Summary
Isolated decreased serum‐immunoglobulin (Ig)M has been associated with severe and/or recurrent infections, atopy and autoimmunity. However, the reported high prevalence of clinical problems in IgM‐deficient patients may reflect the skewed tertiary centre population studied so far. Also, many papers on IgM deficiency have included patients with more abnormalities than simply IgM‐deficiency. We studied truly selective primary IgM deficiency according to the diagnostic criteria of the European Society for Immunodeficiencies (ESID) (true sIgMdef) by reviewing the literature (261 patients with primary decreased serum‐IgM in 46 papers) and analysing retrospectively all patients with decreased serum‐IgM in a large teaching hospital in 's‐Hertogenbosch, the Netherlands [1 July 2005–23 March 2016; n = 8049 IgM < 0·4 g/l; n = 2064 solitary (IgG+IgA normal/IgM < age‐matched reference)]. A total of 359 of 2064 (17%) cases from our cohort had primary isolated decreased serum‐IgM, proven persistent in 45 of 359 (13%) cases; their medical charts were reviewed. Our main finding is that true sIgMdef is probably very rare. Only six of 261 (2%) literature cases and three of 45 (7%) cases from our cohort fulfilled the ESID criteria completely; 63 of 261 (24%) literature cases also had other immunological abnormalities and fulfilled the criteria for unclassified antibody deficiencies (unPAD) instead. The diagnosis was often uncertain (possible sIgMdef): data on IgG subclasses and/or vaccination responses were lacking in 192 of 261 (74%) literature cases and 42 of 45 (93%) cases from our cohort. Our results also illustrate the clinical challenge of determining the relevance of a serum sample with decreased IgM; a larger cohort of true sIgMdef patients is needed to explore fully its clinical consequences. The ESID online Registry would be a useful tool for this.
Keywords: IgM deficiency, immunodeficiency, primary immunodeficiency, primary selective IgM deficiency, unclassified antibody deficiency
Introduction
Immunoglobulin (Ig)M deficiency is, on one hand, reported to be associated with a wide range of clinical presentations, including severe or recurrent infections, atopy, autoimmunity and malignancy 1. On the other hand, there are doubts about its clinical significance 2; studies in healthy populations have shown that genetic polymorphisms as well as environmental factors may influence serum IgM levels 2, 3. Previous studies on the clinical significance of IgM deficiency have been affected by selection bias towards ‘disease’, as mostly symptomatic patients from tertiary centre cohorts have been described 4, 5, 6.
The European Society for Immunodeficiencies (ESID) Registry defines primary selective IgM deficiency (sIgMdef) as a serum IgM level repeatedly below 2 standard deviations (s.d.) of normal with normal levels of serum IgA, IgG and IgG subclasses, normal vaccination responses, absence of T cell defects and absence of causative external factors (http://www.esid.org). Many previously published papers that report on ‘IgM deficiency’ do not fulfil these criteria 7, 8.
To facilitate a clear discussion, we define three categories in our study: (i) truly selective primary IgM deficiency (true sIgMdef) – the ESID criteria are completely fulfilled, which means that serum IgM levels are decreased repeatedly and IgG, IgA, IgG‐subclasses and vaccination responses have been determined and were normal for age; we consider the absence of clinical signs suggesting a T cell defect sufficient; (ii) possible selective primary IgM deficiency (possible sIgMdef) – the diagnosis of true sIgMdef is uncertain, which means that the ESID criteria are not fulfilled completely, because data on IgG subclasses and/or vaccination responses are lacking; and (iii) unclassified primary antibody deficiency (unPAD) – other abnormalities in antibodies are also present: IgG‐subclass deficiency, below‐normal levels of IgG or IgA and/or impaired vaccination responses.
The aim of our study was to learn more about the clinical significance of true sIgMdef. Therefore, we first conducted a scoping review to identify all previously published patients with decreased serum IgM. Secondly, we analysed decreased serum IgM identified through the laboratory files of the Jeroen Bosch Hospital in 's‐Hertogenbosch, the Netherlands, a large teaching hospital (secondary centre). Finally, we analysed whether these fulfilled the criteria for true sIgMdef.
Materials and methods
Literature search
The PubMed database was searched for papers concerning ‘IgM deficiency’ published until 10 May 2017 (no starting date). The search query was defined as {selective OR isolated} AND {IgM OR Immunoglobulin M} AND {deficiency OR low} AND {immunodeficiency syndromes}. We also screened the reference lists of papers identified by our search strategy and added those papers that reported about decreased serum IgM (snowball method). Our search strategy is described in detail in Supporting information, Fig. S1. We considered decreased serum IgM to be secondary in combination with the use of immunosuppressive agents, malignancy (e.g. clear cell sarcoma, promyelocytic leukaemia, multiple myeloma) or gastrointestinal loss (e.g. enteropathy through Crohn's or coeliac disease). Only papers that (also) contained patients with primary decreased serum IgM were included into the study. We analysed whether these patients fulfilled the criteria for true sIgMdef.
Our cohort
Patient selection
All serum immunoglobulin levels determined between 1 July 2005 and 23 March 2016, in the Jeroen Bosch Hospital (JBZ) in 's‐Hertogenbosch, the Netherlands (encatchment area 350 000; 500 000 out‐patient visits and 32 000 admissions per year), were obtained [n = 38 149; 5342 (14%) samples from children and 32 509 (85%) samples from adults, missing age values in 298 samples]. Of these, all samples with serum IgM values < 0·4 g/l were selected (n = 8049, details in Supporting information, Fig. S2). Samples were excluded if serum IgM levels were normal according to age‐matched reference values (these were all young children) 9. To identify all patients with isolated decreased serum IgM, samples with decreased age‐matched IgA and/or IgG values as well as follow‐up samples of serum IgM were excluded. The medical charts were screened regarding patient history and medication use to exclude the samples from those patients in whom decreased serum IgM could be secondary (caused by external factors; for definition, see literature review above). Patients with cystic fibrosis (n = 3) were excluded because their clinical symptoms would be difficult to interpret. Laboratory data of all primary cases were analysed to identify patients in whom serum IgM level was determined only once and in whom serum IgM level was determined repeatedly, but had normalized. Only the medical charts of patients with persistent decreased serum IgM levels were reviewed in detail; this patient group comprises both possible and true primary sIgMdef (for definitions, see Introduction). The Medical Ethical Committee Brabant approved the study.
Data collection
Data on demographics, clinical features, laboratory results and treatment, conclusions written by medical specialists and ICD‐10 codes were derived from our electronic patient system. For clinical evaluation, we collected the type of medical specialist who discovered the decreased serum IgM, reason(s) for determining serum IgM and clinical problems that could be related to antibody deficiency. We considered the following clinical problems to be possibly related to antibody deficiency: infections, atopic and/or autoimmune manifestations, inflammation of the gastrointestinal tract, long‐lasting fatigue, depression and malignancies. Pneumonia required confirmation by thoracic X‐ray. Allergic diseases (allergic rhinoconjunctivitis, food allergy, allergic urticaria, allergic anaphylaxis) required confirmation by skin‐prick testing or radioallergosorbent test (RAST). For immunological evaluation, we collected data on serum IgM, IgG and IgA levels and, if determined, data on IgG subclasses, T cell subsets and function, antibody responses to vaccinations, isohaemagglutinin levels, anti‐nuclear antibodies (ANA) and specific serum IgE directed against inhalant allergens. For interpretation of serum immunoglobulins and lymphocyte subpopulations, age‐matched reference values were used 10. Because our laboratory cut‐off for serum IgM levels is 0·2 g/l, a value of < 0·2 g/l was replaced by 0·1 g/l for calculating mean serum IgM level (n = 4). For interpretation of pneumococcal antibody responses, laboratory specific reference values were used 11. The follow‐up period was defined as the date of the first serum sample with decreased IgM until the date of data extraction. All patient data were encrypted and saved on a protected server using Research Manager software developed by Cloud9 Health Solutions (Deventer, the Netherlands).
Statistical analysis
Statistical analysis was performed using spss for Windows, version 21. Descriptive statistics were used to compute frequencies of categorical variables and mean (with s.d.) or median [with interquartile range (IQR)] of continuous variables, depending on the distribution.
Results
Literature search
Supporting information, Table S1 provides an overview of the identified relevant literature. A total of 261 patients with primary decreased serum IgM were described in 46 papers; eight patients (two adults and six children) fulfilled the criteria for combined immunodeficiency and these were excluded. Only six of 261 patients (2·3%, three adults and three children) fulfilled the ESID criteria completely for true sIgMdef; 63 of 261 (24·1%; 44 adults and 19 children) fulfilled the criteria for unclassified antibody deficiency. In 192 of 261 patients (73·6%, 164 adults and 28 children) the diagnosis was uncertain (possible sIgMdef), due to incomplete laboratory data (Fig. 1).
Figure 1.
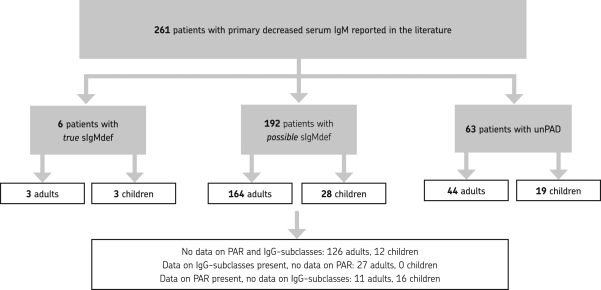
Patients with truly selective primary immunoglobulin (Ig)M deficiency in the literature [according to the European Society for Immunodeficiencies (ESID) Registry clinical diagnosis criteria]. PAR = pneumococcal antibody response; sIgMdef = selective IgM deficiency; unPAD = unclassified primary antibody deficiency.
Clinical and laboratory features of the published adult and paediatric cases with true or possible sIgMdef are summarized in Tables 1 and 2. More than two‐thirds of both adults and children were male (57 of 85 adults, 67% versus 23 of 31 children, 74%). Many patients presented with infectious problems (30 of 62 adults, 48% versus 14 of 15 children, 93%). In three of 62 (5%) of the reported adults, decreased IgM was identified ‘by accident’ as part of laboratory evaluation for ischaemic heart disease, hypertension and visual disturbance. Thirteen of 62 (21%) of the reported adults and one of 15 (7%) children were asymptomatic; this boy was detected during family screening. Serum IgM values were reported in 86 adults and 14 children (mean 0·23 g/l, range 0·004–0·45 g/l for adults versus mean 0·18 g/l, range 0·00–0·36 g/l for children). Undetectable serum IgM levels were reported in two children 12, 13 and four adults 14. Three adults and one baby were treated with intravenous immunoglobulin substitution (IVIG).
Table 1.
Adult patients from the literature
| Year | Reference | Reported patients * |
Age (years/gender) |
Clinical manifestation(s) that could be related to antibody deficiency † | Familial cases | Serum IgM level (g/l) |
IVIG (yes/no) |
|---|---|---|---|---|---|---|---|
| ESID criteria completely fulfilled (true sIgMdef) | |||||||
| 2009 | 4 | 3 | 79/M | Asthma, myalgia, fatigue | No | 0·18 | No |
| 39/F | Recurrent respiratory infections, allergic rhinitis, asthma, myalgia | No | 0·16 | No | |||
| 55/M | Recurrent shingles, myalgia, arthralgia, fatigue | No | 0·39 | No | |||
| ESID criteria not completely fulfilled: data on IgG subclasses and/or pneumococcal antibody responses lacking (possible sIgMdef) | |||||||
| 1967 | 22 | 5 | Adult/M | Asymptomatic | Yes | 0·40 | No |
| Adult/M | Asymptomatic | Yes | 0·40 | No | |||
| Adult/M | Asymptomatic | Yes | 0·45 | No | |||
| Adult/M | Asymptomatic | Yes | 0·30 | No | |||
| Adult/F | Asymptomatic | Yes | 0·30 | No | |||
| 1970 | 24 | 10 | 20/M | Bacterial infections, asthma | n.r. | 0·36 | No |
| 23/M | Allergic rhinitis | n.r. | 0·41 | No | |||
| 28/M | Bacterial infections, asthma | n.r. | 0·42 | No | |||
| 30/M | Bacterial infections, asthma, atopic dermatitis | n.r. | 0·41 | No | |||
| 31/M | Bacterial infections, asthma | n.r. | 0·35 | No | |||
| 33/M | Bacterial infections, atopic dermatitis | n.r. | 0·24 | No | |||
| 48/M | Asthma | n.r. | 0·41 | No | |||
| 50/M | Asthma | n.r. | 0·43 | No | |||
| 56/M | Asthma | n.r. | 0·41 | No | |||
| 75/M | Bacterial infections, asthma | n.r. | 0·35 | No | |||
| 1973 | 25 | 2 | 22/M | CMV hepatitis | Yes | 0·28 | No |
| 20/M | Psittacosis | Yes | 0·33 | No | |||
| 1975 | 17 | 70 | n.r. ‡ | Recurrent respiratory infections(59%), asymptomatic (19%) | n.r. | n.r. | No |
| 1976 | 26 | 2 | 72/M | No | No | 0·15 | No |
| 60/M | Tuberculosis pneumonia | No | 0·04 | No | |||
| 1978 | 27 | 1 | 48/M | Pneumonia, sepsis, rheumatic heart disease | n.r. | 0·21 | No |
| 1981 | 28 | 1 | 21/M | Smallpox, pneumonia, died from infection | No | 0·20 | No |
| 1981 | 29 | 1 | 85/M | No | n.r. | 0·17 | No |
| 1982 | 30 | 1 | 65/M | No | n.r. | 0·01 | No |
| 1984 | 31 | 1 | 66/M | Stomach leiomyoma | n.r. | 0·08 | No |
| 1986 | 32 | 7 | 58/M | Urinary tract infection, pulmonary tuberculosis | n.r. | 0·20 | No |
| 73/F | Urinary tract infection, respiratory infection | n.r. | 0·14 | No | |||
| 71/F | Urinary tract infection, pneumonia | n.r. | 0·11 | No | |||
| 53/F | Urinary tract infection, rheumatoid arthritis | n.r. | 0·17 | No | |||
| 29/F | Urinary tract infection, respiratory infection, SLE | n.r. | 0·25 | No | |||
| 30/M | Urinary tract infection, SLE | n.r. | 0·06 | No | |||
| 48/M | Pneumonia | n.r. | 0·10 | No | |||
| 1987 | 33 | 4 | 44/F | SLE‐like | n.r. | 0·26 | No |
| 62/F | Asthma | n.r. | 0·23 | No | |||
| 60/F | Lymphoma | n.r. | 0·08 | No | |||
| 51/F | SLE | n.r. | 0·10 | No | |||
| 1992 | 34 | 6 | 50/M | Liver abscess, cholangitis, dermatitis | No | 0·18 | No |
| 57/M | Diabetes mellitus | No | 0·06 | No | |||
| 22/M | Streptococcal infection | No | 0·32 | No | |||
| 34/M | Chronic tonsillitis, bronchitis, psoriasis pustulosa | No | 0·01 | No | |||
| 57/M | Diabetes mellitus, polyarthritis | No | 0·004 | No | |||
| 37/F | Asymptomatic | No | 0·34 | No | |||
| 2004 | 35 | 1 | 23/M | Recurrent respiratory infections, allergic rhinitis, asthma | No | 0·28 | Yes |
| 2006 | 5 | 23 | Unknown § | n.a. | No | 0·32 | No |
| 2009 | 4 | 5 | 69/M | Asthma, rhinorrhoea | No | 0·39 | No |
| 44/F | Chronic sinusitis | No | 0·27 | Yes | |||
| 44/F | Recurrent sinus infections, allergic rhinitis, rash | No | 0·28 | No | |||
| 76/M | Recurrent respiratory infections | No | 0·30 | No | |||
| 46/F | Recurrent respiratory infections, rheumatoid arthritis | No | 0·39 | No | |||
| 2009 | 36 | 2 | n.r. | n.r. | n.r. | n.r. | n.r. |
| 2015 | 37 | 1 | 52/M | CEP, pericarditis, allergic rhinitis, asthma, coeliac disease | No | 0·32 | No |
| 2016 | 2 | 11 | 57/M | Asymptomatic | No | 0·19 | No |
| 45/M | Urinary tract infection (×2) | No | 0·29 | No | |||
| 48/M | Atopic dermatitis, allergic rhinitis, food allergy | No | 0·27 | No | |||
| 50/F | Atopic dermatitis, allergic rhinitis | No | 0·25 | No | |||
| 32/M | Atopic dermatitis | No | 0·27 | No | |||
| 55/F | Asymptomatic | No | 0·23 | No | |||
| 63/M | Asymptomatic | No | 0·27 | No | |||
| 57/M | Asymptomatic | No | 0·19 | No | |||
| 48/M | Asymptomatic | No | 0·29 | No | |||
| 50/M | Asymptomatic | No | 0·16 | No | |||
| 30/M | Asymptomatic | No | 0·26 | No | |||
| 2016 | 14 | 10 | Unkown ¶ | n.r. | n.r. | Unknown | n.r. |
The three adults with true and 164 adults with possible selective primary immunoglobulin (Ig)M deficiency from the literature (definition of true selective IgM deficiency (sIgMdef) according to the European Society for Immunodeficiencies (ESID) registry clinical diagnosis criteria). *Only reported patients fulfilling the criteria for reported true or possible primary sIgMdef are described in this table. †The difference between ‘asymptomatic’ and ‘no’ is that ‘no’ refers to patients who were screened for problems not related to antibody deficiency in contrast to asymptomatic patients, who had no clinical problems at all. ‡Seventy patients were reported without specific age indications or exact IgM levels in this paper. §Clinical manifestations of patients were not described separately in this paper. Mean age at diagnosis of the whole group was 54 years; 11 males, 12 females. One patient was treated with intravenous Ig (IVIG) because of refractory asthma. ¶Patient data were not described separately in this paper. Of the 20 described patients, 50% had also specific anti‐polysaccharide antibody deficiency and fulfilled the criteria for ‘unclassified antibody deficiency’. Therefore, these 10 patients were not included in this table. Age range of the whole group: 24–56 years, F : M ratio 1.1 : 1.0, serum IgM range: 0·04 g/l to 0·32 g/l. CEP = chronic eosinophilic pneumonia; CMV = cytomegalovirus; F = female; M = male; n.a. = not applicable; n.r. = not reported; SLE = systemic lupus erythematosus.
Table 2.
Paediatric patients from the literature and our cohort
| Year | Reference | Reported patients |
Age (years/gender) |
Clinical manifestations that could be related to antibody deficiency | Serum IgM level (g/l) |
IVIG (yes/no) |
|---|---|---|---|---|---|---|
| ESID criteria completely fulfilled (true sIgMdef) | ||||||
| Our cohort | 16/M | URTI, growth retardation, verrucae vulgares, RLS | 0·36 | No | ||
| 2008 | 6 | 2 | 10/M | Recurrent otitis media | 0·21 | No |
| 12/M | Pneumonia | 0·30 | No | |||
| 2009 | 38 | 1 | 6/M | Multiple recurrent impetigo | 0·21 | No |
| Data on IgG subclasses present, but no data on pneumococcal antibody responses (possible sIgMdef) | ||||||
| No cases | ||||||
| Data on pneumococcal antibody responses present, but no data on IgG subclasses (possible sIgMdef) | ||||||
| 2013 | 39 | 16 | Unknown * | n.r. | n.r. | n.r. |
| No data on pneumococcal antibody responses and no data on IgG subclasses (possible sIgMdef) | ||||||
| Our cohort | 16/M | Recurrent infections, asthma, verrucae vulgares | 0·28 | No | ||
| Our cohort | 17/M | Depression, long‐lasting fatigue | 0·38 | No | ||
| 1967 | 22 | 1 | 5/M | Meningococcal meningitis, died from infection | 0·12 | No |
| 1971 | 13 | 1 | 0/M | Recurrent pseudomonas infections | 0·00 | No |
| 1973 | 40 | 1 | 2/F | Recurrent otitis media, laryngitis, meningitis | 0·08 | No |
| 1973 | 25 | 1 | 13/M | CMV hepatitis | 0·26 | No |
| 1973 | 41 | 2 | 4/M | Meningitis | 0·34 | No |
| 1/M | Asymptomatic | 0·36 | No | |||
| 1986 | 42 | 1 | 16/F | Disseminated molluscum contagiosum | 0·04 | No |
| 1989 | 12 | 1 | 3/M | Recurrent infections | 0·00 | No |
| 2001 | 43 | 1 | 10/M | Recurrent sinusitis, pneumonia, chronic staphylococci blepharitis | 0·23 | No |
| 2005 | 23 | 1 | 0/M | Pseudomonas septicemia | 0·12 | Yes |
| 2009 | 44 | 1 | 6/M | Chronic recurrent multi‐focal osteomyelitis | 0·20 | No |
| 2010 | 45 | 1 | 16/M | Refractory giardiasis | 0·21 | No |
The three paediatric patients from our cohort and 31 paediatric patients with true or possible selective primary IgM deficiency (sIgMdef) from the literature. *Patients were not described separately in this paper. Median age at diagnosis was 4·2 years; 10 males, six females. CMV = cytomegalovirus; F = female; Ig = immunoglobulin; IVIG = intravenous immunoglobulin; M = male; n.a. = not applicable; n.r. = not reported; URTI = upper respiratory tract infection; RLS = Raynaud‐like symptoms.
Our cross‐sectional retrospective cohort
A total of 2064 patients with isolated decreased serum IgM were identified in the laboratory system of the JBZ (1 July 2005–23 March 2016): 2034 adults and 17 children aged 6–18 years (13 children < 6 years were excluded because the age‐matched reference value was lower than the cut‐off value of the test). The patient selection process is shown in detail in Supporting information, Fig. S2; 1685 of 2034 adults (83%) and seven of 17 (41%) children had secondary isolated decreased serum IgM; 349 of 2034 (17%) adults and 10 of 17 (59%) children had a primary form; of these, serum IgM levels were determined more than once in only 49 of 349 (14%) adults and three of 10 (30%) children. In seven of 49 (14%) of the adults the serum IgM level normalized, yielding persistent isolated decreased serum IgM (possible or true sIgMdef cases) in 42 adults and three children.
More than half the adults (54.8%) and all the children were male. Mean age at the date of the first serum sample with decreased IgM was 61 (range 33–86) years in the adults and 16 (range 16–17) years in the children. Mean follow‐up time was 74·8 (range 20–133) months in the adults and 102·7 (range 82–119) months in the children.
Clinical and laboratory features are described in Tables 2 (three children) and 3 (42 adults). The onset and duration of symptoms could not be determined accurately in the medical files; 24% of the adults and two of the three children were analysed for suspected potential immunodeficiency. The others were detected during analysis for other problems; however, 22% of these adults and one child had a history of symptoms that could be related to antibody deficiency (mainly infections). The majority (72%) of adults without such symptoms remained asymptomatic during follow‐up; 28% developed symptoms that could be related to antibody deficiency. In none of the patients was a family history of immunodeficiency found in the medical charts. Only 7% (two adults and one child) fulfilled the ESID criteria completely for true sIgMdef.
The first serum IgM level in possible or true sIgMdef cases ranged from < 0·2 to 0·39 g/l (mean 0·30 ± 0·84) in the adults and from 0·28 to 0·38 g/l (mean 0·34 ± 0·05) in the children. First serum IgA levels were increased (> 4·0 g/l) in seven adults (17%). Serum IgE levels were determined in six adults and one child (mean 133 ± 182 U/ml; range 5–410 U/ml); they were elevated (> 90 U/ml) in two adults. None of the patients were treated with IVIG or prophylactic antibiotics.
Discussion
We studied true sIgMdef (according to the ESID diagnostic criteria) by reviewing the literature and by analysing decreased serum IgM in our secondary hospital population. Our main finding is that true sIgMdef is probably very rare. Unfortunately, when a decreased serum IgM level is found, it is rarely analysed fully. In most cases in our cohort serum IgM levels were determined only once (86%). When proven persistently decreased, further immunological analysis is often not performed (data on IgG subclasses and/or vaccination responses were lacking in 74% of the literature cases and 93% of the cases in our cohort). Also, different criteria for ‘selective IgM deficiency’ are used in the literature; in a quarter of the cases, the deficiency is not ‘selective’, other immunological abnormalities were present. Eight literature cases even showed clinical and/or laboratory signs fitting combined immunodeficiency; these should not be classified as a form of ‘predominantly antibody deficiency’. Sixty‐three (24%) literature cases fitted the ESID classification ‘unclassified antibody deficiency’. These patients with concomitant defects in specific antibody production (SPAD) and/or IgG subclass deficiencies may be at risk of more severe and frequent infections, comparable to the increased number of lower respiratory tract infections and bronchiectasis in patients with IgA deficiency in combination with IgG subclass deficiency and/or SPAD 15. Patients with recurrent and/or severe infections and decreased serum IgM levels in combination with SPAD have been described to benefit from immunoglobulin treatment 4, 16.
Routine determination of serum IgM is advised in many medical protocols, mainly for adults; we showed in our cohort that this leads to many incidental findings of decreased serum IgM. The relatively common finding of a low serum IgM level in, immunologically speaking, asymptomatic adults (see Table 3), often not followed by further evaluation, warrants re‐evaluation of these medical protocols. In our cohort, secondary decreased serum IgM was five times more prevalent in adults and 2·5 times more prevalent in children than the primary form. Hobbs 17 reported that secondary decreased IgM was 20 times more prevalent than the primary form in 1975. This may be explained by the fact that age‐related reference values have changed over the years, as the sensitivity of the methods used to measure serum IgM increased (Hobbs et al. < 0·47 g/l > 3 years, our cohort < 0·21 g/l < 6 years, < 0·13 g/l < 16 years and < 0·40 g/l ≥ 16 years). In any case, the first reaction to finding a low IgM should be to exclude a secondary cause.
Table 3.
Adult patients from our cohort
| Patient | Age (years/gender) | Reason(s) for determining serum IgM level | Manifestation(s) during follow‐up that could be related to antibody deficiency | First serum IgM level (g/l) | Last serum IgM level (g/l) |
|---|---|---|---|---|---|
| 10 adults analysed for potential immunodeficiency | |||||
| ESID criteria completely fulfilled (true sIgMdef) | |||||
| 1 | 54/F | Recurrent respiratory infections, asthma, AR | Long‐lasting fatigue, keratitis | 0·26 | 0·27 |
| 2 | 41/M | Recurrent respiratory infections, asthma | – | 0·23 | 0·26 |
| Data on IgG subclasses present, but no data on pneumococcal antibody responses (possible sIgMdef) | |||||
| 3 | 33/M | Recurrent respiratory infections, asthma | – | 0·29 | 0·24 |
| 4 | 33/F | Recurrent vaginal candidiasis, weight loss | – | 0·24 | 0·24 |
| 5 | 68/F | Pneumonia | CREST syndrome, ABPA | 0·37 | 0·30 |
| 6 | 73/F | Recurrent pneumonia, bronchiectasis, AR | Chronic sinusitis | 0·36 | 0·29 |
| Data on pneumococcal antibody responses present, but no data on IgG subclasses (possible sIgMdef) | |||||
| 7 * | 34/M | Arthralgia | Erysipelas | <0·20 | <0·20 |
| No data on pneumococcal antibody responses and no data on IgG subclasses (possible sIgMdef) | |||||
| 8 | 53/F | Recurrent UTI, sinusitis | Inflammatory nodular hand osteoarthritis | 0·26 | 0·24 |
| 9 | 71/M | Pneumonia, bronchiectasis | – | 0·26 | 0·22 |
| 10 | 76/F | Non‐healing ulcer on feet | Depression, bronchiectasis, UTI | <0·20 | <0·20 |
| 7 adults diagnosed during analysis for other problems; history of symptoms that could be related to antibody deficiency | |||||
| No data on pneumococcal antibody responses and no data on IgG subclasses (possible sIgMdef) | |||||
| Serum IgM ordered by a neurologist | |||||
| 11 | 45/M | Migraine | – | 0·24 | 0·25 |
| 12 | 79/M | Polyneuropathy | Psoriasis | 0·39 | 0·32 |
| Serum IgM ordered by an internist | |||||
| 13 | 55/F | Liver test abnormalities | – | 0·38 | 0·31 |
| 14 | 58/F | Liver test abnormalities | – | 0·35 | 0·32 |
| 15 | 60/M | Collapsed vertebra | – | 0·23 | 0·23 |
| 16 | 73/M | Renal insufficiency | Chronic Q fever | <0·20 | 0·21 |
| 17 | 51/M | Long‐lasting fatigue, Q fever infection | – | 0·26 | 0·33 |
| 25 adults diagnosed during analysis for other problems; no history of symptoms that could be related to antibody deficiency | |||||
| No data on pneumococcal antibody responses and no data on IgG subclasses (possible sIgMdef) | |||||
| Serum IgM ordered by a rheumatologist | |||||
| 18 | 68/M | Arthralgia, RLS | Cholecystitis, pharyngitis, infected hematoma | 0·28 | 0·27 |
| 19 | 65/M | Arthralgia, myalgia | – | 0·28 | 0·26 |
| 20 | 75/F | Raynaud‐like symptoms | Basal cell carcinoma | <0·20 | 0·22 |
| 21 | 51/M | Arthritis urica | Inflammatory arthritis | 0·38 | 0·30 |
| Serum IgM ordered by an internist | |||||
| 22 | 67/F | Hypoparathyroidism, hypothyroidism | Abscess in thigh, infection of right hip | 0·27 | 0·27 |
| 23 | 70/M | Liver test abnormalities | – | 0·26 | <0·2 |
| 24 | 62/F | Weight loss | – | 0·37 | 0·30 |
| 25 | 52/F | Micro‐albuminuria, hypothyroidism | Chronic Q fever | 0·22 | 0·27 |
| 26 | 43/F | Splenic infarcts, abdominal pain | – | 0·38 | 0·23 |
| 27 | 55/M | Haematuria, recurrent kidney stones | UTI, respiratory infection, cervical lymphadenopathy | 0·35 | 0·37 |
| 28 | 71/F | Renal insufficiency | – | 0·32 | 0·31 |
| 29 | 45/M | Renal insufficiency | – | 0·37 | 0·32 |
| 30 | 69/M | Renal insufficiency | – | 0·37 | 0·32 |
| Serum IgM ordered by a neurologist | |||||
| 31 | 66/M | Polyneuropathy | – | 0·36 | 0·31 |
| 32 | 67/F | Polyneuropathy | – | 0·32 | 0·31 |
| 33 | 68/M | Polyneuropathy | Nodular basal cell carcinoma | 0·39 | 0·37 |
| 34 | 72/F | Polyneuropathy | – | 0·39 | 0·39 |
| 35 | 73/F | Polyneuropathy | – | 0·32 | 0·36 |
| 36 | 74/F | Polyneuropathy | – | 0·37 | 0·36 |
| 37 | 74/M | Polyneuropathy | – | 0·33 | 0·37 |
| 38 | 58/F | Polyneuropathy | – | 0·36 | 0·39 |
| 39 | 84/M | Polyneuropathy | – | 0·37 | 0·36 |
| 40 | 86/M | Polyneuropathy | – | 0·32 | 0·36 |
| 41 | 46/M | Polyneuropathy | – | 0·32 | 0·23 |
| 42 | 63/M | Polyneuropathy | – | 0·35 | 0·27 |
The 42 adult patients with true or possible selective primary IgM deficiency (sIgMdef) from our cohort. *This patient was diagnosed during analysis for rheumatoid arthritis. He was referred to a university centre elsewhere for analysis for potential immunodeficiency when a persistent decreased IgM level was discovered. ABPA = allergic bronchopulmonal aspergillosis; AR = allergic rhinitis; CREST = calcinosis, Raynaud's phenomenon, oesophageal dysmotility, sclerodactyly, teleangiectasia; ESID = European Society for Immunodeficiencies; F = female; Ig = immunoglobulin; M = male; RLS = Raynaud‐like symptoms; UTI = urinary tract infection.
The fact that only a few incidental findings of decreased serum IgM were followed by further evaluation in our cohort suggests that the perceived medical problems were mild. Most of our incidentally diagnosed cases with true or possible sIgMdef did not have a history of symptoms related to antibody deficiency (76%), and that often remained to be the case during follow‐up (72%) (conversely, 28% later developed symptoms that could be related to antibody deficiency). The higher prevalence of various associated diseases in the literature cases 1 is probably related to the fact that these patients had been referred to specialized allergy and immunology clinics 4, 5, 6.
Interestingly, possible or true sIgMdef was observed more frequently in males in our cohort. This parallels the observed male predominance in the literature. However, also among healthy controls low IgM levels are more common in males 18, 19, 20, 21, and there are some reports of low serum IgM levels among fathers of patients 22, 23. It would be of interest to investigate this gender difference further.
The limitation of our study is, of course, its retrospective design. We collected our cohort data from the medical files, which were not collected with a research purpose in mind. Therefore, we could not correct for environmental factors and genetic polymorphisms that may influence serum IgM levels 3. However, although very interesting on a population basis, these factors are probably not very helpful in directing decisions regarding individual patient care in the doctor's consulting room.
In conclusion, our review of the literature and retrospective secondary centre cohort study on decreased serum IgM illustrate the challenge of determining the clinical significance of a serum sample with decreased IgM. The diagnosis could rarely be made with certainty, but truly selective primary IgM deficiency is probably very rare. Our strict definitions and thorough analysis of the available information have yielded the largest cohort study so far. However, a larger cohort of true sIgMdef patients is needed to fully explore the clinical consequences; the ESID online Registry would be a useful tool for this.
Disclosure
There are no conflicts of interest.
Author contributions
L. M. A. J. and E. d. V. designed the study and wrote the paper. L. M. A. J. acquired the data and carried out statistical analyses. E. d. V. supervised and critically reviewed all data collection. T. M., M. C. W. C., J. F. M. P. and J. J. J. E. critically reviewed the results and contributed to the final version of the paper; all authors approved the final paper as submitted.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. Identification of papers that report on patients with decreased serum immunoglobulin (Ig)M (date: 10 May 2017). sIgMdef = selective IgM deficiency; unPAD = unclassified primary antibody deficiency.
Fig. S2. Patient selection. CF = cystic fibrosis; Ig = immunoglobulin; pt = patient; sIgMdef = selective IgM deficiency.
Table S1. Overview of the literature
References
- 1. Louis AG, Gupta S. Primary selective IgM deficiency: an ignored immunodeficiency. Clin Rev Allergy Immunol 2014; 46:104–11. [DOI] [PubMed] [Google Scholar]
- 2. Entezari N, Adab Z, Zeydi M et al The prevalence of Selective Immunoglobulin M Deficiency (SIgMD) in Iranian volunteer blood donors. Hum Immunol 2016; 77:7–11. [DOI] [PubMed] [Google Scholar]
- 3. Yang M, Wu Y, Lu Y et al Genome‐wide scan identifies variant in TNFSF13 associated with serum IgM in a healthy Chinese male population. PLOS ONE 2012; 7:e47990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Yel L, Ramanuja S, Gupta S. Clinical and immunological features in IgM deficiency. Int Arch Allergy Immunol 2009; 150:291–8. [DOI] [PubMed] [Google Scholar]
- 5. Goldstein MF, Goldstein AL, Dunsky EH, Dvorin DJ, Belecanech GA, Shamir K. Selective IgM immunodeficiency: retrospective analysis of 36 adult patients with review of the literature. Ann Allergy Asthma Immunol 2006; 97:717–30. [DOI] [PubMed] [Google Scholar]
- 6. Goldstein MF, Goldstein AL, Dunsky EH, Dvorin DJ, Belecanech GA, Shamir K. Pediatric selective IgM immunodeficiency. Clin Dev Immunol 2008; 2008:624850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Guill MF, Brown DA, Ochs HD, Pyun KH, Moffitt JE. IgM deficiency: clinical spectrum and immunologic assessment. Ann Allergy 1989; 62:547–52. [PubMed] [Google Scholar]
- 8. Hassanein HA, Elbadry MI. Selective immunoglobulin M deficiency in an adult with miliary tuberculosis: a clinically interesting coexistence. A case report and review of the literature. Int J Mycobacteriol 2016; 5:106–10. [DOI] [PubMed] [Google Scholar]
- 9. Kanariou M, Petridou E, Liatsis M, Revinthi K, Mandalenaki‐Lambrou K, Trichopoulos D. Age patterns of immunoglobulins G, A & M in healthy children and the influence of breast feeding and vaccination status. Pediatr Allergy Immunol 1995; 6:24–9. [DOI] [PubMed] [Google Scholar]
- 10. de Vries E. Patient‐centred screening for primary immunodeficiency, a multi‐stage diagnostic protocol designed for non‐immunologists: 2011 update. Clin Exp Immunol 2012; 167:108–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Richtlijn interpretatie van uitslagen van respons op pneumokokken vaccinaties . Laboratorium Translationele Immunologie; 2016. http://www.umcutrecht.nl/getattachment/Ziekenhuis/Professionals/Diagnostiek-aanvragen/laboratorium-translationele-immunologie/medische-immunologie/B2AMI192-Richtlijn-interpretatie-van-uitslagen-van-respons-op-pneumokokken-vaccinaties.pdf.aspx (accessed October 18, 2017).
- 12. Raziuddin S, Elawad ME, Benjamin B. T‐cell abnormalities in antibody deficiency syndromes. Scand J Immunol 1989; 30:419–24. [DOI] [PubMed] [Google Scholar]
- 13. Faulk WP, Kiyasu WS, Cooper MD, Fudenberg HH. Deficiency of IgM. Pediatrics 1971; 47:399–404. [PubMed] [Google Scholar]
- 14. Louis AG, Agrawal S, Gupta S. Analysis of subsets of B cells, Breg, CD4Treg and CD8Treg cells in adult patients with primary selective IgM deficiency. Am J Clin Exp Immunol 2016; 5:21–32. [PMC free article] [PubMed] [Google Scholar]
- 15. Aghamohammadi A, Cheraghi T, Gharagozlou M et al IgA deficiency: correlation between clinical and immunological phenotypes. J Clin Immunol 2009; 29:130–6. [DOI] [PubMed] [Google Scholar]
- 16. Stoelinga GB, van Munster PJ. Antibody deficiency syndrome and autoimmune haemolytic anaemia in a boy with isolated IgM deficiency dysimmunoglobulinaemia type 5. Acta Paediatr Scand 1969; 58:352–62. [DOI] [PubMed] [Google Scholar]
- 17. Hobbs JR. IgM deficiency. Birth Defects Orig Artic Ser 1975; 11:112–6. [PMC free article] [PubMed] [Google Scholar]
- 18. Kacprazak‐Bergman I. Sexual dimorphism of heritability of immunoglobulin levels. Ann Hum Biol 1994; 21:563–9. [DOI] [PubMed] [Google Scholar]
- 19. Hatagima A, Cabello PH, Krieger H. Causal analysis of the variability of IgA, IgG, and IgM immunoglobulin levels. Hum Biol 1999; 71:219–29. [PubMed] [Google Scholar]
- 20. Kardar GA, Shams SH, Pourpak Z, Moin M. Normal value of immunoglobulins IgA, IgG, and IgM in Iranian healthy adults, measured by nephelometry. J Immunoassay Immunochem 2003; 24:359–67. [DOI] [PubMed] [Google Scholar]
- 21. Cassidy JT, Nordby GL. Human serum immunoglobulin concentrations: prevalence of immunoglobulin deficiencies. J Allergy Clin Immunol 1975; 55:35–48. [DOI] [PubMed] [Google Scholar]
- 22. Hobbs JR, Milner RD, Watt PJ. Gamma‐M deficiency predisposing to meningococcal septicaemia. BMJ 1967; 4:583–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zaka‐ur‐Rab Z, Gupta P. Pseudomonas septicemia in selective IgM deficiency. Ind Pediatr; 2005; 42:961–2. [PubMed] [Google Scholar]
- 24. Kaufman HS, Hobbs JR. Immunoglobulin deficiencies in an atopic population. Lancet 1970; 2:1061–3. [DOI] [PubMed] [Google Scholar]
- 25. Silver HK, Shuster J, Gold P, Freedman SO. Leukopenia, leukoagglutinins, and low IgM in a family with severe febrile illnesses. Clin Immunol Immunopathol 1973; 1:220–9. [DOI] [PubMed] [Google Scholar]
- 26. Ross IN, Thompson RA. Severe selective IgM deficiency. J Clin Pathol 1976; 29:773–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dworzack DL, Murray CM, Hodges GR, Barnes WG. Community‐acquired bacteremic Achromobacter xylosoxidans type IIIa pneumonia in a patient with idiopathic IgM deficiency. Am J Clin Pathol 1978; 70:712–7. [DOI] [PubMed] [Google Scholar]
- 28. Brilliant LB, Nakano JH, Kitamura T, Hodakevic LN, Bharucha PB. Occupationally‐acquired smallpox in an IgM‐deficient health worker. Bull World Health Org 1981; 59:99–106. [PMC free article] [PubMed] [Google Scholar]
- 29. Endoh M, Kaneshige H, Tomino Y, Nomoto Y, Sakai H, Arimori S. Selective IgM deficiency: a case study. Tokai J Exp Clin Med 1981; 6:327–31. [PubMed] [Google Scholar]
- 30. Karsh J, Watts CS, Osterland CK. Selective immunoglobulin M deficiency in an adult: assessment of immunoglobulin production by peripheral blood lymphocytes in vitro . Clin Immunol Immunopathol 1982; 25:386–94. [DOI] [PubMed] [Google Scholar]
- 31. Matsushita S, Inoue T, Okubo H. A case of selective IgM deficiency: isotype‐specific suppressor T lymphocytes. Jpn J Med 1984; 23:149–51. [DOI] [PubMed] [Google Scholar]
- 32. Inoue T, Okumura Y, Shirama M, Ishibashi H, Kashiwagi S, Okubo H. Selective partial IgM deficiency: functional assessment of T and B lymphocytes in vitro . J Clin Immunol 1986; 6:130–5. [DOI] [PubMed] [Google Scholar]
- 33. Ohno T, Inaba M, Kuribayashi K, Masuda T, Kanoh T, Uchino H. Selective IgM deficiency in adults: phenotypically and functionally altered profiles of peripheral blood lymphocytes. Clin Exp Immunol 1987; 68:630–7. [PMC free article] [PubMed] [Google Scholar]
- 34. Yamasaki T. Selective IgM deficiency: functional assessment of peripheral blood lymphocytes in vitro. Intern Med 1992; 31:866–70. [DOI] [PubMed] [Google Scholar]
- 35. Fallon KE. Inability to train, recurrent infection, and selective IgM deficiency. Clin J Sport Med 2004; 14:357–9. [DOI] [PubMed] [Google Scholar]
- 36. Kutukculer N, Gulez N. The outcome of patients with unclassified hypogammaglobulinemia in early childhood. Pediatr Allergy Immunol 2009; 20:693–8. [DOI] [PubMed] [Google Scholar]
- 37. Chen S, Shamriz O, Toker O, Fridlender ZG, Tal Y. Recurrent eosinophilic pneumonia in a patient with isolated immunoglobulin M deficiency and celiac disease. Isr Med Assoc J 2015; 17:526–7. [PubMed] [Google Scholar]
- 38. Belgemen T, Suskan E, Dogu F, Ikinciogullari A. Selective immunoglobulin M deficiency presenting with recurrent impetigo: a case report and review of the literature. Int Arch Allergy Immunol 2009; 149:283–8. [DOI] [PubMed] [Google Scholar]
- 39. Cipe FE, Dogu F, Guloglu D et al B‐cell subsets in patients with transient hypogammaglobulinemia of infancy, partial IgA deficiency, and selective IgM deficiency. J Investig Allergol Clin Immunol 2013; 23:94–100. [PubMed] [Google Scholar]
- 40. Ostergaard PA. A girl with recurrent infections, low IgM and an abnormal chromosome number 1. Acta Paediatr Scand 1973; 62:211–5. [DOI] [PubMed] [Google Scholar]
- 41. Jones DM, Tobin BM, Butterworth A. Three cases of meningococcal infection in a family, associated with a deficient immune response. Arch Dis Child 1973; 48:742–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mayumi M, Yamaoka K, Tsutsui T et al Selective immunoglobulin M deficiency associated with disseminated molluscum contagiosum. Eur J Pediatr 1986; 145:99–103. [DOI] [PubMed] [Google Scholar]
- 43. Kiratli HK, Akar Y. Multiple recurrent hordeola associated with selective IgM deficiency. J AAPOS 2001; 5:60–1. [DOI] [PubMed] [Google Scholar]
- 44. Makay B, Unsal E, Anal O et al Chronic recurrent multifocal osteomyelitis in a patient with selective immunoglobulin M deficiency. Rheumatol Int 2009; 29:811–5. [DOI] [PubMed] [Google Scholar]
- 45. Kampitak T. Selective immunoglobulin M deficiency in a patient with refractory giardiasis. J Investig Allergol Clin Immunol 2010; 20:358–60. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. Identification of papers that report on patients with decreased serum immunoglobulin (Ig)M (date: 10 May 2017). sIgMdef = selective IgM deficiency; unPAD = unclassified primary antibody deficiency.
Fig. S2. Patient selection. CF = cystic fibrosis; Ig = immunoglobulin; pt = patient; sIgMdef = selective IgM deficiency.
Table S1. Overview of the literature


