T cells in splenocytes increased significantly in miR‐126KD mice. Moreover, the expression levels of CD69 and CD44 on CD4+ T cells increased significantly and the expression level of CD62L decreased significantly. Of note, adoptive cell transfer assay showed that the pathology of colitis was more serious in carboxyfluorescein succinimidyl ester (CFSE)‐labelled miR‐126KD CD4+ T cell‐transferred group, compared with that in the CFSE‐labelled WT CD4+ T cells transferred group. Consistently, the expression levels of CD69 and CD44 on CFSE+ cells increased significantly. Furthermore, both the proliferation and IFN‐γ secretion of CFSE+ cells also increased significantly in the CFSE‐labelled miR‐126KD CD4+ T cell‐transferred group. Mechanistic evidence showed that the expression of insulin receptor substrate 1 (IRS‐1), as a functional target of miR‐126, was elevated in CD4+ T cells from miR‐126KD mice, accompanied by altered transduction of the extracellular regulated kinase, protein B (AKT) and nuclear factor kappa B (NF‐κB) pathway. Our data revealed a novel role in which miR‐126 was an intrinsic regulator in the function of CD4+ T cells, which provided preliminary basis for exploring further the role of miR‐126 in the development, function of CD4+ T cells and related clinical diseases.
Keywords: CD4+ T cell, cytokines, DSS, IRS‐1, miR‐126
Introduction
MicroRNA‐126 (miRNA‐126, miR‐126) is an important member of microRNA families, localized within intron 7 of epidermal growth factor‐like domain‐containing protein 7 (EGFL7), and expressed highly in endothelial cells of blood vessel, heart and lung, as well as regulating the development of angiogenesis and cardiovascular diseases, etc. 1, 2, 3, 4, 5. Some new evidence has shown further that miR‐126 also plays an important role in the development of various cancers. For instance, Zhang et al. 6 have shown that miR‐126 and miR‐126* inhibits breast cancer metastasis by repressing the recruitment of mesenchymal stem cells and inflammatory monocytes. Other studies have also reported that the expression of miR‐126 is down‐regulated in lung cancer, gastric cancer and ovarian cancer, indicating that it is a promising new tumour suppressor gene 7, 8, 9, 10.
Recently, significant progress also has been made in determining the role of miR‐126 in the regulation of immune‐related diseases. For example, Feng et al. 11 reported that miR‐126 expression is up‐regulated during the development of ulcerative colitis, accompanied by inhibition of its target gene, IκBα. Zhang et al. 12 found that circulating miR‐126 is a potential biomarker to predict the onset of type 2 diabetes mellitus in susceptible individuals. Importantly, numerous studies have documented that miR‐126 also plays an important regulatory role in the function of various immune cells, such as plasmacytoid dendritic cells (pDCs), through changing the expression of vascular endothelial growth factor receptor 2 (VEGFR2) 13. Moreover, Okuyama et al. 14 reported that miR‐126, as a potential alternative to transcriptional factors, regulates the function of B cell myeloid progenitors. Interestingly, Zhao et al. 15 further found that the expression of miR‐126 is up‐regulated in CD4+ T helper type 2 (Th2) cells from systemic lupus erythematosus disease, in which miR‐126 could regulate DNA hypomethylation through DNA methyltransferase 1 (DNMT1). Similarly, our previous evidence also showed that miR‐126 could regulate the function and induction of CD4+forkhead box protein 3 (Foxp3)+ regulatory T cells through the phosphoinositide 3‐kinase/protein kinase B (PI3K/AKT) pathway 16. However, the exact roles and mechanisms of miR‐126 involved in the development and function of CD4+T cells and their distinct subsets remain to be elucidated fully.
In the present study, we first accessed the potential effect of miR‐126 deficiency on the function of CD4+ T cells from miR‐126 knock‐down (KD) mice using the miRNA‐sponge technique. Our data show that the activation and proliferation, as well as the expression of interferon (IFN)‐γ, of CD4+ T cells from miR‐126KD mice, is enhanced significantly in vitro. Importantly, we found that miR‐126 deficiency could also elevate the activation, proliferation and expression of IFN‐γ of CD4+ T cells in vivo, which ultimately promotes the pathology of colitis in the dextran sulphate sodium (DSS)‐induced murine autoimmune colitis mode. Finally, we found that the expression of insulin receptor substrate 1 (IRS‐1), as a functional target of miR‐126, is elevated in CD4+ T cells from miR‐126KD mice, accompanied by altered transduction of the extracellular regulated kinase (ERK), kinase, protein B (AKT) and nuclear factor kappa B (NF‐κB) pathway. Thus, our data reveal a novel role in which miR‐126 is an intrinsic regulator in the function of CD4+ T cells, which provides a preliminary basis for further exploration into the role of miR‐126 in the development and function of CD4+ T and their subsets, as well as related clinical diseases.
Materials and methods
Mice
We established Friend leukaemia virus B (FVB)/N miR‐126KD mice (8–10 weeks old, n = 8) using the plasmids encoding enhanced green fluorescent protein (pEGFP)‐C2‐miR‐126 sponge sequence with the help of Cyagen Biosciences Inc. (Santa Clara, CA, USA). All animals were housed under specific pathogen‐free conditions at Zunyi Medical College, and all animal experiments were performed according to the guidelines for the Care and Use of Laboratory Animals (Ministry of Health, China, 1998). All the experimental procedures were approved by the ethical guidelines of Zunyi Medical College Laboratory Animal Care and Use committee (permit number 2014026).
Preparation of single‐cell suspensions
We collected spleen from female wild‐type (WT) FVB/N mice (8–10 weeks old) and miR‐126KD mice, respectively. Then spleen was put into gentle magnetic affinity cell sorting (MACS) C tubes loaded with 7 ml PBE [PBS + 0.5% fetal bovine serum (FBS) and ethylenediamine tetraacetic acid (EDTA)]. The program of m_spleen_01 was selected and was taken for 56 s. After completion of the program, the cells were filtered using a 200‐mesh sterile cells strainer, and the whole cell suspension was spun down at 265 (×g) and 4°C for 10 min. We discarded the supernatant and added 3 ml red blood cell (RBC) pyrolysis liquid to resuspend the cell pellets on ice for 15 min. Then, a 10 ml PBS suspension of splenocytes was added, filtered by a 200‐mesh sterile cells strainer, repeated twice, into a new 15‐ml centrifuge tube for the next experimental protocol.
CD4+CD62L+ T cells purified by MACS
After the number of single‐cell suspensions from mice spleen was counted, the whole cell suspension was spun down at 265 (×g) and 4°C for 10 min. We discarded the supernatant and resuspended the cell pellets in 40 μl PBS buffer per 107 total cells. First, we added 10 μl of CD4+ T cell biotin–antibody cocktail per 107 total cells, mixed well and incubated in 4°C for 20 min, then added 30 μl 0·5% PBE and 20 μl anti‐biotin MicroBeads per 107 total cells at the same time, mixed well and incubated in 4°C for 20 min, and the whole cell suspension was spun down at 265 (×g) and 4°C for 10 min. The supernatant was discarded and 2 ml 0·5% PBE were added to the resuspended cell pellets for spare parts. Secondly, the LS column was placed into the magnetic field of a suitable MACS separator. The column was prepared by rinsing with 2 ml of 0·5% PBE and the cell suspension was applied onto the column; the column was then washed with 2 ml of 0·5% PBE. The flow‐through containing labelled cells was collected, representing the enriched CD4+ T cells. Thirdly, we added 200 μl CD62 (L‐selectin) MicroBeads and 800 μl 0·5% PBE, mixed well and incubated in 4°C for 20 min; the whole cell suspension was spun down at 265 (×g) and 4°C for 10 min. The supernatant was discarded and 2 ml 0·5% PBE was added to the resuspend cell pellets for spare parts. Lastly, the LD column was place into the magnetic field of a suitable MACS separator. The column was prepared by rinsing with 2 ml of 0·5% PBE and the cell suspension was applied onto the column; the column was then washed with 2 ml of 0·5% PBE. The flow‐through containing unlabelled cells was collected, then 1 ml 0·5% PBE was added to the LD column twice, squeezing the liquid with piston. Then, the flow‐through containing labelled cells was collected, representing the enriched CD4+CD62L+ T cells.
T cell activation, proliferation and electroporation transfection
All cells were cultured in a humidified atmosphere of 5% CO2 at 37°C. Coating with 20 μg/ml anti‐CD3e (eBioscience, San Diego, CA, USA; 16‐0031‐86) for 4°C overnight in plates and cells were co‐stimulated with 4 ng/ml anti‐CD28 (eBioscience; 16‐0281‐85) antibody and 10 ng/ml plus interleukin (IL)‐2 (ProSpec, cyt‐370‐b). For transfection, cells were transfected with IRS‐1 RNAi vector or IRS‐1 RNAi‐negative vector with the mouse T cell nucleofector kit (Lonza, Allendale, NJ, USA). The CD4+ T cell proliferation was evaluated after electroporation transfection using a Cell Counting Kit‐8 (CCK8) kits (Boster Biological Technology Co., Pleasanton, CA, USA). All cells were harvested at the time‐points indicated for the following experiments.
Real‐time polymerase chain reaction (PCR) assay
Conventional primers were obtained from Shanghai Sangon Biological Engineering Co. (Shanghai, China) and the TaqMan probes of miR‐126 (000386) and U6 (001793) were purchased from Life Technologies (Carlsbad, CA, USA); the other reagents were from Takara Bio Inc. (Shiga, Japan). Reverse transcriptase reactions and real‐time PCR assays were performed according to the manufacturers' protocols. All reverse transcriptase reactions, including no‐template controls and reverse transcriptase minus controls, were run in triplicate in Bio‐Rad CFX96 (Bio‐Rad Laboratories, Hercules, CA, USA). The following primer sequences were used: IL‐4 forward: 5'‐AACGAGGTCACAGGAGAA‐3', reverse: 5'‐CCTTGGAAGCCCTACAGA‐3'; IL‐6 forward: 5'‐GGAAATCGTGGAAATGAG‐3', reverse: 5'‐AGGACTCTGGCTTTGTCT‐3'; IL‐10 forward: 5'‐TACAGCCGGGAAGACAATAA‐3', reverse: 5'‐AGGAGTCGGTTAGCAGTATG‐3'; IL‐12 forward: 5'‐CCCCATTCCTACTTCTCC‐3', reverse: 5'‐ACGCACCTTTCTGGTTACAC‐3'; IFN‐γ forward: 5'‐TCTGAGACAATGAACGCTAC‐3', reverse: 5'‐TTCCACATCTATGCCACT‐3'; transforming growth factor (TGF)‐β forward: 5'‐GGCGGTGCTCGCTTTGTA‐3', reverse: 5'‐TCCCGAATGTCTGACGTATTG‐A‐3'; tumour necrosis factor (TNF)‐α forward: 5'‐CAGGGGCCACCACGCTCTTC‐3', reverse: 5'‐TTTGTGAGTGTGAG‐GGTCTGG‐3'; and IRS‐1 forward: 5'‐GGGGTCTGCTACGGTTTG‐3', reverse: 5'‐GGTATTGGTCT‐CACGGTT‐3'. Gene expression levels were quantified using the Bio‐Rad CFX96 detection system (Bio‐Rad Laboratories). Relative expression was calculated with the comparative threshold cycle (Ct) method.
Flow cytometry
Surface markers of CD4+ T cells were evaluated by flow cytometry (FCM) with Beckman Gallios (Beckman Coulter, Inc., Brea, CA, USA). Flow cytometry was performed on Beckman Gallios (Beckman Coulter, Inc.) with Cell Quest Pro software using directly conjugated monoclonal antibodies (mAbs) against the following markers: CD4‐peridinin chlorophyll‐cyanin 5.5 (PerCP‐Cy5.5) (12‐0041‐82), CD62L‐phycoerythrin (PE) (17‐5941‐81), CD44‐allophycocyanin (APC) (17‐5711‐81), CD69‐APC (17‐0691‐82), with corresponding isotype‐matched controls (eBioscience). Ki‐67‐APC (50‐5698‐82), IL‐4‐APC (17‐7041‐81), IL‐17A‐PC5.5 (45‐7177‐82), IFN‐γ‐PC5.5 (45‐7311‐82), p‐AKT‐APC (17‐9715‐42), p‐ERK1/2‐PE (12‐9109‐42), p‐NF‐κB (Cell Signaling Technology, Danvers, MA, USA; no. 3039) and anti‐rabbit immunoglobulin (Ig)G Fab2‐PE (Cell Signaling Technology; no. 8885). Before staining we used intracellular (IC) fixation buffer and permeabilization buffer (eBioscience) to destroy cell membranes. All cells were stained with the corresponding antibodies (1 : 100), respectively, at 4°C for 30 min and washed twice before analysis, according to the manufacturer's protocol.
Induction of autoimmune colitis
Three percent DSS was purchased from MP Biomedicals (Strasbourg, France) in drinking water. Mice were administered orally with distilled water. After drinking for 10 days instead of normal drinking water, the mice were euthanized on day 14 (the mice were only drinking distilled water for contrast). Then, the entire colon was removed and measured for length.
Assessment of the severity of colitis and histology
Pathology and histology were scored using a previously validated scoring system 17, 18. Pathology was scored as follows: (i) weight loss (no change = 0, < 5% = 1, 6–10% = 2, 11–20% = 3, > 20% = 4); (ii) faeces (normal = 0, pasty, semiformed = 2, liquid, sticky or unable to defecate after 5 min = 4), (iii) blood (no blood = 0, visible blood in rectum = 1, visible blood on fur = 2); and (iv) general appearance (normal = 0, piloerection = 1, lethargy and piloerection = 2, motionless, sickly = 4). For histological examination, the colons were removed, rolled around a cotton swab, fixed in 10% formaldehyde and paraffin‐embedded. The paraffin blocks were sectioned serially longitudinally with at a thickness of 5 mm and were stained with haematoxylin and eosin (H&E) to allow histological examination of whole colons at ×100 and ×400 magnification by light microscopy. Histology was scored as follows: (i) inflammation severity: none = 0, slight = 1, moderate = 2, severe = 3; (ii) depth of injury: none = 0, mucosal = 1, mucosal and submucosal = 2, transmural = 3; (iii) crypt damage: none = 0, basal one‐third damaged = 1, basal two‐thirds damaged = 2, only surface epithelium intact = 3, entire crypt and epithelium lost = 4; and (iv) percentage of area involved: none = 0, 1–25% = 1, 26–50% = 2, 51–75% = 3, 76–100% = 4. The final scores are the averages of all individual scores of six pieces per colon.
Adoptive transfer experiment
In the adoptive transfer model, 106 cells/ml CD4+CD62L+ T cells purified from WT or miR‐126KD mice by MACS were resuspended in RPMI‐1640 medium. Then 2 μl carboxyfluorescein succinimidyl ester (CFSE) was added to cell fluid per millilitre of liquid, followed by 2 ml PBS buffer solution. After 30 min at 37°C, ×5 vol liquid PBE was added gently to stop staining. Cells were terminated, respectively, according to 107 cells in each mouse by intravenous (i.v.) tail injection to WT mice and 3% DSS water solution feeding; the mice were euthanized on day 14.
Western blotting
Western blotting was performed on cytosolic cellular extracts. Equal amounts of protein were resolved under reducing conditions on a 10% sodium dodecyl sulphate–polyacrylamide gel (SDS‐PAGE). Protein migration was assessed using protein standards (Bio‐Rad). Transfer to a nitrocellulose membrane was performed for 1 h at 250 mA using a wet transfer system. Equal protein loading was confirmed with Ponceau staining. The membrane was washed in 5% skimmed milk in PBS plus 0·05% Tween 20 (PBST) for 2 h to block non‐specific protein‐binding sites on the membrane. Immunoblotting was performed using a mAb to IRS‐1 (Cell Signaling Technology; no. 2390) and glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) (Cell Signaling Technology; no. 4970) at a dilution of 1 : 800 in a non‐fat milk–Tris buffer. The membrane was then washed in PBST and probed subsequently with a secondary antimouse or rabbit antibody conjugated to horseradish peroxidase (HRP) (Cell Signaling Technology; no. 7074) at a dilution of 1 : 2000. The signal was detected and analysed using the chemiluminescence imaging system (ChemiScope5600; Clinx, Shanghai, China). Each experiment was performed in triplicate.
Construction of the IRS‐1 RNAi vector
We designed a synthesis of the sequence of IRS‐1 RNAi: sense: 5′‐CAGACTCGAACTAT‐TTCACAATTCA‐3′, antisense: 5′‐TGAATTGTGAAATAGTTCGAGTCTG‐3′ and then subcloned into BamHI and EcoRI sites of pLVX‐shRNA1. The plasmid DNA sequence was confirmed by sequencing.
Statistical analyses
Statistical analyses of the data were performed with GraphPad PrismTM (Graphpad Software Inc., San Diego, CA, USA) and the aid of analysis programs in spss version 16.0 software. All data are presented as the mean X ± S. Student's t‐test was used when two conditions were compared, and analysis of variance with Bonferroni or Newman–Keuls correction was used for multiple comparisons. Probability values of < 0·05 were considered significant.
Results
Mir‐126 deficiency enhanced the activation and proliferation of CD4+ T cells in vitro
To investigate the potential role of miR‐126 in the function of CD4+ T cells, we first detected the relative expression of miR‐126 in various organs and tissues. As shown in Supporting information, Fig. S1a, we found that the relative expression level of miR‐126 was higher in spleen than in other immune organs (P < 0·05). However, we further found that the expression of miR‐126 decreased significantly in CD4+ T cell‐deprived splenocytes (Supporting information, Fig. S1b, P < 0·05), indicating that miR‐126 might be expressed predominantly in CD4+ T cells. We then generated miR‐126KD mice using the miRNA‐sponge technique, according to our recent work 19. As shown in Supporting information, Fig. S2, the expression levels of miR‐126 in indicated organs and tissues decreased significantly in miR‐126KD mice compared with those in WT mice (P < 0·05). Importantly, our data showed further that the proportion of CD4+ T cells increased significantly in splenocytes in miR‐126KD mice compared with that in control mice (Supporting information, Fig. S3a,b, P < 0·05). These data suggest that miR‐126 might be involved in the development and function of CD4+ T cells.
To study further the exact role of miR‐126 in the function of CD4+ T cells, we purified CD4+CD62L+ T cells from miR‐126KD mice and observed the possible change in activation and function. Expectedly, our results showed that the relative expression of miR‐126 decreased significantly in CD4+ T cells from miR‐126KD mice compared with the WT mice group (Fig. 1a, P < 0·01). Importantly, we found that the expression levels of surface active molecules, including CD44 and CD69, were elevated in CD4+ T cells from miR‐126KD mice stimulated by anti‐CD3/anti‐CD28 antibody (Fig. 1b,c, P < 0·05). Conversely, these CD4+ T cells expressed lower level of CD62L (Fig. 1b,c, P < 0·05), indicating an enhanced activation phenotype. We further detected the expression of intracellular proliferating nuclear antigen Ki‐67 in CD4+ T cells, and found that the proportion of Ki‐67+ in CD4+ T cells from miR‐126KD mice increased significantly (Fig. 1d,e, P < 0·001). To confirm this finding further, we also detected the proliferation of CD4+ T cells stimulated by concanavalin A (ConA) and obtained similar results (Supporting information, Fig. S4a,b, P < 0·01). Finally, we also found that the apoptosis proportion of CD4+ T cells did not change significantly (Fig. 1f,g, P > 0·05). All these data suggest that miR‐126 deficiency could clearly enhance the activation and proliferation capacity of CD4+ T cells in vitro.
Figure 1.

MicroRNA‐126 (Mir‐126) deficiency enhanced the activation and proliferation of CD4+ T cells. CD4+CD62L+ T cells purified by magnetic‐activated cell sorting (MACS) from splenocytes in miR‐126 knock‐down (KD) mice and wild‐type (WT) mice (8–10 weeks old, n = 6) respectively. Next, cells were cultured in the presence of anti‐CD3 (20 μg/ml)/anti‐CD28 (4 ng/ml) antibody plus interleukin (IL)‐2 (10 ng/ml) for 72 h. Then, the relative expression of miR‐126 was detected by real‐time polymerase chain reaction (RT–PCR) assay (a). The expression levels of CD69, CD62L and CD44 on CD4+ T cells were analysed by fluorescence activated cell sorting (FACS) (b) and calculated (c). Proliferation of CD4+ T cells from miR‐126KD mice and WT mice were assessed by Ki‐67 staining (d) and calculated (e). (f) The apoptosis of CD4+ T cells was also analysed by FACS and calculated (g). One representative example of three independent experiments is shown. *P < 0·05; **P < 0·01; ***P < 0·001. NS=not significant. [Colour figure can be viewed at wileyonlinelibrary.com]
Mir‐126 deficiency affected the expression of cytokines in CD4+ T cells
Next, we investigated the potential effect of miR‐126 deficiency on cytokine expression of CD4+ T cells. Real‐time PCR assay showed a lower‐level expression of cytokines IL‐4 and IL‐10 in CD4+ T cells from miR‐126KD mice compared with WT mice (Fig. 2a, P < 0·05). Conversely, we found that the expression levels of cytokines including IL‐12, TGF‐β, TNF‐α and IFN‐γ were higher in CD4+ T cells from miR‐126KD mice (Fig. 2a, P < 0·05), even though the level of expression of cytokine IL‐6 in CD4+ T cells from miR‐126KD mice did not change significantly compared with WT mice (Fig. 2a, P > 0·05). To confirm this phenomenon, we further detected the proportion of relative cytokines of CD4+ T cells by flow cytometry. As shown in Fig. 2b,c, the proportion of IL‐4+ cells in CD4+ T cells from miR‐126KD mice decreased significantly compared with that in CD4+ T cells from WT mice (P < 0·01). Meanwhile, the proportion of IFN‐γ+ cells increased noticeably (Fig. 2b,c, P < 0·05). In addition, we found that the proportion of IL‐17A+ cells in CD4+ T cells from miR‐126KD mice did not change significantly (Fig. 2b,c, P > 0·05). Collectively, these data suggest that miR‐126 deficiency also could alter secretion of related cytokines in CD4+ T cells in vitro.
Figure 2.
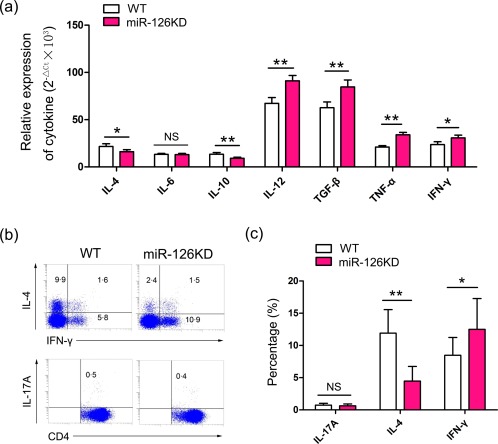
MicroRNA‐126 (Mir‐126) deficiency altered the expression of cytokines in CD4+ T cells. CD4+CD62L+ T cells purified by magnetic‐activated cell sorting (MACS) from splenocytes in miR‐126 knock‐down (KD) mice and wild‐type (WT) mice (8–10 weeks old, n = 6) were cultured in the presence of anti‐CD3 (20 μg/ml)/anti‐CD28 (4 ng/ml) antibody plus interleukin (IL)‐2 (10 ng/ml); 72 h later, the relative expression of cytokines including IL‐4, IL‐6, IL‐10, IL‐12, transforming growth factor (TGF)‐β, tumour necrosis factor (TNF)‐α and interferon (IFN)‐γ were detected by real‐time polymerase chain reaction (RT–PCR) and calculated (a). (b) The expression levels of IFN‐γ, IL‐4 and IL‐17A in CD4+ T cells were analysed by fluorescence activated cell sorting (FACS) and calculated (c). One representative example of three independent experiments is shown. *P < 0·05; **P < 0·01; NS = not significant. [Colour figure can be viewed at wileyonlinelibrary.com]
Mir‐126 deficiency promoted the pathology of DSS‐induced autoimmune colitis
It is well known that DSS‐induced autoimmune colitis is an important animal model platform for the exploration on immune reaction in inflammatory bowel disease in vivo, in which effector CD4+ T cells play a critical role in the pathology of colitis 20. To investigate further whether miR‐126 deficiency could affect the activation and function of CD4+T cells in vivo, we established murine DSS‐induced autoimmune colitis (Fig. 3a) and observed the possible change on pathology of colitis. As shown in Fig. 3b,c (P < 0·05), the clinical and histological scores indicated that the liquid and sticky defecate correlated significantly with weight loss in the miR‐126KD mice group compared with that in the WT mice group, and the mucosal epithelium appeared as a larger ulcer in the miR‐126KD mice group. Moreover, the infiltration of inflammatory cells also clearly increased. Importantly, we found that the percentage and total cell number of CD4+ T cells in splenocytes increased clearly in the miR‐126KD mice group compared with those in the WT mice group (Fig. 3d,e, P < 0·05). Further analysis showed that the expression level of CD62L on CD4+ T cells decreased significantly (Fig. 3f,g, P < 0·05) and the level of CD44 in CD4+ T cells in the miR‐126KD mice group clearly increased (Fig. 3f,g, P < 0·05), indicating an elevated activation phenotype. Combining these data suggested that miR‐126 deficiency could promote the pathology of DSS‐induced autoimmune colitis, which might be related to an elevated activation and function of CD4+ T cells.
Figure 3.

MicroRNA‐126 (Mir‐126) deficiency promoted the pathology of CD4+ T cell‐mediated autoimmune colitis. MiR‐126 knock‐down (KD) mice and wild‐type (WT) mice (8–10 weeks old, n = 6) were treated with 3% dextran sulphate sodium (DSS) water for 10 days. (a) Schematic representation of the animal study. (b) Clinical and histological scores. (c) The pathology of colitis was observed by haematoxylin and eosin (H&E) staining. Magnification: ×100 and ×400. Arrows indicate intestinal tissue loss. (d) The proportion of CD4+ T cells in spleen was analysed by FACS and calculated. (e) The total cell number of CD4+ T cells was calculated. (f) The expression levels of CD62L and CD44 in CD4+ T cells also were analyzed by fluorescence activated cell sorting (FACS) and calculated (g). One representative example of three independent experiments is shown. *P < 0·05. [Colour figure can be viewed at wileyonlinelibrary.com]
MiR‐126 deficiency endowed CD4+ T cells to aggravate the pathology of DSS‐induced autoimmune colitis
To verify the effect of miR‐126 deficiency on the activation and function of CD4+ T cells in vivo, we purified and labelled CD4+CD62L+ T cells from miR‐126KD mice with CFSE, then transferred these cells adoptively into normal WT mice through the tail vein (Fig. 4a). Our data show that the length of colon was shortened significantly in the miR‐126KD CD4+ T cell‐transferred group, compared with that in the WT CD4+ T cell‐transferred group (Fig. 4b,c, P < 0·05). Clinical and histological examination showed that the liquid and sticky defecate correlated significantly with weight loss in the miR‐126KD CD4+T cell‐transferred group compared with that in the WT CD4+ T cell‐transferred group. Furthermore, intestine tissue damage was aggravated and inflammatory cell infiltration increased significantly in the miR‐126KD CD4+ T cell‐transferred group (Fig. 4d,e, P < 0·05). Notably, we found that the expression level of CD62L on CFSE+ cells decreased significantly in the miR‐126KD CD4+ T cell‐transferred group (Fig. 4f,g, P < 0·05), and the level of CD44 on CFSE+ cells clearly increased (Fig 4h,i, P < 0·05), which was consistent with our above data.
Figure 4.
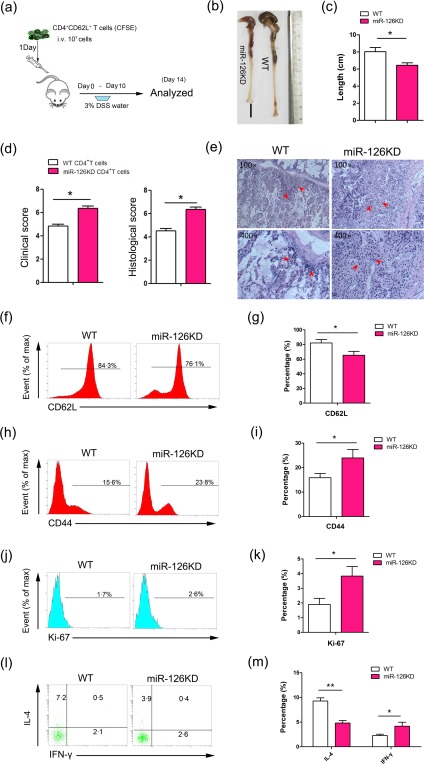
MicroRNA‐126 (Mir‐126) deficiency endowed CD4+ T cells to aggravate the pathology of dextran sulphate sodium (DSS)‐induced autoimmune colitis. CD4+CD62L+ T cells were purified by magnetic‐activated cell sorting (MACS) from miR‐126 knock‐down (KD) mice and wild‐type (WT) mice, respectively. Then, 1 × 107 cells were labelled by carboxyfluorescein succinimidyl ester (CFSE) and transferred adoptively into normal WT mice (8–10 weeks old, n = 6) through tail vein. One day later, mice were treated with 3% DSS water for 10 days. (a) Schematic representation of the animal study. (b) The morphology of intestine and its length was calculated (c). (d) Clinical and histological scores. (e) The histopathology of intestine was observed by haematoxylin and eosin (H&E) staining. Magnification: ×100 and ×400. Arrows indicate intestinal tissue loss. (f–m) The expression levels of CD62L, CD44, Ki‐67, IL‐4 and IFN‐γ in CD4+ T cells were analysed by FACS and calculated respectively. One representative data of three independent experiments was shown. *P < 0·05; **P < 0·01. [Colour figure can be viewed at wileyonlinelibrary.com]
Next, we analysed further the possible change in the proliferation and cytokine secretion of CD4+ T cells in vivo. As shown in Fig. 4j,k, the proportion of Ki‐67‐positive, a type of proliferating cell nuclear antigen, in CFSE+ cells was elevated significantly in the miR‐126KD CD4+ T cell‐transferred group, compared with that in the WT CD4+ T cell‐transferred group (P < 0·05). Furthermore, we found that the proportion of IFN‐γ+ in CFSE+ cells also increased significantly (Fig. 4l,m, P < 0·05). Consistent with our above data, the proportion of IL‐4+ in CFSE+ cells clearly decreased (Fig. 4l,m, P < 0·01). Collectively, all these data demonstrate that miR‐126 deficiency endowed CD4+ T cells with an enhanced activation and function capacity to aggravate the pathology of DSS‐induced autoimmune colitis.
MiR‐126 deficiency altered the expression of IRS‐1
To elucidate the potential molecular mechanism by which miR‐126 deficiency enhanced the activation and function of CD4+ T cells, we used an miRNA target detection program (http://www.microrna.org) to predict the potential target genes of miR‐126 and selected 13 potential candidate genes, including phosphomannomutase 1 (PMM1), target of Myb1 membrane trafficking protein (Tom1), phosphatidylinositol‐4,5‐bisphosphate 3‐kinase catalytic subunit delta (PIK3CD), regulator of G protein signalling 3 (RGS3), tuberous sclerosis 1 (TSC1), insulin receptor substrate 1 (IRS‐1), ADAM metallopeptidase domain 9 (ADAM9), EGF‐like domain multiple 7 (EGFL7), vascular cell adhesion molecule 1 (VCAM1), PHD finger protein 7 (Phf7), solute carrier family 39 member 6 (SLC39a6), Huntingtin interacting protein 1 (HIP1) and poly(ADP‐ribose) polymerase family member 16 (PARP16P). Then, the relative expression of these genes indicated on CD4+ T cells between WT mice and miR‐126KD mice was analysed. As shown in Fig. 5a, among these 13 candidate genes, the expression of IRS‐1 was up‐regulated significantly in CD4+ T cells from miR‐126KD mice compared with that from WT mice.
Figure 5.
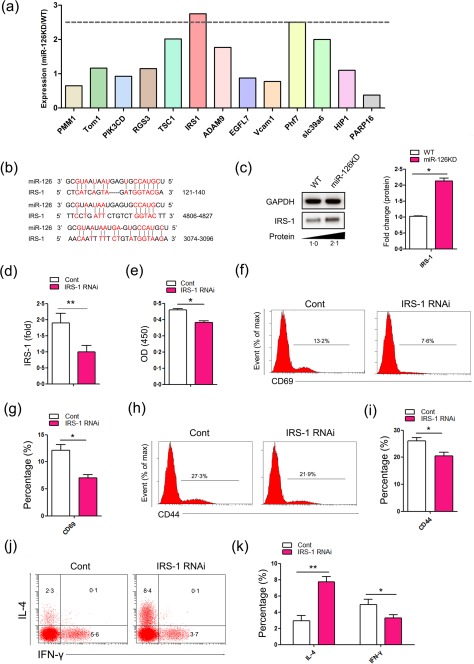
The expression of insulin receptor substrate 1 (IRS‐1) in CD4+ T cells. CD4+CD62L+ T cells were purified by magnetic‐activated cell sorting (MACS) from microRNA‐126 knock‐down (Mir‐126KD) mice and wild‐type (WT) mice (8–10 weeks old, n = 6), respectively. Then, cells were cultured in the presence of anti‐CD3 (20 μg/ml)/anti‐CD28 (4 ng/ml) antibody plus interleukin (IL)‐2 (10 ng/ml) for 48 h. The relative expression of indicated genes, including phosphomannomutase 1 (PMM1), target of Myb1 membrane trafficking protein (Tom1), phosphatidylinositol‐4,5‐bisphosphate 3‐kinase catalytic subunit delta (PIK3CD), regulator of G protein signalling 3 (RGS3), tuberous sclerosis 1 (TSC1), insulin receptor substrate 1 (IRS‐1), ADAM metallopeptidase domain 9 (ADAM9), EGF‐like domain multiple 7 (EGFL7), vascular cell adhesion molecule 1 (VCAM1), PHD finger protein 7 (Phf7), solute carrier family 39 member 6 (SLC39a6), Huntingtin interacting protein 1 (HIP1) and poly(ADP‐ribose) polymerase family member 16 (PARP16P), were analysed by real‐time polymerase chain reaction (RT–PCR) assay and calculated (a). (b) Putative miR‐126‐binding sites in the 3' untranslated region (UTR) of murine IRS‐1. (c) CD4+CD62L+ T cells were purified by MACS from FVB/N6 miR‐126KD mice and WT mice, respectively. Then, cells were cultured in the presence of anti‐CD3 (20 μg/ml)/anti‐CD28 (4 ng/ml) antibody plus interleukin (IL)‐2 (10 ng/ml) for 48 h. The expression of IRS‐1 protein was determined by Western blot and calculated. CD4+CD62L+ T cells were purified by MACS from miR‐126KD mice, Then, cells were transfected with p‐IRS‐1 RNAi (5 ng) and cultured in the presence of anti‐CD3 (20 μg/ml)/anti‐CD28 (4 ng/ml) antibody plus IL‐2 (10 ng/ml) for 48 h. (d) The expression of IRS‐1 was analysed by real‐time polymerase chain reaction (RT–PCR). (e) Call counting kit‐8 assay. The expression levels of CD69 and CD44 were analysed by fluorescence activated cell sorting (FACS) and the percentage was calculated (f–i). (j) The expression levels of interferon (IFN)‐γ and IL‐4 were analysed by FACS and the percentage was calculated (k). One representative example of three independent experiments is shown. *P < 0·05; **P < 0·01. [Colour figure can be viewed at wileyonlinelibrary.com]
Previous work has shown that miR‐126 could regulate the biological characters of various cells, including cancer cells, through IRS‐1 21, 22, 23, and IRS‐1 was also reported to play an important role in the function of various immune cells 24. We performed a sequence alignment analysis and found expectedly that miR‐126‐binding sites in the 3'UTR of IRS‐1 (Fig. 5b). Next, we detected the protein level of IRS‐1 in CD4+ T cells from miR‐126KD mice. As shown in Fig. 5c, the level of IRS‐1 protein in CD4+ T cells from miR‐126KD mice was increased significantly, compared with that in WT mice (P < 0·05). Finally, to verify the role of IRS‐1 in the effect of miR‐126 deficiency on the activation and function of CD4+ T cells, we also transfected IRS‐1 RNAi into CD4+ T cells from miR‐126KD mice. As shown in Fig. 5d, the relative expression of IRS‐1 in CD4+ T cells in the IRS‐1 RNAi‐transfected group decreased significantly, compared with that in the control group (P < 0·01). Furthermore, the proliferation of CD4+ T cells in the IRS‐1 RNAi‐transfected group also clearly decreased (Fig. 5e, P < 0·05). Importantly, we also found that the expression levels of CD69 and CD44 in CD4+ T cells decreased significantly (Fig. 5f–i, P < 0·05), indicating that down‐regulation of IRS‐1 could impair the CD4+ T cell activation in miR‐126KD mice. To confirm this phenomenon, we also detected the expression of related cytokines in CD4+ T cells. Consistently, we found that the expression of IFN‐γ in CD4+ T cells in the IRS‐1 RNAi‐transfected group decreased significantly compared with those in the control group (Fig. 5j,k, P < 0·05). Conversely, the expression level of cytokine IL‐4 was higher in CD4+ T cells in the IRS‐1 RNAi‐transfected group (Fig. 5j,k, P < 0·05). Collectively, our data indicate that miR‐126 deficiency enhances the activation and function of CD4+ T cells, which is due closely to the up‐regulation expression of its target IRS‐1.
MiR‐126 deficiency altered the transduction of related signalling pathways in CD4+ T cells
It is well known that multiple signalling pathways are related to the activation and function of CD4+ T cells, including ERK, AKT, NF‐κB, etc. 25, 26, 27, 28, 29. Moreover, IRS‐1 was also reported to be related closely to the transduction of these signalling pathways 22, 30, 31, 32. Thus, to elucidate whether miR‐126 deficiency affected the activation and function of CD4+ T cells was related to the change in these signalling pathways, the expression levels of phosphorylation of AKT, ERK and NF‐κB were analysed in CD4+T cells purified from miR‐126KD mice and WT mice, respectively. As shown in Fig. 6a,b, the ERK phosphorylation expression level in CD4+ T cells from miR‐126KD mice was elevated compared with that from WT mice (P < 0·05). Moreover, we found that the level of phosphorylation of AKT in these CD4+ T cells increased significantly (Fig. 6a,b, P < 0·01). Finally, we analysed the expression level of phosphorylation of NF‐κB in CD4+ T cells from miR‐126KD mice and obtained similar results (Fig. 6a,b, P < 0·01). Combining these results suggested that the effects of miR‐126 deficiency on the activation and function of CD4+ T cells are related closely to the altered transduction of ERK, AKT and NF‐κB signalling pathway (Fig. 6c).
Figure 6.
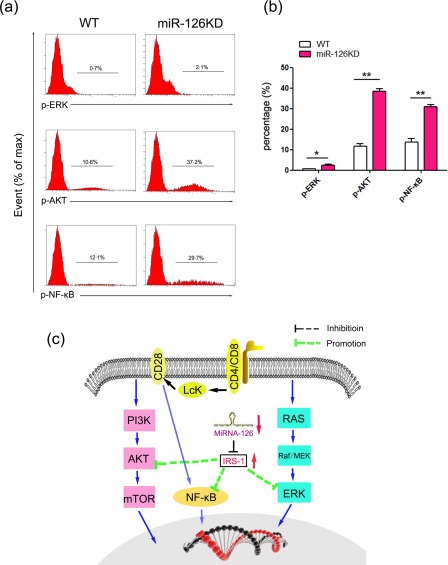
The effect of microRNA‐126 (Mir‐126) deficiency on the transduction of related signalling pathway. CD4+CD62L+ T cells purified by magnetic‐activated cell sorting (MACS) from miR‐126 knock‐down (KD) mice and wild‐type (WT) mice (8–10 weeks old, n = 6), respectively, Then, cells were cultured in the presence of anti‐CD3 (20 μg/ml)/anti‐CD28 (4 ng/ml) antibody plus interleukin (IL)‐2 (10 ng/ml) for 48 h. (a) The expression levels of phospho‐extracellular regulated kinase (p‐ERK), phospho‐protein kinase B (p‐AKT) and phospho‐nuclear factor kinase kappa B (p‐NF‐κB) were determined by fluorescence activated cell sorting (FACS) and calculated (b). (c) Schematic representation of the underlying mechanism of miR‐126 deficiency on the activation and function of CD4+ T cells. miR‐126 deficiency leads to up‐regulation of insulin receptor substrate 1 (IRS‐1), which could successively alter the transduction of the AKT, ERK and NF‐κB signalling pathway. [Colour figure can be viewed at wileyonlinelibrary.com]
Discussion
In this study, this is the first time, to our knowledge, that the potential effect of miR‐126 deficiency on the function of CD4+ T cells has been investigated. Our data show that miR‐126 deficiency could clearly enhance the activation and proliferation, as well as IFN‐γ secretion, of CD4+ T cells in vitro and in vivo. Furthermore, miR‐126 deficiency could endow CD4+ T cells to promote the pathology of autoimmune colitis inflammation. Finally, we found that IRS‐1, a functional target of miR‐126, was up‐regulated in CD4+ T cells with miR‐126 deficiency, accompanied by altered transduction of the ERK, AKT and NF‐κB pathway.
CD4+ T cells are an important subpopulation of T cells which play a central role in innate immune responses, maintaining the balance of anti‐inflammatory and proinflammatory responses, promoting activation of antigen‐presenting cells (APCs) and B cells as well as promoting the accumulation of immune cells 33, 34, 35, 36, 37. Increasing evidence demonstrates that the distinct miRNA molecule plays a critical regulatory role in the development and function of various immune cells, including CD4+ T cells, which affect the pathogenesis and development of related clinical diseases 38, 39, 40, 41, 42, 43, 44. For example, Zeng et al. 45 reported that down‐regulation of miR‐451a affects the activation and proliferation of CD4+ T cells by targeting the transcription factor myelocytomatosis oncogene (Myc) in dilated cardiomyopathy (DCM) patients, which contribute to the immunopathogenesis of DCM. Our new research work also reports that miR‐7 deficiency alters the proportion and absolute number of CD4+ T cells in bronchoalveolar lavage (BAL), while it is related to ameliorated pathologies of acute lung injury 19. In the present study, we extend previous findings by demonstrating that miR‐126 deficiency could clearly elevate activation and proliferation, as well as IFN‐γ secretion, in CD4+ T cells, indicating that miR‐126 might be a novel negative factor in CD4+ T cell function. Similarly, Okuyama et al. 14 reported that miR‐126 is a critical regulator in the development and function of B cells. Combining these literatures might highlight that miR‐126 is an important intrinsic regulator in the generation and biological function of immune cells. It should be noted that our previous work reported that miR‐126 could be involved in the induction and function of CD4+regulatory T cells (Tregs). Interestingly, Zhao et al. 15 also reported that miR‐126 is expressed highly in CD4+ Th2 cells from systemic lupus erythematosus (SLE) patients. Similarly, in the present study, we found that miR‐126 deficiency could alter the expression of IFN‐γ and IL‐4, two critical representative cytokines for Th1 and Th2 subsets, suggesting that miR‐126 is also critical for the biology of distinct CD4+ T cell subsets. Therefore, successive research work into the possible role of miR‐126 in these CD4+ T cell subsets, such as CD4+Th1 or Th2 cells, is extremely important for verification of the exact biological role of miR‐126 in the immune system.
Previous studies have documented that the change in biological function of CD4+ T cells are related closely to the development of inflammatory bowel disease (IBD) 46, 47. Moreover, accumulating evidence shows the irreplaceable role of distinct miRNA molecules in the occurrence and development of IBD 17, 48, 49. For instance, Runtsch et al. 50 reported that miR‐146a was involved in constraining intestinal barrier function. Moreover, miR‐146a deficiency was resistant to DSS‐induced colitis. In our study, we found that miR‐126 deficiency could promote the pathological change of colitis significantly in DSS‐induced autoimmune colitis model mice. Simultaneously, the percentage and total number of CD4+ T cells displayed an elevated activation phenotype, clearly increased in DSS‐induced autoimmune colitis model mice. Most importantly, adoptive cell transfer assay showed further that miR‐126 deficiency could endow CD4+ T cells with an elevated activation, proliferation and IFN‐γ secretion capacity to aggravate the pathology of colitis in the DSS‐induced autoimmune colitis model. In line with our findings, Holmkvist et al. 20 reported that the state of activation and function of CD4+ T cells is correlated closely with the development of T cell‐mediated immune colitis. Combining these data suggests strongly that miR‐126 might be a novel potential regulator in the development of autoimmune colitis, at least partially through regulating the function of CD4+ T cells. Hence, further studies on the correlation between miR‐126 expression and clinical IBD patients, which were not investigated in the current study, would be very valuable for exploration into the exact role of miR‐126 in the development of clinical IBD.
IRS‐1, a distinct member of the IRS family, is an important factor in the biological character of some types of cells, including cancer cells, which are related closely to transduction of the ERK, AKT and NF‐κB pathway 30. Regarding immune cells, Li et al. 48 reported that over‐expression of IRS‐1 could protect T cells from activation‐induced cell death (AICD). Moreover, Stentz and Kitabchi 49 found that up‐regulation of IRS‐1 is involved in activation of CD4+ T cells in response to hyperglycaemia. In the present study, our data show that, in miR‐126 deficiency, the expression of IRS‐1 in CD4+ T cells is elevated, which is consistent with previous findings that miR‐126 could regulate the biological character of various cells through IRS‐1. Furthermore, we found that miR‐126 deficiency also alters the transduction of the ERK, AKT and NF‐κB pathway. Given the important role of this signal pathway in the activation, proliferation and cytokine secretion of CD4+ T cells, we presume that miR‐126 deficiency affects the activation and function of CD4+ T cells, which is related to the up‐regulation of IRS‐1 and subsequently alters the transduction of the related signalling pathway. However, because of the complex of miRNA–target networks, whether or not miR‐126 regulates the activation and function of CD4+ T cells through controlling the expression of other potential molecules still remains to be elucidated fully in future.
Altogether, our present data reveal the unknown biological role of miR‐126 to be a novel intrinsic negative regulator in the function of CD4+ T cells, which provides a new fundamental basis for exploring further the role of miR‐126 in the development and function of immune cells and their subsets, as well as the occurrence and development of related diseases.
Disclosure
All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author contributions
F. C. and Y. H. performed the experiments, analysed the data, wrote the paper; Y. Z. and M. G. performed the experiments and analysed the data; J. L., W. Z. and H. X. performed the experiments; J. Z. wrote the paper; L. X. conceived and designed the experiments, analysed the data and wrote the paper; all authors reviewed the paper.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. The relationship between microRNA‐126 (Mir‐126) and CD4+T cells. Kidney, liver, lung, brain, intestine, heart, spleen, thymus, lymph node, bone, muscle and skin were collected from wild‐type (WT) mice (6–8 weeks old, n = 6). (a) The relative expression of miR‐126 in those 12 tissues and organs were analysed by real‐time polymerase chain reaction (RT–PCR) and calculated. (b) CD4+ T cells were purified by magnetic‐activated cell sorting (MACS). Then, the relative expression of miR‐126 in CD4+ T cells and CD4+ T cell‐deprived splenocytes were measured by RT–PCR assay and calculated. *P < 0·05.
Fig. S2. The relative expression of microRNA‐126 (Mir‐126) in wild‐type (WT) mice and miR‐126 knock‐down (KD) mice. Kidney, liver, lung, brain, intestine, heart, spleen, thymus, lymph node, bone and muscle, as well as skin, were collected from WT mice (6–8 weeks old, n = 6) and miR‐126KD mice (6–8 weeks old, n = 6). The relative expression of miR‐126 in those indicated tissues and organs were analysed by real‐time polymerase chain reaction (RT–PCR) and calculated. *P < 0·05; **P < 0·01.
Fig. S3. Change on the proportion of CD4+T cells in wild‐type (WT) mice and microRNA‐126 knock‐down (Mir‐126KD) mice. CD4+CD62L+ T cells were purified by magnetic‐activated cell sorting (MACS) from splenocytes in miR‐126KD mice (6–8 weeks old, n = 6) and WT mice (6–8 weeks old, n = 6) respectively. Then, cells were cultured in the presence of anti‐CD3 (20 μg/ml)/anti‐CD28 (4 ng/ml) antibody plus interleukin (IL)‐2 (10 ng/ml) for 72 h. (a) The proportion of CD4+ T cells in WT mice and miR‐126KD mice was analysed by fluorescence activated cell sorting (FACS) and calculated (b). One of three independent experiments is shown. *P < 0·05.
Fig. S4. Change in the proliferation of CD4+ T cell in wild‐type (WT) mice and microRNA‐126 knock‐down (Mir‐126KD) mice upon concanavalin A (ConA) stimulation. CD4+CD62L+ T cells purified by magnetic‐activated cell sorting (MACS) from splenocytes in miR‐126KD mice and WT mice (6–8 weeks old, n = 6) were cultured in the presence of ConA (5 μg/ml) for 72 h. (a) Proliferation of CD4+ T cells from miR‐126KD mice and WT mice is assessed by Ki‐67 staining and calculated (b). One representative example of three independent experiments is shown. **P < 0·01.
Acknowledgements
This work was supported by the Program for High level innovative talents in Guizhou Province (QKH‐RC‐2016‐4031), the National Natural Science foundation of China (31370918, 31760258), the Program for New Century Excellent Talents in University, Ministry of Education of China (NCET‐12–0661), the Program for Excellent Young Talents of Zunyi Medical University (15ZY‐001) and the Project of Guizhou Provincial Department of Science and Technology (2009C491).
References
- 1. Lagos‐Quintana M, Rauhut R, Yalcin A et al Identification of tissue‐specific microRNAs from mouse. Curr Biol 2002; 12:735–9. [DOI] [PubMed] [Google Scholar]
- 2. Harris TA, Yamakuchi M, Ferlito M et al MicroRNA‐126 regulates endothelial expression of vascular cell adhesion molecule 1. Proc Natl Acad Sci USA 2008; 105:1516–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Landgraf P, Rusu M, Sheridan R et al A mammalian microRNA expression atlas based on small RNA library sequencing. Cell 2007; 129:1401–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang S, Aurora AB, Johnson BA et al An endothelial‐specific microRNA governs vascular integrity and angiogenesis. Dev Cell 2008; 15:261–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Goerke SM, Kiefer LS, Stark GB et al Mir‐126 modulates angiogenic growth parameters of peripheral blood endothelial progenitor cells. Biol Chem 2014; 396:245–52. [DOI] [PubMed] [Google Scholar]
- 6. Zhang Y, Yang P, Sun T et al miR‐126 and miR‐126* repress recruitment of mesenchymal stem cells and inflammatory monocytes to inhibit breast cancer metastasis. Nat Cell Biol 2013; 15:284–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Meister J, Schmidt MH. miR‐126 and miR‐126*: new players in cancer. Sci World J 2010; 10:2090–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Feng R, Chen X, Yu Y et al miR‐126 functions as a tumour suppressor in human gastric cancer. Cancer Lett 2010; 298:50–63. [DOI] [PubMed] [Google Scholar]
- 9. Barshack I, Meiri E, Rosenwald S et al Differential diagnosis of hepatocellular carcinoma from metastatic tumors in the liver using microRNA expression. Int J Biochem Cell Biol 2010; 42:1355–62. [DOI] [PubMed] [Google Scholar]
- 10. Snowdon J, Boag S, Feilotter H et al A pilot study of urinary microRNA as a biomarker for urothelial cancer. Can Urol Assoc J 2012; 6:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feng X, Tan W, Cheng S et al Upregulation of microRNA‐126 in hepatic stellate cells may affect pathogenesis of liver fibrosis through the NF‐κB pathway. DNA Cell Biol 2015; 34:470–80. [DOI] [PubMed] [Google Scholar]
- 12. Zhang T, Li L, Shang Q et al Circulating miR‐126 is a potential biomarker to predict the onset of type 2 diabetes mellitus in susceptible individuals. Biochem Biophys Res Commun 2015; 463:60–3. [DOI] [PubMed] [Google Scholar]
- 13. Agudo J, Ruzo A, Tung N et al The miR‐126–VEGFR2 axis controls the innate response to pathogen‐associated nucleic acids. Nat Immunol 2014; 15:54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Okuyama K, Ikawa T, Gentner B et al MicroRNA‐126‐mediated control of cell fate in B‐cell myeloid progenitors as a potential alternative to transcriptional factors. Proc Natl Acad Sci USA 2013; 110:13410–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhao S, Wang Y, Liang Y et al MicroRNA‐126 regulates DNA methylation in CD4+ T cells and contributes to systemic lupus erythematosus by targeting DNA methyltransferase 1. Arthritis Rheum 2011; 63:1376–86. [DOI] [PubMed] [Google Scholar]
- 16. Qin A, Wen Z, Zhou Y et al MicroRNA‐126 regulates the induction and function of CD4(+) Foxp3(+) regulatory T cells through PI3K/AKT pathway. J Cell Mol Med 2013; 17:252–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Taylor BC, Zaph C, Troy AE et al TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J Exp Med 2009; 206:655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liu T, Ren J, Wang W et al Treatment of dextran sodium sulfate‐induced experimental colitis by adoptive transfer of peritoneal cells. Sci Rep 2015; 5:16760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhao J, Chen C, Guo M et al MicroRNA‐7 deficiency ameliorates the pathologies of acute lung injury through elevating KLF4. Front Immunol 2016; 7:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Holmkvist P, Pool L, Hägerbrand K et al IL‐18Rα‐deficient CD4+T cells induce intestinal inflammation in the CD45RB(hi), transfer model of colitis despite impaired innate responsiveness. Eur J Immunol 2016; 46:1371–82. [DOI] [PubMed] [Google Scholar]
- 21. Luan Y, Zuo L, Zhang S et al MicroRNA‐126 acts as a tumor suppressor in glioma cells by targeting insulin receptor substrate 1 (IRS‐1). Int J Clin Exp Pathol 2015; 8:10345–54. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 22. Zhou Y, Feng X, Liu YL et al Down‐regulation of miR‐126 is associated with colorectal cancer cells proliferation, migration and invasion by targeting IRS‐1 via the AKT and ERK1/2 signaling pathways. PLOS ONE 2013; 8:e81203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang J, Du YY, Lin YF et al The cell growth suppressor, mir‐126, targets IRS‐1. Biochem Biophys Res Commun 2008; 377:136–40. [DOI] [PubMed] [Google Scholar]
- 24. Hamada M, Abe M, Miyake T et al B cell‐activating factor controls the production of adipokines and induces insulin resistance. Obesity 2011; 19:1915–22. [DOI] [PubMed] [Google Scholar]
- 25. Hu Y, Wang C, Li Y et al MiR‐21 controls in situ expansion of CCR6+ regulatory T cells through PTEN/AKT pathway in breast cancer. Immunol Cell Biol 2015; 93:753–64. [DOI] [PubMed] [Google Scholar]
- 26. Ye H, Zhang J, Wang J et al CD4 T‐cell transcriptome analysis reveals aberrant regulation of STAT3 and Wnt signaling pathways in rheumatoid arthritis: evidence from a case–control study. Arthritis Res Ther 2015; 17:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Anderson AE, Pratt AG, Sedhom MA et al IL‐6‐driven STAT signalling in circulating CD4+ lymphocytes is a marker for early anticitrullinated peptide antibody‐negative rheumatoid arthritis. Ann Rheum Dis 2016; 75:466–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Laky K, Evans S, Perez‐Diez A et al Notch signaling regulates antigen sensitivity of naive CD4+ T cells by tuning co‐stimulation. Immunity 2015; 42:80–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Han JM, Patterson SJ, Levings MK. The role of the PI3K signaling pathway in CD4+ T cell differentiation and function. Front Immunol 2012; 3:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bailey KL, Agarwal E, Chowdhury S et al TGF‐γ/Smad3 regulates proliferation and apoptosis through IRS‐1 inhibition in colon cancer cells. PLoS One 2017; 12:e0176096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mokashi P, Khanna A, Pandita N. Flavonoids from Enicostema littorale blume enhances glucose uptake of cells in insulin resistant human liver cancer (HepG2) cell line via IRS‐1/PI3K/Akt pathway. Biomed Pharmacother 2017; 90:268–77. [DOI] [PubMed] [Google Scholar]
- 32. Tang CY, Man XF, Guo Y et al IRS‐2 partially compensates for the insulin signal defects in IRS‐1–/– mice mediated by miR‐33. Mol Cells 2017; 40:123–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cui P, Hu Y, Tao Y et al miR‐126 knockdown enhances the activity of murine CD4+ T cells in vivo and promotes their differentiation into Th1 cells. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2016; 32:347–51. [PubMed] [Google Scholar]
- 34. Maehara T, Moriyama M, Hayashida JN et al Selective localization of T helper subsets in labial salivary glands from primary Sjögren's syndrome patients. Clin Exp Immunol 2012; 169:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Graf T, Enver T. Forcing cells to change lineages. Nature 2009; 462:587–94. [DOI] [PubMed] [Google Scholar]
- 36. de Araújo‐Souza PS, Hanschke SC, Viola JP. Epigenetic control of interferon‐gamma expression in CD8 T cells. J Immunol Res 2015; 2015:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Crotty S. A brief history of T cell help to B cells. Nat Rev Immunol 2015; 15:185–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Fu Y, Yi Z, Li J et al Deregulated microRNAs in CD4+ T cells from individuals with latent tuberculosis versus active tuberculosis. J Cell Mol Med 2014; 18:503–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li W, Kong LB, Li JT et al MiR‐568 inhibits the activation and function of CD4+T cells and Treg cells by targeting NFAT5. Int Immunol 2014; 26:269–81. [DOI] [PubMed] [Google Scholar]
- 40. Zhao J, Li Y, Hu Y et al MicroRNAs expression profile in CCR6+ regulatory T cells. Peer J 2014; 2:e575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xiao C, Calado DP, Galler G et al MiR‐150 controls B cell differentiation by targeting the transcription factor c‐Myb. Cell 2016; 165:1027. [DOI] [PubMed] [Google Scholar]
- 42. Marega LF, Teocchi MA, Dos Santos Vilela MM. Differential regulation of miR‐146a/FAS and miR‐21/FASLG axes in autoimmune lymphoproliferative syndrome due to FAS mutation (ALPS‐FAS). Clin Exp Immunol 2016; 185:148–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nakahama T, Hanieh H, Nguyen NT et al Aryl hydrocarbon receptor‐mediated induction of the microRNA‐132/212 cluster promotes interleukin‐17‐producing T‐helper cell differentiation. Proc Natl Acad Sci USA 2013; 110:11964–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Lai NS, Koo M, Yu CL et al Immunopathogenesis of systemic lupus erythematosus and rheumatoid arthritis: the role of aberrant expression of non‐coding RNAs in T cells. Clin Exp Immunol 2017; 187:327–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zeng Z, Ke W, Li Y et al Down‐regulation of microRNA‐451a facilitates the activation and proliferation of CD4+ T cells by targeting Myc in patients with dilated cardiomyopathy. J Biol Chem 2017; 292:6004–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Chen WX, Ren LH, Shi RH. Implication of miRNAs for inflammatory bowel disease treatment:Systematic review. World J Gastrointest Pathophysiol 2014; 5:63–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Feng X, Wang H, Ye S et al Up‐regulation of microRNA‐126 may contribute to pathogenesis of ulcerative colitis via regulating NF‐kappaB inhibitor IκBα. PLoS One 2012; 7:e52782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Li L, Qi X, Williams M et al Overexpression of insulin receptor substrate‐1, but not insulin receptor substrate‐2, protects a T cell hybridoma from activation‐induced cell death. J Immunol 2002; 168:6215–23. [DOI] [PubMed] [Google Scholar]
- 49. Stentz FB, Kitabchi AE. Hyperglycemia‐induced activation of human T‐lymphocytes with de novo emergence of insulin receptors and generation of reactive oxygen species. Biochem Biophys Res Commun 2005; 335:491–5. [DOI] [PubMed] [Google Scholar]
- 50. Runtsch MC, Hu R, Alexander M et al MicroRNA‐146a constrains multiple parameters of intestinal immunity and increases susceptibility to DSS colitis. Oncotarget 2015; 6:28556–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. The relationship between microRNA‐126 (Mir‐126) and CD4+T cells. Kidney, liver, lung, brain, intestine, heart, spleen, thymus, lymph node, bone, muscle and skin were collected from wild‐type (WT) mice (6–8 weeks old, n = 6). (a) The relative expression of miR‐126 in those 12 tissues and organs were analysed by real‐time polymerase chain reaction (RT–PCR) and calculated. (b) CD4+ T cells were purified by magnetic‐activated cell sorting (MACS). Then, the relative expression of miR‐126 in CD4+ T cells and CD4+ T cell‐deprived splenocytes were measured by RT–PCR assay and calculated. *P < 0·05.
Fig. S2. The relative expression of microRNA‐126 (Mir‐126) in wild‐type (WT) mice and miR‐126 knock‐down (KD) mice. Kidney, liver, lung, brain, intestine, heart, spleen, thymus, lymph node, bone and muscle, as well as skin, were collected from WT mice (6–8 weeks old, n = 6) and miR‐126KD mice (6–8 weeks old, n = 6). The relative expression of miR‐126 in those indicated tissues and organs were analysed by real‐time polymerase chain reaction (RT–PCR) and calculated. *P < 0·05; **P < 0·01.
Fig. S3. Change on the proportion of CD4+T cells in wild‐type (WT) mice and microRNA‐126 knock‐down (Mir‐126KD) mice. CD4+CD62L+ T cells were purified by magnetic‐activated cell sorting (MACS) from splenocytes in miR‐126KD mice (6–8 weeks old, n = 6) and WT mice (6–8 weeks old, n = 6) respectively. Then, cells were cultured in the presence of anti‐CD3 (20 μg/ml)/anti‐CD28 (4 ng/ml) antibody plus interleukin (IL)‐2 (10 ng/ml) for 72 h. (a) The proportion of CD4+ T cells in WT mice and miR‐126KD mice was analysed by fluorescence activated cell sorting (FACS) and calculated (b). One of three independent experiments is shown. *P < 0·05.
Fig. S4. Change in the proliferation of CD4+ T cell in wild‐type (WT) mice and microRNA‐126 knock‐down (Mir‐126KD) mice upon concanavalin A (ConA) stimulation. CD4+CD62L+ T cells purified by magnetic‐activated cell sorting (MACS) from splenocytes in miR‐126KD mice and WT mice (6–8 weeks old, n = 6) were cultured in the presence of ConA (5 μg/ml) for 72 h. (a) Proliferation of CD4+ T cells from miR‐126KD mice and WT mice is assessed by Ki‐67 staining and calculated (b). One representative example of three independent experiments is shown. **P < 0·01.


