Summary
Itolizumab is a humanized anti‐CD6 monoclonal antibody (mAb) that has previously shown encouraging results, in terms of safety and positive clinical effects, in a 6‐week monotherapy clinical trial conducted in rheumatoid arthritis (RA) patients. The current Phase I study evaluated the safety and clinical response for a longer treatment of 12 itolizumab intravenous doses in subjects with active RA despite previous disease‐modifying anti‐rheumatic drug (DMARD) therapy. Twenty‐one subjects were enrolled into four dosage groups (0·1, 0·2, 0·4 and 0·8 mg/kg). Efficacy end‐points including American College of Rheumatology (ACR)20, ACR50 and ACR70 response rates and disease activity score in 28 joints (DAS28) were monitored at baseline and at specific time‐points during a 10‐week follow‐up period. Itolizumab was well tolerated up to the highest tested dose. No related serious adverse events were reported and most adverse events were mild. Remarkably, itolizumab treatment did not produce lymphopenia and, therefore, was not associated with infections. All patients achieved a clinical response (ACR20) at least once during the study. Eleven subjects (55%) achieved at least a 20% improvement in ACR just 1 week after the first itolizumab administration. The clinical response was observed from the beginning of the treatment and was sustained during 24 weeks. The efficacy profile of this 12‐week treatment was similar to that of the previous study (6‐week treatment). These results reinforce the safety profile of itolizumab and provide further evidence on the clinical benefit from the use of this anti‐CD6 mAb in RA patients.
Keywords: CD6, clinical trial, itolizumab, Phase I, rheumatoid arthritis
Introduction
Rheumatoid arthritis (RA) is a systemic immune‐mediated inflammatory autoimmune disease (AD) that affects approximately 1% of the population worldwide 1. In Cuba, musculoskeletal pain is highly prevalent, with RA in particular having a prevalence of 1·24% 2. This disease is characterized by a chronic synovial inflammation, which progresses to joint destruction and bone erosions 3. Consequently, its final evolution is towards a complete loss of mobility and functioning, leading to a tremendous negative impact on the ability to perform daily activities and health‐related quality of life. It is now well established that RA contributes significantly to morbidity and mortality 4, 5.
Although the pathogenic mechanisms underlying the disease are not elucidated fully, it is known that joint inflammation is mediated by infiltration of immune cells, including T cells, into the synovial fluid. These activated T cells proliferate and recruit other immune cells, leading to the production of proinflammatory cytokines 6, 7, 8, 9.
Hence, biological agents targeting molecules that are involved in the autoimmune inflammatory process have been introduced into clinical practice, revolutionizing the therapeutic approach for RA. However, a substantial proportion of patients achieve only partial responses and do not reach clinical remission 10. Some others remain refractory or become non‐responders to the treatment 11, 12, 13, 14. Moreover, concerns remain regarding the immunosuppressive effects of these biological agents and the associated increased risk of infection 15. These issues underscore the imperative need to identify alternative RA treatments that exploit novel therapeutic targets with high efficacy over time and minimized toxicity.
The cell surface glycoprotein CD6 was one of the first T cell antigens to be identified 16. However, its role in T cell signalling pathways is complex and still controversial. It has been suggested that CD6 plays a dual role in T cells by promoting T cell activation through strong adhesion to other immune cells and inhibiting T cell receptor (TCR) signalling 17, 18, 19.
Several studies link CD6 with the pathogenesis of human AD, including multiple sclerosis, Sjögren's syndrome and RA 20, 21, 22, 23, 24, although it remains unclear how CD6 is implicated in the pathogenesis of these AD. In particular, CD166, the predominant ligand of CD6 25, as well as 3A11, another CD6 ligand, have been shown to mediate interactions between synovial fibroblasts and T lymphocytes in bone and joint tissues 26, 27. In consequence, CD6 represents a candidate target for the treatment of patients with AD. Nonetheless, few therapeutic approaches use this molecule as a target in the clinical setting 28, itolizumab being the only CD6‐targeting drug that has been used so far to treat AD patients.
Also known as T1h, itolizumab is a humanized version of the murine monoclonal antibody ior T1 29, which selectively targets the extracellular, membrane‐distal domain 1 of human CD6 30. A series of in‐vitro tests with peripheral blood mononuclear cells (PBMC) from healthy donors demonstrated that itolizumab inhibits CD6‐mediated co‐stimulation of human T lymphocytes, reducing their proliferation and proinflammatory cytokine production 31. A clinical study with itolizumab in patients with psoriasis corroborated these results 32.
An early clinical trial with the parental murine antibody using daily intravenous (i.v.) injections over 7 days provided a proof of concept in the clinical scenario of RA, showing favourable results but with undesired secondary effects because of the murine origin of the antibody. A dose‐dependent adverse event (AE) incidence was observed, with higher doses being associated with more serious toxicity 33. Later, an exploratory study with itolizumab demonstrated a striking absence of adverse reactions and reinforced a possible efficiency in a similar clinical scenario 34. However, this study did not allow definition of a therapeutic dose based on efficacy results, due to the small sample size and the short 6‐week treatment period.
As proof‐of‐concept trials in RA require at least 3 months of treatment to demonstrate an improvement in the manifestations of the active disease 35, the aim of the current study was to investigate a long‐lasting schedule of monotherapy with itolizumab in a larger patient cohort.
Thus, in the current study we examined the safety profile (primary objective), pharmacokinetics (PK), immunogenicity and preliminary effect (secondary objectives) of 12 i.v. doses of itolizumab used as monotherapy, in subjects with active moderate to severe RA despite previous therapies with disease‐modifying antirheumatic drugs (DMARDs).
Methods
Patients
Eligible patients were aged between 18 and 65 years who fulfilled the American College of Rheumatology (ACR) 1987 revised criteria for RA 36. Enrolment criteria required patients to have been diagnosed with RA at least 1 year before the screening, to have active disease despite being on a stable regimen of anti‐rheumatic therapy (at least one DMARD) and to complete an appropriate washout period after discontinuation of any previous treatment [4 weeks for DMARDs and steroids; 2 weeks for non‐steroidal anti‐inflammatory drugs (NSAIDs)]. Additionally, patients were required to have at least eight swollen and tender joints (66/68 joint count) to initiate treatment with the antibody. Serum chemistry and haematology results were required to be within acceptable limits. A protocol amendment was made to set up the lower limit of haemoglobin at 80 g/l, as haematological disorders are common in patients who suffer an active RA. Men or women with reproductive potential were required to be using a medically accepted form of contraception at the time of enrolment and were instructed to continue its use throughout the study.
Main exclusion criteria were the presence of recurrent chronic infection or any significant medical condition that would predispose to an unacceptable risk, and the presence of an inflammatory joint disease other than RA, or other systemic autoimmune disorder, or any overlapping syndrome. Female patients were excluded if they were pregnant or nursing, while women of childbearing potential had to show a negative result on a urine pregnancy test prior to receiving the study medication. All patients were given oral and written information concerning the trial and provided a written informed consent before undergoing any screening procedure. An institutional review board committee (IRB) safeguarded the rights, safety and wellbeing of all trial subjects.
Study design and assessment
This was a 24‐week, open‐label, non‐controlled, dose‐finding, pharmacokinetic, single‐centre, Phase I trial of itolizumab in adults with moderate to severe active RA. The study was conducted from January 2008 to May 2009 at a single clinical centre (National Service for Rheumatology) in Havana, Cuba. The protocol and related documents were reviewed and approved by the institutional review board (IRB) at the participating site, the study was authorized by the National Regulatory Agency [Center for State Control of the Quality of Drugs (CECMED)] and was carried out in compliance with the Declaration of Helsinki and good clinical practice guidelines. The trial was registered at the Cuban Public Registry of Clinical Trials in registroclinico.sld.cu under registration no. RPCEC00000035.
The study consisted of a washout period of 4 weeks, a 14‐week treatment period and a 10‐week follow‐up period. After a washout period, patients were assigned sequentially to one of four cohorts of five patients each, receiving an itolizumab dose of either 0·1, 0·2, 0·4 or 0·8 milligrams per kilogram of body weight. An ascending‐dose design was selected to ensure patient safety. Once three enrolled patients at a given dose level received at least two doses without showing any serious adverse reactions, the next‐higher dosing regimen could begin. The treatment duration and dose range were selected based on previous studies 33, 34, 37. As in our previous trial in RA patients 34, we chose a weekly administration frequency based on the positive results obtained with this scheme.
Patients received 12 i.v. doses of itolizumab, each in a 2‐h administration. Itolizumab was administered on day 1, followed by a 21‐day washout period to allow for single‐dose pharmacokinetic evaluation. From day 22 onwards, each patient received weekly administrations of the antibody. Patients were followed for an additional 10 weeks after the last dose. Concurrent treatment with any RA‐modifying drugs was forbidden throughout the treatment period and up to 4 weeks of follow‐up (week 18), when rescue therapy could be administered as per physician criteria if the disease flared. Nonetheless, patients were allowed to continue taking analgesic drugs (acetaminophen) during the study.
The primary end‐points were safety and tolerability. Safety was assessed by the occurrence of AEs and serious adverse events (SAEs), and by monitoring biochemical, haematological and urinalysis parameters during the entire study, up to week 24. AEs were given grades using the Common Toxicity Criteria (CTC) version 3.0 as a guideline. Secondary exploratory efficacy end‐points included the proportion of patients having American College of Rheumatology criteria improvement ≥ 20% (ACR20), ≥ 50% (ACR50) and ≥ 70% (ACR70) 38.
For each patient, all efficacy parameters were assessed on day 1, prior to the administration of itolizumab (baseline) and then 1 and 10 weeks after finishing the 12‐dose treatment (weeks 15 and 24 of the study, respectively). In addition, a retrospective data analysis determined changes in disease activity using the disease activity score of 28 joints (DAS 28) calculated from ACR elements. The DAS 28‐ESR calculation was based on the erythrocyte sedimentation rate (ESR) 39.
Phenotypical analysis of peripheral blood lymphocyte subsets
Peripheral blood mononuclear cells (PBMCs) isolated from fresh blood were studied at baseline (week 0) and weeks 9, 14 and 24. Cytofluorimetric analysis of lymphocyte populations was performed using an immunophenotypical panel (CD3+, CD4+, CD8+). A minimum of 100 000 events in the lymphocyte gate were acquired and analysed (BD Facscan, CellQuest software; Becton‐ Dickinson, San Diego, CA, USA). The total number of lymphocytes was determined using the values of complete blood counts performed as routine, when available.
Immunogenicity
Serum levels of immunoglobulin (Ig)G antibodies against the variable region of the humanized antibody itolizumab were measured, as the IgG response is predominant after prolonged exposure to the biological agent. Blood samples were taken at baseline (week 0) and at 9, 14 and 24 weeks. Anti‐idiotypical antibody response in human serum samples were measured by enzyme‐linked immunosorbent assay (ELISA), as described previously 32. Briefly, plates were coated with itolizumab F(ab′)2 and sera were assayed at 1 : 100 dilution. Pooled sera from Cercopitecus aethiops monkeys immunized with itolizumab and having a known high reactivity were used as positive reference 29. All samples were processed in duplicate. A cut‐off value of twofold the signal for the pre‐immune sera was taken to define a positive response.
Statistical analysis
No formal sample size calculation was performed, as the primary end‐point was safety and tolerability. All data analyses were conducted using descriptive statistics. The safety population, comprising all enrolled patients who received at least one dose of itolizumab, was used for safety analysis; the evaluable population for assessing the immunogenicity included all subjects with at least one valid immunogenicity test; the population to explore clinical benefit included all subjects who completed at least six itolizumab doses.
Patients who did not achieve an ACR20 were considered as non‐responders. Patients who dropped out of the study after receiving the drug or did not attend physician evaluation at the time‐point set to assess the clinical effect, regardless of the reason, were considered as non‐responders in the analysis of categorical end‐points (ACR).
Data on patients' disposition (number of patients enrolled, number of dropouts and reasons for dropping out), demographics (i.e. gender, age, skin) and other baseline characteristics are summarized as median and range (min–max) when the variable of examination is continuous, or using counts and percentages when the variable of examination is categorical (Table 1). The incidence of AEs and the proportion of patients achieving clinical benefit (ACR20/50/70) are presented in terms of number and percentage of patients. Results are summarized separately for the overall population (pooled across all dosage levels) and for each of the four dose cohorts.
Table 1.
Demographic indicators and baseline disease characteristics of rheumatoid arthritis (RA) patients in the intent‐to‐treat population enrolled in the trial by treatment group
| Itolizumab dose levels | |||||
|---|---|---|---|---|---|
| Characteristic | 0·1 mg/kg (n = 5) | 0·2 mg/kg (n = 5) | 0·4 mg/kg (n = 5) | 0·8 mg/kg (n = 5) | Total (n = 20) |
| Sex, no. (%) female | 5 (100) | 5 (100) | 4 (80) | 4 (80) | 18 (90) |
| Skin, no. (%) | |||||
| White | 2 (40) | 3 (60) | 2 (40) | 2 (40) | 9 (45) |
| Black | 2 (40) | 1 (20) | 2 (40) | 0 | 5 (25) |
| Other | 1 (20) | 1 (20) | 1 (20) | 3 (60) | 6 (30) |
| Age, years, median (range) | 59 (34–65) | 48 (35–64) | 41 (20–61) | 59 (32–64) | 56 (20–65) |
| RA duration, years, median (range) | 12 (3–41) | 8 (2–10) | 6 (1–23) | 5 (1–11) | 6 (1–41) |
| RA activity, moderate, no. (%) | 3 (60) | 4 (80) | 5 (100) | 4 (80) | 16 (80) |
| SJC, 66 joints * , median (range) | 32 (10–34) | 25 (12–29) | 9 (8–29) | 9 (8–21) | 14·5 (8–34) |
| TJC, 68 joints * , median (range) | 33 (10–44) | 31 (22–38) | 10 (8–30) | 12 (10–24) | 23 (8–44) |
| PAP * , median (range) | 9 (6–10) | 10 (8–10) | 8 (7–9) | 8 (8–10) | 9 (6–10) |
| GDAP * , median (range) | 9 (6–10) | 10 (6–10) | 8 (7–9) | 8 (7–10) | 9 (6–10) |
| GDAO * , median (range) | 10 (5–10) | 10 (7–10) | 8 (7–9) | 8 (7–10) | 9 (5–10) |
| HAQ * , median (range) | 2·1 (1·2–3) | 2 (0·8–2·6) | 1·3 (0·8–1·6) | 1·1 (0·3–2) | 1·6 (0·3–3) |
| ESR * , median (range) | 61 (13–117) | 55 (11–128) | 80 (20–105) | 50 (38–91) | 60 (11–128) |
| RF positive * , no. (%) | 4 (60) | 4 (80) | 4 (80·0) | 5 (100) | 17 (85) |
| CRP positive, no. (%) | 5 (100) | 4 (80) | 5 (100·0) | 5 (100) | 5 (100) |
| DAS28, median (range) | 8·1 (4·3–9·0) | 7·8 (6·4–8·0) | 6·2 (5·7–7·5) | 6·1 (5·6–6·9) | 6·9 (4·3–9·0) |
| DMARD failures, no. (%) | |||||
| ≥ 2 DMARDs | 5 (100) | 5 (100) | 4 (80) | 5 (100) | 19 (95) |
| Oral corticosteroid | 3(60) | 5 (100) | 5 (100) | 5 (100) | 18 (90) |
*Clinical indicators after the washout period were considered as baseline. CRP = C‐reactive protein; DAS 28 = disease activity score in 28 joints; DMARDs = disease‐modifying anti‐rheumatic drugs; ESR = erythrocyte sedimentation rate; GDAO = global disease assessment by observer; GDAP = global disease assessment by patient; HAQ = health assessment questionnaire; hb = haemoglobin; PAP = patient assessment of pain; RF = rheumatoid factor; SJC = swollen joint count; TJC = tender joint count.
Results
Patient baseline characteristics and disposition
This report is based on 20 of 21 enrolled patients who received ≥ 1 dose of itolizumab (Fig. 1). One patient assigned to the 0·1 mg/kg itolizumab dose fell into the exclusion criteria during the washout period, before receiving treatment. This patient was judged to have not achieved end‐points and was then replaced.
Figure 1.
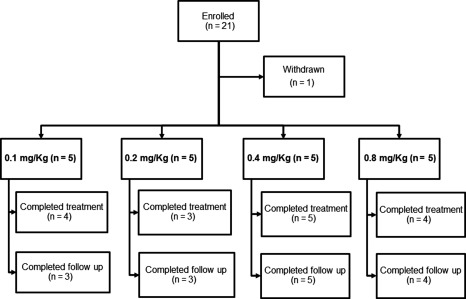
Patient disposition. A total of 21 patients were enrolled. One patient was excluded before starting treatment. Five other patients withdrew from the study at different time‐points.
Patients were predominantly white (45%) women (90%), with a median age of 56 years and median disease duration of 6 years. All the male patients (two) and nine of 18 women (50%) were of reproductive age (up to 65 and < 50 years, respectively). The population was biologically naive and showed active disease (moderate, 80% and severe, 20%) despite previous DMARD therapy; 95% of patients had previously received two or more DMARDs: 18 patients (90%) received methotrexate prior to entering the study. In addition, 15 (75%) patients had used anti‐malarial drugs (chloroquine or hydroxychloroquine), 12 (60%) sulfasalazine, seven (35%) azathioprine, four (20%) penicillamine and 18 patients (90%) used glucocorticoids (prednisone). Five patients (25%) had used non‐steroidal anti‐inflammatory drugs. Patient characteristics in this study were similar to those reported in an earlier overall study of the Cuban RA population 34. Baseline DAS 28‐ESR scores ranged from 4·3 to 9·0 (Table 1).
There were no differences in baseline demographic characteristics between any of the treatment groups. However, patients in the lower dose groups showed slightly higher median values for some disease characteristics compared to patients in the higher‐dose groups (i.e. RA duration: 12 versus 5 years, swollen joint count: 32 versus 9, tender joint count: 33 versus 12, health assessment questionnaire (HAQ)‐DI: 2·1 versus 1·1, DAS 28‐ESR: 8·1 versus 6·1), reflecting differences in baseline disease severity (Table 1). Such an imbalance may be attributed in part to sequential assignation.
Overall, 16 patients (80%) completed treatment to week 14 (12 administrations of itolizumab) and 15 patients (75%) completed the week 10 follow‐up visits (the entire 24‐week study). Five patients (25%) withdrew early from the study, most of them dropping out during the treatment period (80%). The relatively high dropout rate was not associated with safety reasons. The most common reason for discontinuation was patient's decision (50%). A comparable retention rate between dose groups was observed, with the lowest rate at 0·4 mg/kg (Table 2).
Table 2.
Frequently reported adverse events (in ≥ 10% of patients) and laboratory abnormalities through week 24 (by treatment group)
| Itolizumab dose levels | |||||
|---|---|---|---|---|---|
| 0·1 mg/kg (n = 5) | 0·2 mg/kg (n = 5) | 0·4 mg/kg (n = 5) | 0·8 mg/kg (n = 5) | Total (n = 20) | |
| Total number of AEs | 60 | 38 | 32 | 23 | 153 |
| Total subjects reporting ≥ 1 AEs | 5 (100) | 5 (100) | 5 (100) | 5 (100) | 20 (100) |
| Most frequent adverse events related to study drug | |||||
| Pyrexia | 4 (80) | 4 (80) | 5 (100) | 4 (80) | 17 (85) |
| Chills | 4 (80) | 1 (20) | 3 (60) | 4 (80) | 12 (60) |
| Headache | 3 (60) | 2 (40) | 0 | 1 (20) | 6 (30) |
| Pruritus | 2 (40) | 1 (20) | 0 | 1 (20) | 4 (20) |
| Shaking chills | 1 (20) | 0 | 2 (40) | 1 (20) | 4 (20) |
| Rash | 2 (40) | 0 | 0 | 1 (20) | 3 (15) |
| Nausea | 0 | 1 (20) | 0 | 1 (20) | 2 (10) |
| Most frequent adverse events unrelated to study drug | |||||
| Pyrexia | 2 (40) | 2 (40) | 1 (20) | 0 | 5 (25) |
| Cough | 2 (40) | 0 | 1 (20) | 0 | 3 (15) |
| Diarrhoea | 0 | 1 (20) | 1 (20) | 0 | 2 (10) |
| Anorexia | 0 | 1 (20) | 1 (20) | 0 | 2 (10) |
| Infections | |||||
| Common cold | 0 | 1 (20) | 0 | 0 | 1 (5) |
| Molar access | 0 | 0 | 1 (20) | 0 | 1 (5) |
| Infected bronchiectasis | 1 (20) | 0 | 0 | 0 | 1 (5) |
| Keratoconjunctivitis | 0 | 0 | 1 (20) | 0 | 1 (5) |
| Blepharoconjunctivitis | 0 | 0 | 1 (20) | 0 | 1 (5) |
| Urinary infection | 0 | 0 | 0 | 1 (20) | 1 (5) |
| Laboratory abnormalities | |||||
| Anaemia | 5 (100) | 3 (60) | 4 (80) | 3 (60) | 15 (75) |
| Thrombocytosis | 5 (100) | 5 (100) | 2 (40) | 3 (60) | 15 (75) |
| Decreased WBC | 2 (40) | 0 | 1 (20) | 0 | 3 (15) |
| Decreased ALC | 1 (20) | 0 | 0 | 1 (20) | 2 (10) |
| Early study discontinuation | |||||
| Use of restricted drugs | 0 | 1 (20) | 0 | 0 | 1 (5) |
| Consent withdrawn | 1 (20) | 1 (20) | 0 | 1 (20) | 3 (15) |
| Lost to follow‐up | 1 (20) | 0 | 0 | 0 | 1 (5) |
Given values correspond to the number of patients, followed by the percent they represent within their dose cohort. Subjects were counted only once for each referred term, regardless of how many events the subject reported. AE = adverse event; WBC = white blood cells; ALC = absolute lymphocyte count.
Safety
Twenty subjects who received at least one dose of itolizumab were included in the safety population; one patient who was enrolled but never treated was not analysed.
No serious or severe related AEs were reported. No AEs resulted in either definitive discontinuation or reduction of the antibody dose. All subjects experienced at least one AE during the study, but no relationship was established between the itolizumab dose and the nature, duration, frequency or severity of the reported AEs.
Of all the 153 AEs reported during the study, 93 events (60·7%) were considered to be related to the study drug. In general, AEs were transient and mild (97·8%). The most common one (incidence > 10% of total population) was pyrexia (37·6% of reported AEs), observed in 17 patients (85%), followed by chills (12·9%) in 12 patients (60%) and headache (12·9%) in six patients (30%) (Table 2). It is worth noting that we did not observe differences in the obtained results between patients in reproductive age and older patients (data not shown).
Concerning drug tolerability, 63 events (41%) were reported in 95% of patients on administration days. All these events were classified as related to the study drug administration and administration‐related reactions. The majority of them (41 AEs, 65%) occurred on the first administration day, with a subsequent decline in frequency (data not shown).
Three patients needed a temporary treatment interruption because of AEs of mild or moderate intensity, which could be managed feasibly without permanent interruption of treatment. These events were considered unrelated to treatment. Six infections occurred in five patients (25%), none of them being severe or considered to be drug‐related events. Patients treated with 0·4 mg/kg itolizumab showed a higher infection incidence (60%) compared to other treatment groups. Four patients recovered completely before the end of the study and one patient withdrew voluntarily from the study while being treated with specific medications (for infected bronchiectasis) (Table 2).
Overall, no changes considered to be of clinical relevance were observed during the study in regard to haematology, chemistry and urinalysis parameters, with the exception of a few laboratory parameters linked with disease activity. The most frequently detected haematological abnormalities were anaemia and thrombocytosis, reported in 15 patients (75%). In general, values tended to be within normal ranges throughout the treatment (Fig. 2a,b). In particular, median haemoglobin (Hb) levels decreased during the washout period and the subsequent 21‐day washout needed for first‐dose PK evaluations, which was probably associated with RA exacerbation as a consequence of the restricted use of DMARDs. However, after treatment restart at week 4, median Hb stabilized at approximately 110 g/l, keeping within the normal range until the end of the study (Fig. 2a). Notably, the first itolizumab administration produced a visible effect in all the groups, both in stopping Hb decrease (Fig. 2a) and in reducing platelet levels (Fig. 2b).
Figure 2.
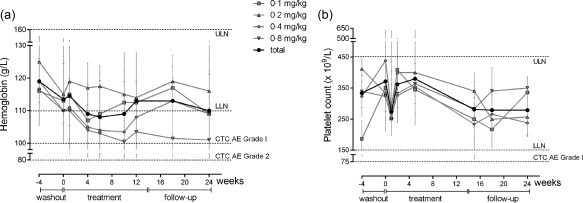
Laboratory markers of rheumatoid arthritis (RA) disease activity, per group and total median values (IQR). (a) Changes in haemoglobin (Hb) levels. (b) Changes in platelet counts. The graphs show also the normal laboratory reference ranges and the clinically significant ranges as per Common Terminology Criteria for Adverse Events (CTCAE) version 3. LLN = lower limit of normal; ULN = upper limit of normal; IQR = interquartile range.
Other laboratory parameters related with the itolizumab mechanism of action, such as white blood cell (WBC) population and absolute lymphocyte (ALC) population were within their normal reference ranges, and means were stable across the entire study for most patients (Fig. 3a,b). Approximately 60% of patients experienced some reduction in their ESR levels and 30% in C‐reactive protein (CRP) values (data not shown).
Figure 3.

Peripheral blood cell levels during the study, per group and total median values (IQR). (a) Total white blood cells (WBC), (b) absolute lymphocyte count (ALC), (c) CD4+ T cells and (d) CD8+ T cells. The graphs show also the normal laboratory reference ranges and the clinically significant ranges as per Common Terminology Criteria for Adverse Events (CTCAE) version 3. LLN = lower limit of normal; ULN = upper limit of normal; IQR = interquartile range.
Although treatment with DMARDs, glucocorticoids or NSAIDs was allowed after week 18 (4 weeks after treatment), only one patient from the 0·2 mg/kg dose group was medicated with low doses of oral corticosteroid at week 24, because of disease flares.
Immunophenotyping
As itolizumab binds to a T lymphocyte marker, special attention was given to characterize the effect of treatment on the immune system. In particular, we performed a phenotypical analysis of peripheral blood lymphocyte subsets (CD4+ and CD8+ T cells). In some patients, a transitory reduction of the CD4+ or CD8+ T cells was observed, but no apparent safety‐related significance was attributed to these effects. Moreover, the median peripheral blood CD4+ T cell counts decreased slightly throughout the treatment, but increased following the last dose (week 15), reaching almost baseline levels by week 24 (Fig. 3c). In contrast, median CD8+ T cell counts were stable (Fig. 3d). In general, no significant reduction in the numbers of CD4+ and CD8+ T cells was observe, either in the full set or in any of the cohorts. Hence, T cell subsets were not affected significantly by itolizumab treatment.
Immunogenicity
The population for the immunogenicity analysis comprised 20 subjects. The presence in the serum of anti‐idiotypical antibodies against itolizumab was tested throughout the study following administration on weeks 4, 9 and 14. No patient throughout the different dosage cohorts developed a significant anti‐idiotypical response (Fig. 4).
Figure 4.
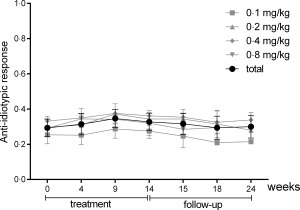
Anti‐idiotypical responses during itolizumab therapy, per group and total mean values [standard deviation (s.d.)]. A response was considered positive when the ratio post‐treatment optical density (OD)/pretreatment OD was > 2.
Efficacy evaluation
The efficacy analysis was performed 1 and 10 weeks after the last itolizumab dose (weeks 15 and 24, respectively). The overall study cohort analysis (total of patients) showed a substantial amelioration in the severity of active RA, as evidenced by the marked decrease of median DAS 28‐ESR from a baseline level of 6·9 at week 0 to 3·6 at week 24 (Fig. 5a), and by the percentage of patients (calculated for the intention‐to‐treat population) reaching each ACR level at weeks 15 and 24 (Fig. 5b), for the full set and by dose group.
Figure 5.
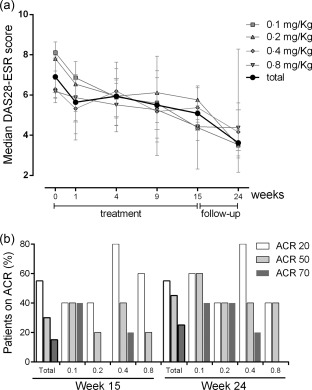
Efficacy outcomes. (a) Disease activity score in 28 joints (DAS)28‐erythrocyte sedimentation rate (ESR) assessed at baseline and during itolizumab therapy, per group and total median values (IQR). (b) Proportion of patients with improvement in American College of Rheumatology (ACR) criteria. IQR = interquartile range.
By the start of the study, almost all patients (19 of 20, 95%) exhibited high disease activity, evidenced by their high (≥ 5·1) DAS 28‐ESR values (Table 1). While modest improvements were observed at week 15, the situation changed considerably by week 24, when only 25% (four of 16) of the patients showed high DAS 28‐ESR and 25% (four of 16) exhibited low disease activity (DAS 28‐ESR ≤ 301832) (see Supporting information, Table S1).
Remarkably, both swollen and tender joint counts showed improvement as early as 1 week after treatment start, which was sustained over time (Supporting information, Fig. S1). Substantial reduction of the swollen joints preceded pain relief. These results correspond with the number of patients reaching the different ACR levels. By week 24, 79% (11 of 14) of patients in the per‐protocol population showed an ACR20 response, while 64% (nine of 14) and 36% (five of 14) showed ACR50 and ACR70, respectively. A high proportion of non‐responder patients (45%) was reported which resulted, to a large extent, from the relatively high number of non‐available patients at weeks 15 and 24 (20 and 30%, respectively). However, none of the dropouts was due to increased disease activity and two of the three patients who withdrew before completion of the 12‐week treatment period achieved ACR20 and ACR70 responses, respectively, by the last recorded visit (data not shown).
Discussion
The 24‐week findings from this open‐label, non‐controlled, single‐centre, dose‐finding, 12‐week treatment, prospective Phase I study are consistent with the safety and efficacy profiles seen in the previously reported 6‐week monotherapy Phase I study with itolizumab, conducted in biologically naive patients with active moderate to severe RA, despite previous DMARD therapy 34.
Given that the safety issue is a critical aspect for treatment decision in RA, the incidence rate of AEs was the primary intention of this study. In this regard, itolizumab showed a favourable safety and tolerability profile. Overall, AEs were usually mild, occurring mainly on the first administration day, with a considerable decline in frequency with subsequent administrations. The most frequent AEs were pyrexia, chills and headache. None of the tested doses was considered dose‐limiting for this clinical indication (up to 0·8 mg/kg administered weekly intravenously, 12 doses in total). In line with these results, itolizumab administration did not induce a measurable anti‐idiotypical antibody response, as also observed in previous studies 32, 34, 40.
Several biological therapies succeed by employing depleting strategies to eradicate autoreactive immune responses. One of the primary concerns when employing such a rationale is the resulting immunosuppressive effect and the associated increased risk of infection 41. In this study, however, itolizumab monotherapy was not associated with infections or any serious AEs. This observation is connected with a stable lymphocyte population within the normal reference range during the entire study and the absence of any other signs or symptoms which could be interpreted as immunosuppression induced by the antibody. These data support the previously stated thesis that itolizumab does not induce in‐vitro T cell depletion mediated by complement‐dependent cytotoxicity (CDC), antibody‐dependent cell‐mediated cytotoxicity (ADCC) or apoptosis 30, 31, 42. Nevertheless, as this was a short‐term clinical trial, additional long‐term studies are needed to characterize fully the adverse reaction profile of itolizumab.
In the current study, the evidences of clinical benefit were associated with improvements in disease activity and physical function, as measured by a variety of clinical end‐points, including ACR20/ACR50/ACR70 response rates, DAS 28‐ESR and HAQ‐DI. The onset of the clinical response was observed across all subgroups from week 1 following the first itolizumab administration, and was sustained during the 24 weeks. No patient withdrew due to insufficient clinical response. The observed reduced inflammation and RA activity were accompanied by a decrease in ESR, CRP and platelet counts, together with an increase in Hb levels. The latter is consistent with previous observations that, while the rate of anaemia is threefold higher in RA patients than in the general population 43, treatment of the inflammatory disease is associated with an increase in Hb levels 44.
We can state confidently that the increasing efficacy observed during the 10‐week follow‐up after itolizumab treatment was not due to the use of additional medications during this period. Indeed, none of the itolizumab‐treated patients received any DMARDs during the 24 weeks of the study. This is an important fact to note, as in the absence of a control group it serves as proof that the observed improvements are attributable to the antibody treatment, thus highlighting the anti‐inflammatory effects of itolizumab in RA patients.
Based on a previous report focusing on RA patients treated during 6 weeks with itolizumab 34, we proposed that an appropriate extensive use of itolizumab (12 weeks of treatment) would have a stronger impact in disease activity compared to the short treatment period. Nevertheless, in contrast with what we expected, the long‐term treatment performed, in terms of overall benefit, as well as the short‐treatment schedule. In particular, the ACR responses we observed at week 24 were in the same range of those reported in the previous study 45.
Differences in the incidence of adverse effects and clinical responses (ACR20) were observed among the four dose groups. However, the small number of subjects in each group, the differences in baseline clinical parameters between these groups and the relatively high dropout rate preclude drawing conclusions on dose–response efficacy. In addition, the lack of pharmacokinetic data does not allow to define an optimal dose level. Hence, further studies are needed to define the most effective itolizumab dose in RA patients.
The molecular mechanisms behind itolizumab's clinical effects are not understood completely. Several reports have stated that itolizumab does not block CD6–CD166 binding, based on competition binding assays in which a soluble form of CD6 was used 30, 31, 32, 34. In a recent paper, however, we give support to the idea that itolizumab may cause a steric blocking of the CD6–CD166 interaction in the actual cellular context, based on site mutagenesis, structural and modelling data 45.
A second CD6 ligand, called 3A11, was found a few years ago on cells from joint tissues 24, 26. This novel ligand is up‐regulated in synovial fibroblasts by interferon (IFN)‐γ 27. Although the binding site for the 3A11 ligand on CD6 remains unknown, the same reasoning followed previously 46 leads us to speculate that itolizumab might also block the binding of CD6 to 3A11, thus having an additional modulatory effect on the joint cells displaying this molecule.
Conversely, the function of CD6 in lymphocyte biology is controversial, playing a bimodal role as described recently 18, 19, 45. Thus, while the T cell‐activating properties of CD6 depend upon binding to CD166, its inhibitory effects have been shown to be independent of the interaction with this ligand. Furthermore, ligand‐dependent localization of CD6 at the synapse region is not required for the inhibitory functions, whereas it is required for the T cell‐activating functions of CD6 17.
Interestingly, a high expression of CD6 is found in T helper type 17 (Th17) cells from AD patients 47. These cells play a major role during development and aggravation of RA and produce high amounts of interleukin (IL)‐17 and IL‐6, in addition to tumour necrosis factor (TNF)‐α 48, 49. In this regard, previous studies have shown that itolizumab reduces the production of the proinflammatory cytokines IL‐6 and TNF‐α 31, 32, 40. Further research is needed to determine whether itolizumab halts T cell activation or promotes T cell inhibition. Either way, our clinical studies provide new evidence on the role of CD6 in autoimmunity and that modulating the activity of this receptor may provide a clinical benefit.
Within the limitations of an uncontrolled Phase I trial, and taking into account that the low number of subjects constrains interpretation of the obtained data, this study showed that itolizumab is well tolerated and suggests that treatment with this antibody may be effective in DMARD‐refractory active RA. Altogether, our results are encouraging and provide additional support for a further placebo‐controlled investigation.
Disclosure
The authors declare no conflicts of interest.
Author contributions
P. C. R. conceived and designed the study, analyzed and interpreted part of the data and critically reviewed the manuscript; D. M. P., C. M., A. M. L., J. A. G, I. M. H., J. P. M, Y. R., J. M. M, M. V. H. and R. T. recruited and managed the patients; E. M.‡ contributed substantially to the analysis of the data and to the writing and critical revision of the manuscript; L. E. A. performed HAMA studies and critically reviewed the manuscript; Y. A. and Y. B. collected the clinical data; E. M.¶ helped to design the study, analysed and interpreted part of the data; P. H. conducted the trial, collected, analysed and interpreted the data, and drafted the manuscript. All authors reviewed and approved the final version of the manuscript prior to its submission for publication.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Table S1. Proportion of patients with improvement in American College of Rheumatology (ACR) criteria (ACR20, ACR50, ACR70 = 20, 50 and 70% improvement, respectively, in ACR criteria for assessment of rheumatoid arthritis). Values correspond to the number of patients, followed by the percentage within the dose cohort. NR = non‐responders; NA = not available. The change in disease activity score using 28 joint counts from baseline (DAS 28)‐erythrocyte sedimentation rate (ESR).
Fig. S1. Changes in swollen (SJC) and tender (TJC) joint count with itolizumab treatment during the study, per group and total median values (IQR). IQR = interquartile range.
Acknowledgements
The authors thank Kamal Moudgil for critical reading of the manuscript and Roque M. Garcıa, Osmell Martınez and Eduardo Suarez for their assistance in the conduction of the study. We especially thank the patients, investigators and support personnel who made this trial possible.
References
- 1. Alamanos Y, Voulgari PV, Drosos AA. Incidence and prevalence of rheumatoid arthritis, based on the 1987 American College of Rheumatology criteria: a systematic review. Semin Arthritis Rheum 2006; 36:182–8. [DOI] [PubMed] [Google Scholar]
- 2. Reyes‐Llerena GA, Guibert‐Toledano M, Penedo‐Coello A et al Community‐based study to estimate prevalence and burden of illness of rheumatic diseases in Cuba: a COPCORD study. J Clin Rheumatol 2009; 5:51. [DOI] [PubMed] [Google Scholar]
- 3. Schneider M, Manabile E, Tikly M. Social aspects of living with rheumatoid arthritis: a qualitative descriptive study in Soweto, South Africa – a low resource context. Health Qual Life Outcomes 2008; 24:54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sokka T. Long‐term outcomes of rheumatoid arthritis. Curr Opin Rheumatol 2009; 21:284–290. [DOI] [PubMed] [Google Scholar]
- 5. Salaffi F, Sarzi‐Puttini P, Girolimetti R, Atzeni F, Gasparini S, Grassi W. Health‐related quality of life in fibromyalgia patients: a comparison with rheumatoid arthritis patients and the general population using the SF‐36 health survey. Clin Exp Rheumatol 2009; 27:S67–74. [PubMed] [Google Scholar]
- 6. Kusaba M, Honda J, Fukuda T, Oizumi K. Analysis of type 1 and type 2 T cells in synovial fluid and peripheral blood of patients with rheumatoid arthritis. J Rheumatol 1998; 25:1466–71. [PubMed] [Google Scholar]
- 7. Choy EH, Panayi GS. Cytokine pathways and joint inflammation in rheumatoid arthritis. N Engl J Med 2001; 344:907–916. [DOI] [PubMed] [Google Scholar]
- 8. McInnes I. Leukotrienes, mast cells, and T cells. Arthritis Res Ther 2003; 5:288–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Burmester GR, Feist E, Dorner T. Emerging cell and cytokine targets in rheumatoid arthritis. Nat Rev Rheumatol 2014; 10:77–88. [DOI] [PubMed] [Google Scholar]
- 10. Pope J, Combe B. Unmet needs in the treatment of rheumatoid arthritis. Open J Rheumatol Autoimmune Dis 2013; 3:65–78. [Google Scholar]
- 11. Khan IH, Krishnan VV, Ziman M et al A comparison of multiplex suspension array large‐panel kits for profiling cytokines and chemokines in rheumatoid arthritis patients. Cytometry B Clin Cytom 2009; 76:159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Weinblatt ME, Keystone EC, Furst DE et al Adalimumab, a fully human anti‐tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum 2003; 48:35–45. [DOI] [PubMed] [Google Scholar]
- 13. Rubbert‐Roth A, Finckh A. Treatment options in patients with rheumatoid arthritis failing initial TNF inhibitor therapy: a critical review. Arthritis Res Ther 2009; 11:S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hetland ML, Christensen IJ, Tarp U et al Direct comparison of treatment responses, remission rates, and drug adherence in patients with rheumatoid arthritis treated with adalimumab, etanercept, or infliximab: results from eight years of surveillance of clinical practice in the nationwide Danish DANBIO registry. Arthritis Rheum 2010; 62:22–32. [DOI] [PubMed] [Google Scholar]
- 15. Gomez‐Reino JJ, Carmona L, Valverde VR, Mola EM, Montero MD. BIOBADASER Group. Treatment of rheumatoid arthritis with tumor necrosis factor inhibitors may predispose to significant increase in tuberculosis risk: a multicenter active surveillance report. Arthritis Rheum 2003; 48:2122–2127. [DOI] [PubMed] [Google Scholar]
- 16. Kamoun M, Kadin ME, Martin PJ, Nettleton J, Hansen JA. A novel human T cell antigen preferentially expressed on mature T cells and shared by both well and poorly differentiated B cell leukemias and lymphomas. J Immunol 1981; 127:987–91. [PubMed] [Google Scholar]
- 17. Oliveira MI, Goncalves CM, Pinto M et al CD6 attenuates early and late signaling events, setting thresholds for T‐cell activation. Eur J Immunol 2012; 42:195–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Da Glória VG, de Araújo MM, Santos MA et al T cell activation regulates CD6 alternative splicing by transcription dynamics and SRSF1. J Immunol 2014; 193:391–9. [DOI] [PubMed] [Google Scholar]
- 19. Santos RF, Oliveira L, Carmo AM. Tuning T cell activation: the function of CD6 at the immunological synapse and in T cell responses. Curr Drug Targets 2016; 17:629–38. [DOI] [PubMed] [Google Scholar]
- 20. Ramos‐Casals M, Font J, Garcia‐Carrasco M et al High circulating levels of soluble scavenger receptors (sCD5 and sCD6) in patients with primary Sjogren's syndrome. Rheumatology (Oxford) 2001; 40:1056–9. [DOI] [PubMed] [Google Scholar]
- 21. Alonso R, Buors C, Le Dantec C et al Aberrant expression of CD6 on B‐cell subsets from patients with Sjogren's syndrome. J Autoimmun 2010; 35:336–41. [DOI] [PubMed] [Google Scholar]
- 22. De Jager PL, Jia X, Wang J et al Meta‐analysis of genome scans and replication identify CD6, IRF8 and TNFRSF1A as new multiple sclerosis susceptibility loci. Nat Genet 2009; 41:776–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Krintel SB, Essioux L, Wool A et al Hetland ML, CD6 and syntaxin binding protein 6 variants and response to tumor necrosis factor alpha inhibitors in Danish patients with rheumatoid arthritis. PLoS One 2012; 7:e38539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Joo YS, Singer NG, Endres JL et al Evidence for the expression of a second CD6 ligand by synovial fibroblasts. Arthritis Rheum 2000; 43:329–35. [DOI] [PubMed] [Google Scholar]
- 25. Levesque MC, Heinly CS, Whichard LP, Patel DD. Cytokine regulated expression of activated leukocyte cell adhesion molecule (CD166) on monocyte‐lineage cells and in rheumatoid arthritis synovium. Arthritis Rheum 1998; 41:2221–9. [DOI] [PubMed] [Google Scholar]
- 26. Saifullah MK, Fox DA, Sarkar S et al Expression and characterization of a novel CD6 ligand in cells derived from joint and epithelial tissues. J Immunol 2004; 173:6125–33. [DOI] [PubMed] [Google Scholar]
- 27. Stanley KT, VanDort C, Motyl C, Endres J, Fox DA. Immunocompetent properties of human osteoblasts: interactions with T lymphocytes. J Bone Miner Res 2006; 21:29–36. [DOI] [PubMed] [Google Scholar]
- 28. Hafler DA, Fallis RJ, Dawson DM et al Immunologic responses of progressive multiple sclerosis patients treated with an anti‐T‐cell monoclonal antibody, anti‐T12. Neurology 1986; 36:777–84. [DOI] [PubMed] [Google Scholar]
- 29. Roque‐Navarro L, Mateo C, Lombardero J et al Humanization of predicted T‐cell epitopes reduces the immunogenicity of chimeric antibodies: new evidence supporting a simple method. Hybrid Hybridomics 2003; 22:245–57. [DOI] [PubMed] [Google Scholar]
- 30. Alonso R, Huerta V, de Leon J et al Towards the definition of a chimpanzee and human conserved CD6 domain 1 epitope recognized by T1 monoclonal antibody. Hybridoma (Larchmt) 2008; 27:291–301. [DOI] [PubMed] [Google Scholar]
- 31. Nair P, Melarkode R, Rajkumar D, Montero E. CD6 synergistic costimulation promoting proinflammatory response is modulated without interfering with the activated leucocyte cell adhesion molecule interaction. Clin Exp Immunol 2010; 162:116–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aira LE, López‐Requena A, Fuentes D et al Immunological and histological evaluation of clinical samples from psoriasis patients treated with anti‐CD6 itolizumab. MAbs 2014; 6:783–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Montero E, Reyes G, Guibert M et al Immunodiagnosis and therapeutic immunosuppression in rheumatoid arthritis with IORT1 (anti‐CD6) monoclonal antibody. Arthritis Res Ther 2002; 4:114. [Google Scholar]
- 34. Rodriguez PC, Torres‐Moya R, Reyes G et al A clinical exploratory study with itolizumab, an anti‐CD6 antibody, in patients with rheumatoid arthritis. Results Immunol 2012; 2:204–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Strand V, Sokolove J. Randomized controlled trial design in rheumatoid arthritis: the past decade. Arthritis Res Ther 2009; 11:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arnett FC, Edworthy SM, Bloch DA et al The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988; 31:315–324. [DOI] [PubMed] [Google Scholar]
- 37. Montero E, Falcon L, Morera Y, Delgado J, Amador JF, Perez R. CD6 molecule may be important in the pathological mechanisms of lymphocytes adhesion to human skin in psoriasis and ior T1 MAb a possible new approach to treat this disease. Autoimmunity 1999; 29:155–156. [DOI] [PubMed] [Google Scholar]
- 38. Felson DT, Anderson JJ, Boers M et al American College of Rheumatology: preliminary definition of improvement in rheumatoid arthritis. Arthritis Rheum 1995; 38:727–35. [DOI] [PubMed] [Google Scholar]
- 39. Prevoo ML, van't Hof MA, Kuper HH et al Modified disease activity scores that include twenty‐eight‐joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum 1995; 38:44–8. [DOI] [PubMed] [Google Scholar]
- 40. Aira LE, Hernández P, Prada D et al Immunological evaluation of rheumatoid arthritis patients treated with itolizumab. MAbs 2016; 8:187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Smilek DE, Ehlers MR, Nepom GT. Restoring the balance: immunotherapeutic combinations for autoimmune disease. Dis Model Mech 2014; 7:503–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Beck A, Wurch T, Reichert JM. 6th Annual European Antibody Congress 2010: November 29–December 1, 2010, Geneva, Switzerland. MAbs 2011; 3:111–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wolfe F, Michaud K. Anemia and renal function in patients with rheumatoid arthritis. J Rheumatol 2006; 33:1516–1522. [PubMed] [Google Scholar]
- 44. Calisto Pérez C, León R, León F, Ng SL. Rheumatoid arthritis and anemia: the impact of different anti‐inflammatory therapies on hemoglobin levels. An observational study. Bol Asoc Med P R 2012; 104:34–41. [PubMed] [Google Scholar]
- 45. Hernández P, Moreno E, Aira LE, Rodríguez PC. Therapeutic targeting of CD6 in autoimmune diseases: a review of Cuban clinical studies with the antibodies IOR‐T1 and itolizumab. Curr Drug Targets 2016; 17:666–77. [DOI] [PubMed] [Google Scholar]
- 46. Pinto M, Carmo AM. CD6 as a therapeutic target in autoimmune diseases: successes and challenges. BioDrugs 2013; 27:191–202. [DOI] [PubMed] [Google Scholar]
- 47. Brucklacher‐Waldert V, Steinbach K, Lioznov M, Kolster M, Holscher C, Tolosa E. Phenotypical characterization of human Th17 cells unambiguously identified by surface IL‐17A expression. J Immunol 2009; 183:5494–501. [DOI] [PubMed] [Google Scholar]
- 48. Leipe J, Grunke M, Dechant C et al Role of Th17 cells in human autoimmune arthritis. Arthritis Rheum 2010; 62:2876–85. [DOI] [PubMed] [Google Scholar]
- 49. van Hamburg JP, Asmawidjaja PS, Davelaar N et al Th17 cells, but not Th1 cells, from patients with early rheumatoid arthritis are potent inducers of matrix metalloproteinases and proinflammatory cytokines upon synovial fibroblast interaction, including autocrine interleukin‐17A production. Arthritis Rheum 2011; 63:73–83. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Table S1. Proportion of patients with improvement in American College of Rheumatology (ACR) criteria (ACR20, ACR50, ACR70 = 20, 50 and 70% improvement, respectively, in ACR criteria for assessment of rheumatoid arthritis). Values correspond to the number of patients, followed by the percentage within the dose cohort. NR = non‐responders; NA = not available. The change in disease activity score using 28 joint counts from baseline (DAS 28)‐erythrocyte sedimentation rate (ESR).
Fig. S1. Changes in swollen (SJC) and tender (TJC) joint count with itolizumab treatment during the study, per group and total median values (IQR). IQR = interquartile range.


