Abstract
Background and Purpose
Berberine, a compound from rhizome coptidis, is traditionally used to treat gastrointestinal infections, such as bacterial diarrhoea. Recently, berberine was shown to have hypoglycaemic and hypolipidaemic effects. We investigated the mechanisms by which berberine regulates hepatic lipid metabolism and energy expenditure in mice.
Experimental Approach
Liver‐specific SIRT1 knockout mice and their wild‐type littermates were fed a high‐fat, high‐sucrose (HFHS) diet and treated with berberine by i.p. injection for five weeks. Mouse primary hepatocytes and human HepG2 cells were treated with berberine and then subjected to immunoblotting analysis and Oil Red O staining.
Key Results
Berberine attenuated hepatic steatosis and controlled energy balance in mice by inducing autophagy and FGF21. These beneficial effects of berberine on autophagy and hepatic steatosis were abolished by a deficiency of the nutrient sensor SIRT1 in the liver of HFHS diet‐fed obese mice and in mouse primary hepatocytes. SIRT1 is essential for berberine to potentiate autophagy and inhibit lipid storage in mouse livers in response to fasting. Mechanistically, the berberine stimulates SIRT1 deacetylation activity and induces autophagy in an autophagy protein 5‐dependent manner. Moreover, the administration of berberine was shown to promote hepatic gene expression and circulating levels of FGF21 and ketone bodies in mice in a SIRT1‐dependent manner.
Conclusions and Implications
Berberine acts in the liver to regulate lipid utilization and maintain whole‐body energy metabolism by mediating autophagy and FGF21 activation. Hence, it has therapeutic potential for treating metabolic defects under nutritional overload, such as fatty liver diseases, type 2 diabetes and obesity.
Abbreviations
- Atg5
autophagy protein 5
- BAT
brown adipose tissue
- FGF21
fibroblast growth factor 21
- LKO
liver‐specific knockout
- SIRT1
mammalian orthologue of the yeast silent information regulator 2
- WAT
white adipose tissue
- WT
wild type
Introduction
The liver is the central metabolic organ that regulates lipid homeostasis such as de novo lipogenesis, fatty acid β‐oxidation in mitochondria, lipoprotein uptake and secretion in response to nutritional and hormonal signals (Cohen et al., 2011). Autophagy is a cellular quality control pathway that degrades cytoplasmic contents in the lysosomes (Levine and Kroemer, 2008; Mizushima et al., 2008). Autophagy‐related proteins (Atgs) regulate different steps of autophagy, including the expansion of the isolation membrane and autophagosomes production that sequester cytoplasmic cargo for lysosomal degradation (Settembre and Ballabio, 2014). Lipophagy is a specific degradation of lipids by autophagy (Singh et al., 2009), in which lipid droplets are hydrolysed to release free fatty acids for mitochondrial oxidation and the tricarboxylic acid cycle to produce energy (Kaur and Debnath, 2015). Lipophagy has been shown to regulate intracellular lipid homeostasis in response to various nutrient signals, and impaired lipophagy can cause excessive lipid accumulation in the liver (Liu and Czaja, 2013).
Berberine, a commonly used drug in traditional Chinese medicine, has received increasing attention for its therapeutic potential for treating hepatic steatosis, dyslipidaemia and diabetes (Jun et al., 2008). Berberine or its derivative was shown to decrease hepatic steatosis in HepG2 cells (Brusq et al., 2006), in high‐fat diet‐fed rats (Turner et al., 2008; Chang et al., 2010; Xia et al., 2011; Yuan et al., 2015) and in a randomized, placebo‐controlled trial in patients with non‐alcoholic fatty liver disease (NAFLD) (Yan et al., 2015), and to lower serum LDL cholesterol in hypercholesterolaemic patients (Kong et al., 2004). Although the beneficial effects of berberine appear to be partially mediated by activating AMPK (Lee et al., 2006; Hardie, 2011) or up‐regulating LDLR expression (Kong et al., 2004), the underlying mechanism for the effects of berberine on hepatic lipid metabolism remains incompletely understood.
The NAD+‐dependent deacetylase sirtuin 1 (SIRT1), which induces longevity in yeast, worms and flies and may mediate the beneficial effects of caloric restriction (Haigis and Guarente, 2006; Michan and Sinclair, 2007), has emerged as a critical regulator of hepatic lipid homeostasis. Hepatic‐specific SIRT1 deficiency causes hepatic steatosis in fasted and diet‐induced obese (DIO) mice (Purushotham et al., 2009). Hepatic overexpression of SIRT1 represses endoplasmic reticulum stress and insulin resistance in DIO and ob/ob mice (Li et al., 2011a). Resveratrol, a pharmacological SIRT1 activator, represses hepatic lipid accumulation and protects liver function in DIO mice (Baur et al., 2006). In addition, FGF21, a liver‐derived hormone, has emerged as a key metabolic regulator. FGF21 improves hepatic insulin sensitivity and maintains systemic glucose homeostasis in insulin‐resistant mice (Kharitonenkov et al., 2005; Gong et al., 2016), which is similar to the observations obtained in diabetic mice, where it was found to act as an insulin sensitizer and lower blood glucose levels (Ahmadian et al., 2013; Suh et al., 2014). Interestingly, FGF21 mediates SIRT1's salutary effects on alleviating hepatic steatosis and inducing energy expenditure (Li et al., 2014b). However, the relative contribution of SIRT1 or FGF21 to berberine's effects on hepatic lipid and energy metabolism has not been investigated.
Our recent studies demonstrated that berberine ameliorates hepatic steatosis in rodents and patients with NAFLD (Chang et al., 2010; Yan et al., 2015) and hepatic SIRT1 is a key regulator for hepatic lipid metabolism (Li et al., 2011a). In the present study, we demonstrated that SIRT1 has a critical role in the mediation of berberine's salutary effects on improving hepatic steatosis by inducing autophagy and promoting energy expenditure by stimulating FGF21. These in vivo and in vitro studies indicate that (i) administration of berberine enhances systemic energy expenditure and ameliorates obesity; (ii) hepatic SIRT1 is required for berberine‐induced production of FGF21; (iii) berberine stimulates autophagy to ameliorate hepatic steatosis in mice; and (iv) SIRT1 is required for the activation by berberine of autophagy in mice with diet‐induced obesity or in response to fasting.
Methods
Animals
All animal experimental protocols were approved by Institutional Animal Care and Use Committee at the Institute for Nutritional Sciences, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath and Lilley, 2015). Hepatocyte‐specific deletion of SIRT1 [liver‐specific SIRT1 knockout (SIRT1 LKO)] mice were generated by crossing albumin–Cre recombinase transgenic mice with floxed SIRT1 mice containing the deleted SIRT1 exon4 as described previously (Chen et al., 2008). Eight‐week‐old male C57BL/6 mice were purchased from Shanghai Laboratory Animal Co. Ltd ( Shanghai, China). Mice were killed under isoflurane anaesthesia. Tissues were rapidly excised, freshly frozen in liquid nitrogen and stored at −80°C until needed for immunoblots. Other parts of tissues were fixed for histological analysis. The mice were housed in the research animal facility at Shanghai Institutes for Biological Sciences with correct husbandry care and maintained at 23°C under a 12:12 h light/dark. Mice were allowed access to the diets and water ad libitum. The experimenters were blinded to group assignment and outcome assessment. Diet‐induced or fasted mice are animal models that are routinely used to study the pathophysiological mechanisms of metabolic diseases such as hepatic steatosis (Li et al., 2011b; 2014b). The data obtained from these mice have translational potential for human purposes.
Experimental procedures
Mice were fed without or with high‐fat, high‐sucrose (HFHS) diet (D12327; Research Diets, New Brunswick, NJ, USA) consisting of 40% fat, 40% carbohydrate and 20% protein, randomly divided into groups as indicated and then treated with berberine (5 mg·kg−1·day−1, Sigma‐Aldrich, St. Louis, MO, USA) or vehicle (PBS) by i.p. injection once daily for 5 weeks. In vivo autophagy inhibition assay was performed as described previously (Ding et al., 2010), and the mice were i.p. injected with chloroquine (60 mg·kg−1, Sigma‐Aldrich) or vehicle (PBS) for 24 h. Fasting analysis was performed in mice as described previously (Li et al., 2014b; Chen et al., 2016). Mice were treated with berberine (5 mg·kg−1·day−1) or vehicle (PBS) by i.p. injection once daily for 4 weeks and then randomly divided into groups: fed, fasted and refed. The fed group was placed on a normal chow diet, and the fasted group was deprived of food for 24 h, and the refed group was deprived of food for 24 h and then provided with food for 6 h before the end of the experiments.
Liver histological analysis
Livers were fixed in 10% phosphate‐buffered formalin acetate at 4°C overnight and embedded in paraffin wax. Paraffin sections (5 μm) were cut and mounted on glass slides for haematoxylin and eosin (H&E) staining as described previously (Li et al., 2011b; 2013). Livers embedded in optimum cutting temperature compound (Tissue‐Tek; Laborimpex, Vorst, Belgium) were used for Oil Red O staining for the assessment of hepatic steatosis according to the manufacturer's instructions (American MasterTech, Lodi, CA, USA).
Small interfering RNA knockdown
Knockdown experiments of Atg5 in human HepG2 cells were performed using small interfering RNA (siRNA) oligonucleotides from GenePharma (Shanghai, China). The sense sequences of the siRNA oligos are as follows: siAtg5‐938, GACCUUUCAUUCAGAAGCUTT; siAtg5‐695, GUCCAUCUAAGGAUGCAAUTT; and negative control siRNA, UUCUCCGAACGUGUCACGUTT. Cells were transfected with siRNAs using Lipofectamine 2000 (Life Technologies, Carlsbad, CA, USA).
In vitro lipid accumulation
Lipid accumulation in hepatocytes was performed as described previously (Gómez‐Lechón et al., 2007). The HepG2 hepatocytes were starved in serum‐free DMEM (with 1% penicillin/streptomycin) for 24 h and treated for an additional 20 h in DMEM containing 100 μM palmitate, without or with berberine (10 μM), EX527 (5 μM) or chloroquine (50 μM) as indicated. The accumulation of lipid droplets was visualized by Oil Red O staining or quantified by chloroform/methanol extraction analysis.
Cell treatment
Primary mouse hepatocytes, human HepG2, HEK293 and HeLa cells, SIRT1+/+ or SIRT1−/− hepatocytes and mouse embryo fibroblasts (MEFs) were cultured and treated as described previously (Li et al., 2011b; Chen et al., 2016; Gong et al., 2016). SIRT1+/+ or SIRT1−/− hepatocytes were isolated from wild‐type (WT) or SIRT1 LKO mice. MEFs were isolated from floxed SIRT1 mice containing the deleted SIRT1 exon4 and then infected with adenoviral Cre or green fluorescent protein (GFP) control for 72 h.
Statistical analysis
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). Data are expressed as mean ± SEM. Statistical significance was evaluated using Student's unpaired two‐tailed t‐test and among more than two groups by analysis of one‐way ANOVA with Bonferroni's post hoc test. Post hoc test was run only if F achieved P < 0.05, and there was no significant variance in homogeneity. Data were analysed with GraphPad Prism software, version 5.0 (GraphPad Software Inc., La Jolla, CA, USA).
Nomenclature of targets and ligands
Key protein targets and ligands in this article are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2017/18 (Alexander et al., 2017a,b).
Results
Hepatic‐specific deletion of SIRT1 abolishes the berberine‐induced reduction in hepatic steatosis and body weight in HFHS diet‐fed mice
To determine whether SIRT1 plays a role in berberine's effects on hepatic steatosis and obesity, WT littermates or liver‐specific SIRT1 deficient mice were fed a diet consisting of HFHS (Li et al., 2011b; Gong et al., 2016), followed by treatment with berberine or vehicle. In the WT mice, administration of berberine caused a potent reduction in hepatic steatosis as demonstrated by a significant reduction of liver triglyceride levels, H&E and Oil Red O staining and decreased stained areas (Figure 1A–D), suggesting berberine can affect hepatic lipid metabolism. Notably, hepatic cholesterol levels were comparable between these groups. Moreover, berberine‐treated mice had reduced body weight with no significant effect on liver weight (Figure 1E and Supporting Information Figure S1A). These results are consistent with berberine or its derivative ameliorating hepatic steatosis and reducing body weight when used to treat obese rodents and hepatocytes (Brusq et al., 2006; Turner et al., 2008; Chang et al., 2010). Strikingly, compared with WT mice, the beneficial effects of berberine, lowering hepatic steatosis and body weight, were largely diminished in SIRT1 LKO mice, suggesting a critical role of SIRT1 in mediating berberine's effects on hepatic lipid metabolism and body weight gain.
Figure 1.
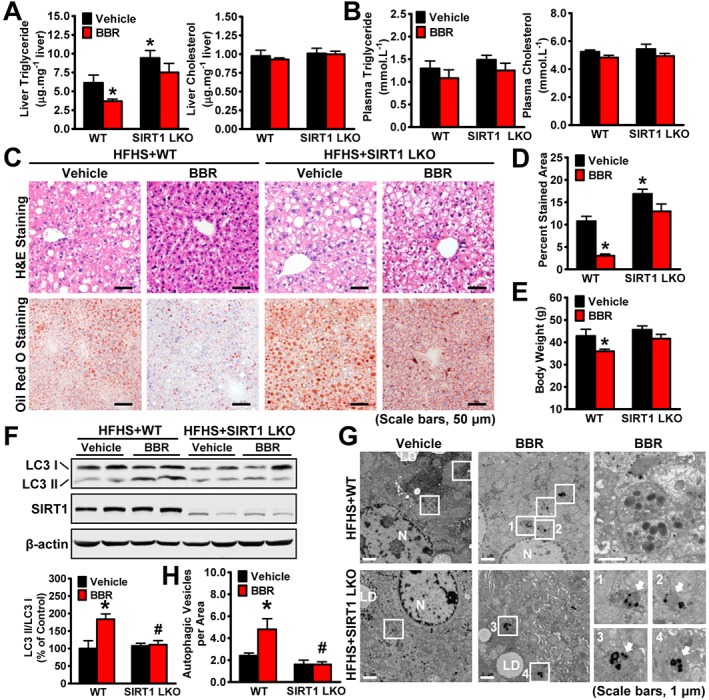
The beneficial effects of berberine (BBR) on body weight and hepatic steatosis are compromised by hepatic‐specific deletion of SIRT1 in HFHS diet‐fed mice. WT and SIRT1 LKO male mice at 16 weeks old were fed on a HFHS diet for 12 weeks and then treated with berberine (5 mg·kg−1·day−1) or vehicle (PBS) by i.p. injection once daily for 5 weeks. (A) Liver and (B) plasma triglyceride and cholesterol levels were assessed in mice. (C) Representative H&E and Oil Red O staining and (D) quantification of Oil Red O‐stained areas are shown, n = 6. (E) The body weight was measured, n = 6. (F‐H) SIRT1 is necessary for berberine‐induced autophagy in the liver of HFHS diet‐fed mice. (F) Berberine‐stimulated conversion of LC3‐I to LC3‐II is reduced in SIRT1 LKO mice fed a HFHS diet. Band intensity of LC3‐II was quantified by densitometry and normalized to the levels of LC3‐I for unwanted sources of variation and presented as the mean ± SEM, n = 6. *P < 0.05, versus WT and vehicle; #P < 0.05, versus WT mice treated with berberine. (G) Electron micrographs of the liver tissue. Images 1–4 showing AVs are high magnifications of the ‘white box’ area indicated in the images of berberine‐treated WT or SIRT1 LKO mice fed a HFHS diet. Arrows denote autophagic vacuoles (AVs). N, nucleus; LD, lipid droplet. (H) The average number of AVs was quantified from a randomly selected pool of five fields under each condition. The data are presented as the mean ± SEM, n = 5. *P < 0.05, versus WT and vehicle; #P < 0.05, versus WT mice treated with berberine.
Berberine stimulates autophagy activation in a SIRT1‐dependent manner in the liver of HFHS diet‐fed mice
Recently, studies have demonstrated that berberine induces the nutrient sensor SIRT1 to promote muscle function (Gomes et al., 2012) and stimulates autophagy to regulate cellular apoptosis (Wang et al., 2010; Yu et al., 2014). To determine whether SIRT1 plays a role in mediating berberine's effects on autophagy in the liver, an immunoblotting analysis was performed in the liver of mice fed the HFHS diet. As shown in Figure 1F, the administration of berberine induced a significant elevation of autophagy, as demonstrated by an increase in the membrane‐associated, phosphatidylethanolamine‐conjugated form of LC3 (LC3‐II). Strikingly, the ability of berberine to induce autophagy was abolished by a deficit of SIRT1; the LC3‐II was significantly reduced in the liver of SIRT1 LKO mice fed the HFHS diet. Importantly, electron microscopic (EM) analysis indicated that the number of cytosolic autophagic vacuoles (AVs), observed as double membrane structures containing undigested cytoplasmic contents, was increased in the berberine‐treated mouse livers, but was diminished in those with a hepatic SIRT1 deficiency (Figure 1G, H).
To determine whether berberine induces autophagic flux in vivo, mice were administered the lysosome inhibitor chloroquine. As shown in Figure 2A, administration of 60 mg·kg−1 chloroquine increased the expression levels of LC3‐II in the liver of mice, which is consistent with previous observations (Ding et al., 2010). Strikingly, the stimulating effects of berberine on LC3‐II were further increased in the liver when lysosome‐mediated protein degradation was inhibited by chloroquine treatment, consistent with the EM analysis showing that berberine induced AVs (Figure 2B). Given that a significant increase in LC3‐II or AVs was induced by berberine in the presence of chloroquine, these data suggest that berberine is able to promote the synthesis of autophagy in the liver of mice fed a HFHS diet.
Figure 2.
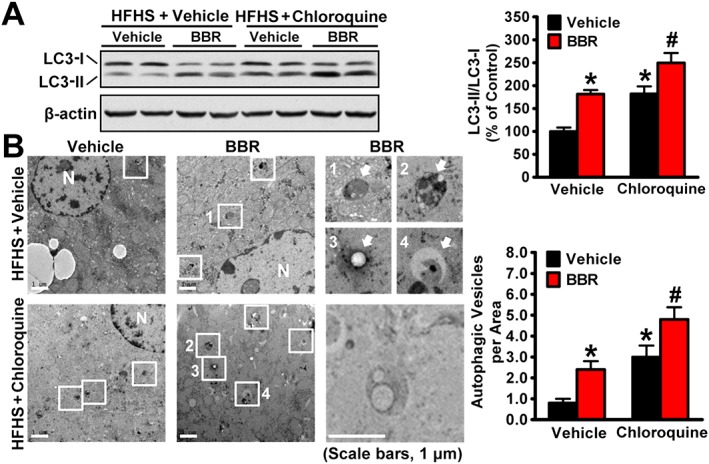
Berberine (BBR) is able to induce autophagic flux in the livers of HFHS diet‐fed mice. (A) Eight‐week‐old male C57BL/6 mice were fed on a HFHS diet for 9 weeks and then treated with berberine (5 mg·kg−1·day−1) once daily for 4 weeks, followed by treatment with chloroquine (60 mg·kg−1) i.p. for 24 h before the kill. (A) Berberine stimulates conversion of LC3‐I to LC3‐II under chloroquine treatment in the liver of mice fed with HFHS diet. Band intensity of LC3‐II was quantified by densitometry and normalized to the levels of LC3‐I for unwanted sources of variation and presented as the mean ± SEM, n = 5. *P < 0.05, versus WT and vehicle; #P < 0.05, versus WT mice treated with berberine. (B) Electron micrographs of the liver tissue. Images 1–4 showing AVs are high magnifications of the ‘white box’ area indicated in the images of berberine‐treated or chloroquine‐treated mice fed a HFHS diet. Arrows denote autophagic vacuoles (AVs). N, nucleus. The average numbers of AVs were quantified from a randomly selected pool of five fields under each condition. The data are presented as the mean ± SEM, n = 5. *P < 0.05, versus vehicle; #P < 0.05, versus vehicle and chloroquine.
Berberine is able to induce autophagy and supress lipid accumulation in hepatocytes
To investigate whether berberine has the ability to induce autophagy and improve hepatic steatosis in vitro, the effects of berberine treatment were assessed in various hepatocytes. As shown in Figure 3A, berberine treatment caused an induction of LC3‐II in a dose‐dependent manner in HepG2 cells, suggesting it increased autophagy. Notably, the expression levels of LC3‐II were increased by serum starvation. Importantly, the ability of berberine to stimulate autophagy were confirmed in mouse primary hepatocytes, as it increased LC3‐II expression in these cells (Figure 3B). In HeLa cells berberine increased the cytosolic redistribution of GFP‐LC3 fusion protein to form puncta (Figure 3C).
Figure 3.
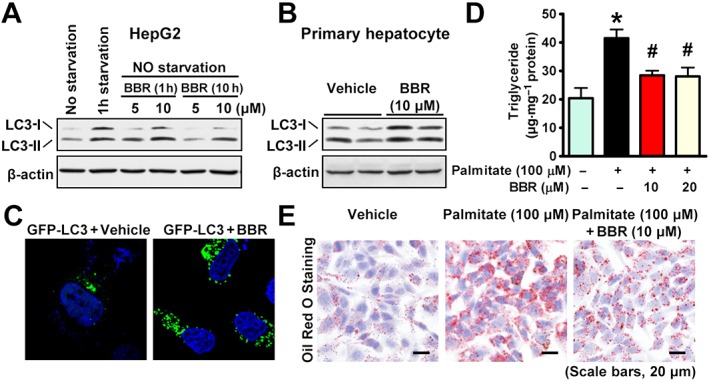
Berberine (BBR) stimulates autophagy to repress lipid accumulation in hepatocytes. Berberine stimulates the conversion of LC3‐I to LC3‐II in (A) HepG2 cells and (B) primary mouse hepatocytes. (C) The redistribution of GFP‐LC3 to form puncta is enhanced by berberine in HeLa cells. Cells were transfected with expressing plasmid encoding GFP‐LC3 fusion protein, followed by treatment without or with 10 μM berberine for 10 h. Berberine decreased lipid accumulation in HepG2 cells exposed to palmitate, as reflected by triglyceride levels determined by a (D) colorimetric enzymatic assay and (E) Oil Red O staining. The data are presented as the mean ± SEM, n = 6. *P < 0.05, versus control; #P < 0.05, versus palmitate.
To further test the functional effect of berberine on hepatic steatosis in vitro, hepatocytes were treated with fatty acid palmitate to induce intracellular lipid accumulation, which has been shown to mimic hepatic steatosis in HepG2 and Huh7 cells (Wobser et al., 2009; Liu et al., 2011). Strikingly, treatment with berberine supressed the palmitate‐induced lipid accumulation in a dose‐dependent manner, as demonstrated by triglyceride levels measured by the chloroform/methanol extraction method (Figure 3D) and by a reduction of lipid staining using Oil Red O staining (Figure 3E). Consistent with the in vivo results, these observations suggest that berberine is able to induce autophagy and inhibit lipid accumulation in hepatocytes.
SIRT1 is necessary for berberine to induce autophagy and inhibit lipid accumulation in hepatocytes
We next elucidated a causal relationship between SIRT1 and autophagy and their effects on lipid accumulation in SIRT1‐deficient hepatocytes. Consistent with the in vivo results, SIRT1 deletion caused a profound reduction in LC3‐II in mouse embryonic fibroblasts (Figure 4A) and attenuated berberine‐induced increase in LC3‐II in primary hepatocytes (Figure 4B). Notably, the SIRT1 deficiency was confirmed by immunoblotting, which showed the expected deleted mutant protein in SIRT1‐deficient hepatocytes, which migrated slightly faster than that in WT cells.
Figure 4.
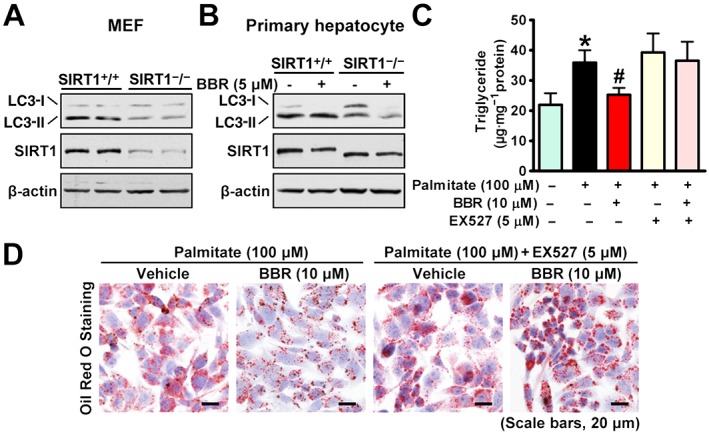
Berberine (BBR) induces autophagy to inhibit lipid accumulation through SIRT1 in hepatocytes. (A) Effects of SIRT1 deficiency on LC3 in MEFs. MEFs of floxed SIRT1 mice were infected with adenoviral Cre for 72 h. (B) SIRT1 deficiency diminishes berberine‐stimulated conversion of LC3‐I to LC3‐II in primary hepatocytes. Primary hepatocytes were isolated from 8‐week‐old WT or SIRT1 LKO mice and treated as indicated. The lipid‐lowering effects of berberine were abolished by a pharmacological SIRT1 inhibitor EX527 in HepG2 cells treated with palmitate, as determined by (C) triglyceride levels and (D) Oil Red O staining. The data are presented as the mean ± SEM, n = 6. *P < 0.05, versus control; #P < 0.05, versus palmitate.
To test the functional consequence of SIRT1 inhibition on the lipid‐lowering effects of berberine in hepatocytes, lipid quantification was measured in HepG2 cells treated with palmitate. Strikingly, the reduction of lipid accumulation caused by berberine was largely blocked by treatment with the specific SIRT1 inhibitor EX527 (Rodgers et al., 2005), as assessed by triglyceride levels (Figure 4C) and Oil Red O staining (Figure 4D). Together, these data indicate that SIRT1 is required for berberine to induce autophagy and inhibit lipid accumulation in vitro.
Berberine alleviates hepatic steatosis in an autophagy‐dependent manner
Next, the mechanism by which berberine affects SIRT1‐dependent activation of autophagy and its effects on hepatic steatosis were extensively explored in vitro. As shown in Figure 5A, treatment with berberine decreased acetylation levels of PGC‐1α, a key downstream substrate of SIRT1, in HEK293 cells stably expressing mouse FLAG‐tagged PGC‐1α, suggesting berberine increases SIRT1 activity. Notably, treatment with the SIRT1‐specific inhibitor EX527 (Peck et al., 2010) increased PGC‐1α acetylation.
Figure 5.
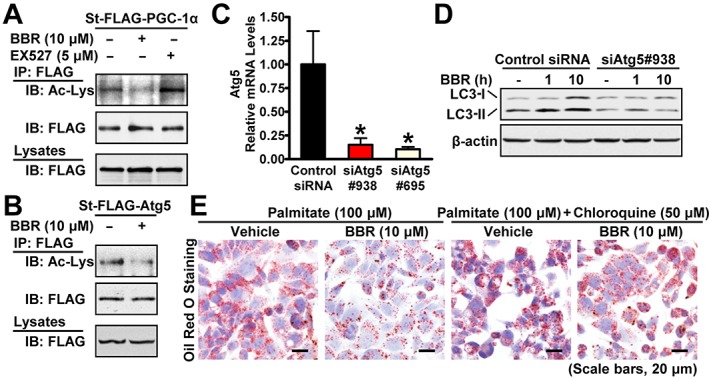
Berberine (BBR) stimulates SIRT1 deacetylation activity and induces autophagy in an Atg5‐dependent manner. Berberine induces deacetylation of (A) PGC‐1α or (B) Atg5. HEK293 cells stably expressing mouse FLAG‐tagged PGC‐1α, human FLAG‐tagged Atg5 (St‐FLAG‐Atg5) or empty vector were treated with 10 μM berberine or 5 μM EX527 for 10 h or 6 h, respectively, followed by immunoprecipitation and immunoblots with the indicated antibodies. (C) The levels of mRNA for Atg5 are decreased by Atg5 knockdown in HepG2 cells. The data were normalized to β‐actin for unwanted sources of variation and presented as the mean ± SEM, n = 5. *P < 0.05, versus control siRNA. (D) Berberine‐stimulated conversion of LC3‐I to LC3‐II is abolished by Atg5 knockdown in HepG2 cells. Cells were transfected with siAtg5 and control siRNAs for 24 h and incubated in serum‐free DMEM containing 5.5 mM glucose overnight, followed by treatment without or with 10 μM berberine for 1 h and 10 h. (E) Berberine's lipid‐lowering effects are attenuated by the autophagy inhibitor chloroquine in HepG2 cells treated with palmitate as reflected by Oil Red O staining.
Recently, SIRT1 was shown to regulate autophagy through deacetylating Atg5 and Atg7 (Lee et al., 2008). Given that SIRT1 is necessary for berberine‐induced autophagy, we hypothesized that berberine regulates autophagy through SIRT1‐mediated deacetylation of Atg proteins. As expected, treatment with berberine decreased acetylation levels of Atg5 in HEK293 cells (Figure 5B). These data suggest that SIRT1‐dependent deacetylation of Atg5 may contribute to berberine‐induced autophagy activation. We next determined whether Atg5 is necessary for berberine to induce autophagy in HepG2 cells by using siRNA‐mediated knockdown of Atg5. As shown in Figure 5C, D, knockdown of Atg5 by siRNA blocked autophagy in hepatocytes (Singh et al., 2009) and also reduced the berberine‐induced accumulation of LC3‐II.
Next, an autophagy inhibitor chloroquine was used to test the functional consequence of autophagy inhibition on the lipid‐lowering effects of berberine in HepG2 cells treated with palmitate. Strikingly, the administration of chloroquine largely abolished berberine's effects on lipid accumulation in palmitate‐treated HepG2 cells as evidenced by Oil Red O staining (Figure 5E). Taken together, these studies indicate that berberine stimulates autophagy and improves hepatic steatosis in hepatocytes.
Berberine increases fasting‐induced autophagy through hepatic SIRT1 in mice
To determine whether autophagy is regulated by nutritional status, an immunoblotting analysis was performed in the liver of mice under fed, fasted or refed conditions. Consistent with previous observations showing increased autophagy in fasted mice (Ezaki et al., 2011), fasting for 24 h caused a profound increase in autophagy in mouse livers, as indicated by the increased conversion of LC3‐I to LC3‐II (Figure 6A). As shown in Figure 6B, C, compared with the fed state, fasting caused an increase in lipid deposits in mouse livers as indicated by Oil Red O staining, which is consistent with our recent observations in mice (Chen et al., 2016). Strikingly, berberine treatment caused an additional increase in the LC3‐I to LC3‐II ratio and reduced p62 expression in fasted mice liver compared with that in the control group, which was correlated with an attenuation of fasting‐induced hepatic steatosis, suggesting that berberine lowers the fasting‐induced lipid accumulation probably through stimulating autophagy. To investigate whether SIRT1 is required for fasting‐induced autophagy in the liver, an immunoblotting analysis was performed using mouse livers. Consistent with the effects of SIRT1 deficiency in the liver of HFHS diet‐fed mice, berberine‐stimulated autophagy was abolished in SIRT1 LKO mice in response to fasting, as indicated by a significant reduction in the conversion of LC3‐I to LC3‐II, no obvious change in p62 expression was observed (Figure 6D), but the number of cytosolic autophagic vacuoles was also reduced (Figure 6E, F). The mRNA levels of autophagic markers, such as BECN1 for initiation and p62 for degradation, were measured (Figure 6G). Compared with vehicle, no significant changes were observed in mouse livers treated with berberine, suggesting that berberine does not regulate autophagy at the transcriptional level. Future studies using the tandem sensor RFP‐GFP will be used to determine, in depth, the autophagy flux. Moreover, these results are correlated with the deletion of berberine's effects on improving steatotic phenotypes by hepatic SIRT1 deficiency, as indicated by Oil Red O staining (Supporting Information Figure S1B, C). Together, these data indicate that SIRT1 is indeed necessary for berberine‐induced autophagy and lipid‐lowering effects in the liver of fasted mice.
Figure 6.
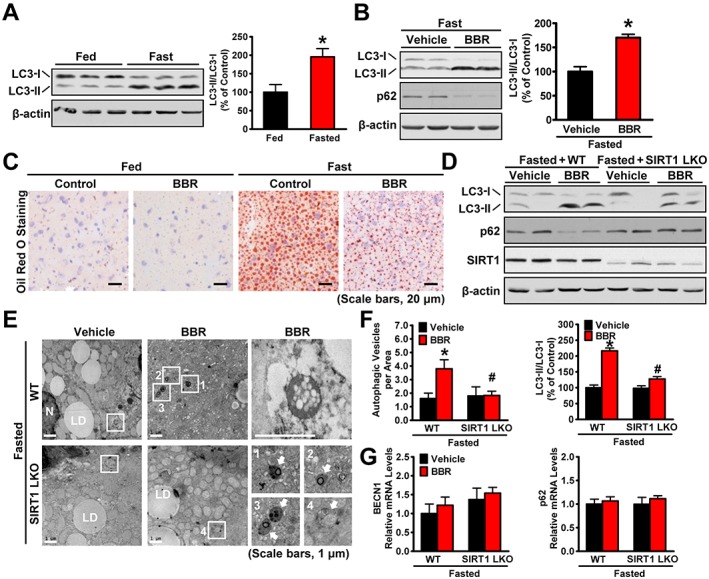
SIRT1 is required for berberine (BBR) to potentiate fasting‐induced autophagy in mouse livers. Male C57BL/6 mice at 8 weeks of age and SIRT1 LKO or WT mice at 16 weeks of age were treated with berberine (5 mg·kg−1·day−1) or vehicle (PBS) by i.p. injection once daily for 4 weeks and then subjected to feeding (fed) or fasting for 24 h (fasted). (A) The conversion of LC3‐I to LC3‐II was increased by fasting in mice. Band intensity of LC3‐II was quantified by densitometry and normalized to the levels of LC3‐I for unwanted sources of variation and presented as the mean ± SEM, n = 5. *P < 0.05, versus fed mice. (B) Berberine stimulates the conversion of LC3‐I to LC3‐II and reduces the p62 in mice in response to fasting, n = 5. (C) Hepatic steatosis was assessed by Oil Red O staining (scale bars: 50 μm). (D) SIRT1 deficiency attenuated berberine‐induced conversion of LC3‐I to LC3‐II and the reduction of p62 in the liver of fasted mice, n = 6. (E) Electron micrographs and the (F) average numbers of autophagic vacuoles (AVs) in the liver tissue were measured. Images 1–4 showing AVs are high magnifications of the ‘white box’ area indicated in the images of berberine‐treated WT or SIRT1 LKO mice in response to fasting. Arrows denote AVs. N, nucleus; LD, lipid droplet. The average numbers of AVs were quantified from a randomly selected pool of five fields under each condition. The data are presented as the mean ± SEM, n = 5. *P < 0.05, versus WT and vehicle; #P < 0.05, versus WT and berberine. (G) The mRNA levels of BECN1 and p62 were measured by real‐time PCR. The data were normalized to β‐actin for unwanted sources of variation and presented as the mean ± SEM, n = 5.
The production of FGF21 and whole‐body energy expenditure is increased by berberine
Our recent study indicates that SIRT1 increases the key metabolic regulator FGF21 and promotes energy expenditure and weight loss in mice, suggesting a potential role of FGF21 in mediating berberine's body weight‐lowering effects. We next determined whether berberine affects the expression levels of FGF21 in mice in response to fasting. As shown in Figure 7A, mRNA levels of FGF21 were increased robustly eightfold in livers of vehicle‐treated mice upon fasting and decreased upon refeeding. Consistently, circulating levels of FGF21 was increased eightfold in vehicle‐treated mice upon fasting, and this increase was decreased upon refeeding. These data are consistent with previous observations (Inagaki et al., 2007). Interestingly, compared with vehicle, treatment with berberine caused an additional increase in the production and secretion of FGF21 in mice. Notably, an induction of plasma β‐hydroxybutyrate concentrations in berberine‐treated mice was observed (Figure 7B), which is consistent the essential role of FGF21 in ketogenesis (Badman et al., 2007; Inagaki et al., 2007). We next investigated whether SIRT1 is necessary for berberine‐induced increase in FGF21 expression. As shown in Figure 7C, D, berberine‐induced expression and secretion of FGF21 were abolished by SIRT1 deficiency or the SIRT1 inhibitor EX527 in hepatocytes, respectively, suggesting a cell‐autonomous regulation of FGF21 by treatment with berberine.
Figure 7.
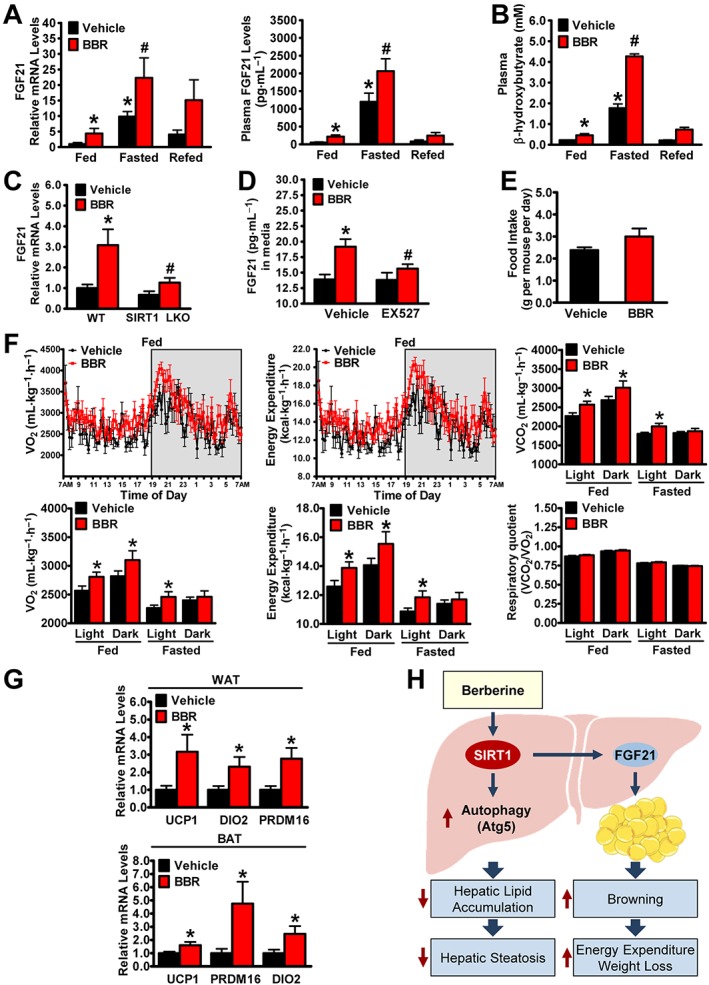
The administration of berberine (BBR) induces the production and secretion of FGF21 and promotes whole‐body energy expenditure in mice. Male C57BL/6 mice at 8 weeks of age were treated with berberine (5 mg·kg−1·day−1) or vehicle (PBS) via i.p. injection for 4 weeks and then subjected to feeding (fed), fasting for 24 h (fasted) or refeeding for 6 h after a 24 h fast (refed). (A) Administration of berberine is able to promote hepatic gene expression and circulating levels of FGF21 in mice. The mRNA levels were normalized to β‐actin for unwanted sources of variation, n = 5. (B) Circulating ketone bodies levels were robustly induced by treatment with berberine. The data are presented as the mean ± SEM, n = 5. *P < 0.05, versus fed and vehicle; #P < 0.05, versus fasted and vehicle. (C) SIRT1 deficiency abolishes berberine‐induced expression of FGF21 in mouse primary hepatocytes. The mRNA levels were normalized to β‐actin for unwanted sources of variation and presented as the mean ± SEM, n = 6. *P < 0.05, versus WT and vehicle; #P < 0.05, versus WT and berberine. (D) Effects of SIRT1 inhibitor EX527 on FGF21 secretion in hepatocytes. Media FGF21 levels were measured in HepG2 cells treated with berberine (10 μM) and EX527 (5 μM). The data are presented as the mean ± SEM, n = 5. *P < 0.05, versus vehicle; #P < 0.05, versus vehicle and berberine. (E) Food intake was measured during the fed state. (F) The rate of VO2, energy expenditure, VCO2 and respiratory quotient were measured by comprehensive metabolic monitoring in mice over a 24 h period with food and over a 24 h fast and normalized to lean body mass. (G) Administration of berberine increases the transcription of brown‐like genes in WAT and BAT in mice. The mRNA levels were normalized to β‐actin for unwanted sources of variation and presented as the mean ± SEM, n = 8. *P < 0.05, versus vehicle. (H) The proposed model for SIRT1 in mediating berberine's amelioration of hepatic steatosis and obesity in diet‐induced insulin‐resistant mice. Administration of berberine stimulates hepatic SIRT1, which in turn increases autophagic function to regulate intracellular lipid stores, and improves fatty liver disease. Activation of the berberine‐SIRT1‐FGF21 axis may represent a new approach to treat obesity by promoting white fat browning and stimulating energy expenditure.
To understand the mechanisms by which berberine‐treated mice are resistant to developing obesity, the major components of energy metabolism such as oxygen consumption and energy expenditure were examined. As shown in Figure 7F, comprehensive metabolic cage studies indicated that the rates of oxygen consumption (VO2) and the calculated energy expenditure were increased in response to berberine treatment during the light and dark phases of the fed and fasted mice. Consistent with the rates of VO2 and energy expenditure, VCO2 rates were significantly higher in the mice treated with berberine. Moreover, the respiratory quotients (respiratory quotient = VCO2/VO2) appeared indistinguishable in vehicle and berberine‐treated mice in fed and fasted conditions, probably due to the combined increase in both VO2 and VCO2. These results indicate the relatively equal use of carbohydrates versus lipids as an energy source in whole body. Notably, no significant alterations in daily food intake or physical activity were observed between berberine‐ and vehicle‐treated mice (Figure 7E and Supporting Information Figure S2).
Given that FGF21 regulates the browning of white adipose tissue (WAT) in adaptive thermogenesis (Fisher et al., 2012), we further investigated whether berberine alters the transcription of browning genes in WAT or thermogenic genes in brown adipose tissue (BAT). Strikingly, the administration of berberine caused a significant induction of key genes related to browning and thermogenesis, such as uncoupling protein 1 (UCP1), and the BAT programme coactivator PRD1‐BF1‐RIZ1 homologous domain containing 16, as well as expression of other BAT markers, type II iodothyronine deiodinase (Figure 7G). Taken together, these findings suggest that berberine ameliorates adiposity of HFHS diet‐fed mice, possibly through increasing FGF21 signalling, improving whole body energy expenditure and the browning of WAT.
Discussion
Although pharmacological studies have demonstrated therapeutic actions of berberine on hepatic steatosis, insulin resistance and obesity, the downstream signalling pathways mediating these activities remain poorly understood. The administration of berberine or berberine's derivative protects against hepatic steatosis in hepatocytes (Brusq et al., 2006), rodents (Turner et al., 2008; Chang et al., 2010) and in patients with NAFLD (Yan et al., 2015) suggesting an obligatory role of the liver in berberine‐mediated lipid homeostasis. In the present study, we provide both in vivo and in vitro evidence that berberine induces autophagy to ameliorate hepatic steatosis in a SIRT1‐dependent manner in the liver of mice subjected to nutrient overload or in response to fasting. Moreover, berberine's effects on FGF21 and energy expenditure may represent a molecular mechanism by which pharmacological administration of berberine protects against obesity (Figure 7H).
The liver is a direct target tissue for mediating berberine's effects on autophagy and lipid metabolism
In vivo and in vitro approaches were utilized in the present study to demonstrate that berberine induces autophagy in the liver. Firstly, administration of berberine induced the conversion of LC3‐I to LC3‐II in livers of mice fed a HFHS diet or mice in response to nutrient deprivation. Secondly, the data from pharmacological administration of berberine and genetic manipulation of autophagy signalling confirmed the important role of hepatocytes in the activation of autophagy induced by berberine in HepG2 and primary mouse hepatocytes. The efficacy of berberine at augmenting autophagy in the liver is consistent with previous findings showing that berberine treatment induces autophagy to regulate apoptosis in a number of carcinoma cell lines (Wang et al., 2010; Yu et al., 2014), protects against cardiac hypertrophy in transverse aortic contraction‐treated rats (Li et al., 2014a) and inhibits macrophage‐mediated inflammation and has the potential to alleviate atherosclerosis (Fan et al., 2015). Importantly, treatment with an autophagy inhibitor, chloroquine, which blocks autophagy degradation in the lysosome largely attenuated the reduction in lipid accumulation induced by berberine in HepG2 cells, suggesting that an augmentation of autophagy mediates the berberine‐induced amelioration of hepatic steatosis. These results are consistent with recent findings showing that genetic manipulation‐ or nutrient deprivation‐stimulated autophagy induces the hydrolysis of lipid droplets in the liver to release free fatty acid for mitochondrial oxidation, leading to attenuated hepatic steatosis in obese ob/ob or fasted mice (Singh et al., 2009; Yang et al., 2010; Liu and Czaja, 2013). Berberine has also been shown to inhibit autophagy in 3T3‐L1 adipocytes. This may, therefore, reflect the deregulation of autophagic signalling in the adipose tissue under these conditions or differential signalling in these cells.
Moreover, autophagy is an adaptive process for intracellular component degradation and recycling, especially when the cell is challenged with a disordered nutrient status. Cellular metabolism is tightly correlated to autophagy. Nutrient deprivation induces autophagy, contributing to maintain the energy balance by recycling the proteins, lipids and glycogen (Singh et al., 2009; Ezaki et al., 2011). Our results show that autophagy is induced in mouse livers by fasting, which is consistent with previous observations (Lee et al., 2014). Importantly, the administration of berberine potentiated the fasting‐induced autophagy synergistically, suggesting that berberine is able to stimulate autophagy and maintain cellular metabolism in the liver during physiological conditions.
Berberine regulates lipid metabolism in the liver during nutrient overload in a SIRT1‐dependent manner
From the results of the present study, we identified, for the first time, crosstalk between berberine and the nutrient sensor SIRT1 directly in the liver. The finding that berberine's augmentation of autophagy and amelioration of steatotic phenotypes was abolished in SIRT1 LKO mice suggests a critical role of SIRT1 in mediating berberine's actions in the liver. Moreover, SIRT1 loss‐of‐function approaches in numerous cells, including primary hepatocytes, HepG2 and MEFs, further confirmed that SIRT1 is necessary for berberine to induce autophagy and inhibit lipid accumulation in a cell‐autonomous manner. Mechanistically, we demonstrated that berberine deacetylates the key autophagy gene Atg5 to enhance autophagy probably through SIRT1 activation, which is consistent with previous observations that SIRT1 positively regulates autophagy in mouse livers and numerous cell lines, including HeLa, HEK293, embryonic stem cells and liver (Lee et al., 2008; Dong et al., 2013; Ou et al., 2014; Qin et al., 2016; Lin et al., 2017). The efficacy of berberine to induce autophagy and regulate liver ischaemia–reperfusion injury was shown to be attenuated by the SIRT1 inhibitors sirtinol and EX527 (Qin et al., 2016; Lin et al., 2017). However, sirtinol and EX527 are known to have off target effects and inhibit other members of the sirtuin family deacetylase activity, such as SIRT2 (Grozinger et al., 2001; Peck et al., 2010). Moreover, pharmacological administration of sirtinol or EX527 causes systemic effects indirectly through other tissues not related to the liver, which may contribute to the net hepatic phenotypes. Our study using SIRT1 LKO mice and SIRT1−/− hepatocytes to define berberine as an activator of SIRT1 and demonstrate an essential role of SIRT1 in mediating berberine's reduction of hepatic fat storage.
In support of our results, recent studies indicate that berberine improves muscle mitochondrial function and insulin sensitivity through SIRT1 (Gomes et al., 2012), although other studies support a role of mitochondrial inhibition by berberine (Jun et al., 2008). Taken together, these findings indicate that in addition to improving insulin sensitivity and regulating glucose metabolism via activation of AMPK (Jun et al., 2008), hepatic SIRT1 is critically involved in the effects of berberine on autophagy flux and hepatic lipid metabolism in the liver and hepatocytes.
Regulation of browning of white adipose tissue by berberine‐induced FGF21
The most important finding of the current paper is the identification that treatment of berberine induces the production and secretion of FGF21 and this contributes to enhanced energy expenditure and weight loss probably through promoting the browning of white fat. Given that FGF21 acts as a key regulator in energy metabolism (Xu et al., 2009; Fisher et al., 2012; Owen Bryn et al., 2014) and SIRT1 loss‐of‐function approaches blocked the ability of berberine to induce FGF21, our results suggest that berberine's effects on energy metabolism and weight loss are likely to be mediated via SIRT1 activation. These results are consistent with recent observations that berberine up‐regulates the development of brown‐like adipocytes in the WAT and enhances energy metabolism in obese db/db mice (Zhang et al., 2014). Our findings that berberine can regulate FGF21 establish a crosstalk between the liver and adipose tissue, which may represent a therapeutic strategy for the development of drugs to combat metabolic disorders during obesity.
In conclusion, the ability of berberine to prevent the deleterious effects of excess caloric intake on hepatic steatosis and obesity suggests that berberine and its derivative with similar properties might be a promising new approach for treating these metabolic disorders. Given that berberine administration is safe in humans, these studies also highlight the therapeutic relevance of targeting the hepatic SIRT1‐autophagy axis to reverse hepatic lipid deregulation and improve lipid homeostasis in obesity and type 2 diabetes.
Author contributions
Y.S., X.G. and Y.L. contributed to the experiment design; Y.S., M.X., H.Y., Y.H., F.Z., Z.H., A.C., F.M., Z.L., X.C., Q.G., J.G. and H.B. contributed to the acquisition and analysis of data; Y.T. reviewed the manuscript; X.G. and Y.L. obtained the funding; and Y.S., X.G. and Y.L. wrote the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Figure S1 The effects of hepatic SIRT1 deficiency on the liver weight or hepatic steatosis in HFHS diet‐fed or fasted mice, respectively. (A) The liver weight of the mice was measured. Wild type (WT) and SIRT1 LKO male mice at sixteen‐week‐old were fed on a HFHS diet for 12 weeks, and then treated with berberine (5 mg·kg−1·day−1) or vehicle (PBS) by intraperitoneal injection once‐daily for five weeks. The data are presented as the mean ± SEM, n = 6. (B‐C) The beneficial effect of berberine on improving hepatic steatosis is compromised by hepatic specific deletion of SIRT1 in fasted mice. Male SIRT1 LKO or WT mice were treated with berberine (5 mg·kg−1·day−1) or vehicle (PBS) by intraperitoneal injection once‐daily for four weeks, and then subjected to fasting for 24 hours (Fasted). Hepatic steatosis was assessed by Oil Red O staining (B) and quantification of Oil Red O stained areas (C). The data are presented as the mean ± SEM, n = 5, *P < 0.05, versus WT mice treated with vehicle.
Figure S2 Effects of berberine on physical activity in mice. Male C57BL/6 mice were treated with berberine (5 mg·kg−1·day−1) or vehicle (PBS) by intraperitoneal injection once‐daily for four weeks. The physical activity were measured by comprehensive metabolic monitoring in mice over a 24‐hour period with food and over a 24‐hour fast, and normalized to lean body mass. The bar graph of physical activity is expressed as total counts measured by summing X and Y beam breaks over a 12‐hour block of light and dark phases in the fed and fasted conditions. The data are presented as the mean ± SEM, n = 6.
Table S1 Quantitative RT‐PCR primers.
Acknowledgements
This work was supported by grants from the National Key Basic Research Programme of China (2012CB524906) and the National Natural Science Foundation of China (81270933 and 81570718) to X.G. This work was also supported by grants from the National Key R&D Programme of China (2017YFC0909601), the National Natural Science Foundation of China (31471129 and 31671224) and the Chinese Academy of Sciences (ZDBS‐SSW‐DQC‐02 and 2013OHTP04) to Y.L. We thank Drs Leonard Guarente at Massachusetts Institute of Technology and Mengwei Zang at Boston University for SIRT1 LKO mice, Drs Jiandie Lin and Chang Liu for providing FLAG‐tagged PGC1α plasmid, Dr Chuangui Wang for providing FLAG‐tagged Atg5 plasmid and Dr Zhixue Liu for providing pCDH‐CMV‐3xFLAG‐SBP and pEGFP‐C1‐LC3 plasmids. We would like to thank Fengling Qin for electron microscopy analysis, Dr Yi Yang for body composition analysis and Zhonghui Weng for animal studies.
Sun, Y. , Xia, M. , Yan, H. , Han, Y. , Zhang, F. , Hu, Z. , Cui, A. , Ma, F. , Liu, Z. , Gong, Q. , Chen, X. , Gao, J. , Bian, H. , Tan, Y. , Li, Y. , and Gao, X. (2018) Berberine attenuates hepatic steatosis and enhances energy expenditure in mice by inducing autophagy and fibroblast growth factor 21. British Journal of Pharmacology, 175: 374–387. doi: 10.1111/bph.14079.
Contributor Information
Yu Li, Email: liyu@sibs.ac.cn.
Xin Gao, Email: happy20061208@126.com, Email: gao.xin@zs-hospital.sh.cn.
References
- Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M et al (2013). PPARγ signaling and metabolism: the good, the bad and the future. Nat Med 99: 557–566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion NV, Peters JA, Faccenda E et al (2017a). The Concise Guide to PHARMACOLOGY 2017/18: Enzymes. Br J Pharmacol 174: S272–S359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion NV, Peters JA, Faccenda E, Harding SD et al (2017b). The Concise Guide to PHARMACOLOGY 2017/18: Transporters. Br J Pharmacol 174: S360–S446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos‐Flier E (2007). Hepatic fibroblast growth factor 21 is regulated by PPARα and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metab 5: 426–437. [DOI] [PubMed] [Google Scholar]
- Baur JA, Pearson KJ, Price NL, Jamieson HA, Lerin C, Kalra A et al (2006). Resveratrol improves health and survival of mice on a high‐calorie diet. Nature 444: 337–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusq J‐M, Ancellin N, Grondin P, Guillard R, Martin S, Saintillan Y et al (2006). Inhibition of lipid synthesis through activation of AMP kinase: an additional mechanism for the hypolipidemic effects of berberine. J Lipid Res 47: 1281–1288. [DOI] [PubMed] [Google Scholar]
- Chang X, Yan H, Fei J, Jiang M, Zhu H, Lu D et al (2010). Berberine reduces methylation of the MTTP promoter and alleviates fatty liver induced by a high‐fat diet in rats. J Lipid Res 51: 2504–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen D, Bruno J, Easlon E, Lin S‐J, Cheng H‐L, Alt FW et al (2008). Tissue‐specific regulation of SIRT1 by calorie restriction. Genes Dev 22: 1753–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Zhang F, Gong Q, Cui A, Zhuo S, Hu Z et al (2016). Hepatic ATF6 increases fatty acid oxidation to attenuate hepatic steatosis in mice through peroxisome proliferator‐activated receptor α. Diabetes 65: 1904–1915. [DOI] [PubMed] [Google Scholar]
- Cohen JC, Horton JD, Hobbs HH (2011). Human fatty liver disease: old questions and new insights. Science 332: 1519–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SPA, Giembycz MA et al (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding WX, Li M, Chen X, Ni HM, Lin CW, Gao W et al (2010). Autophagy reduces acute ethanol‐induced hepatotoxicity and steatosis in mice. Gastroenterology 139: 1740–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S, Jia C, Zhang S, Fan G, Li Y, Shan P et al (2013). The REGγ proteasome regulates hepatic lipid metabolism through inhibition of autophagy. Cell Metab 18: 380–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezaki J, Matsumoto N, Takeda‐Ezaki M, Komatsu M, Takahashi K, Hiraoka Y et al (2011). Liver autophagy contributes to the maintenance of blood glucose and amino acid levels. Autophagy 7: 727–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan X, Wang J, Hou J, Lin C, Bensoussan A, Chang D et al (2015). Berberine alleviates ox‐LDL induced inflammatory factors by up‐regulation of autophagy via AMPK/mTOR signaling pathway. J Transl Med 13: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F et al (2012). FGF21 regulates PGC‐1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev 26: 271–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gómez‐Lechón MJ, Donato MT, Martínez‐Romero A, Jiménez N, Castell JV, O'Connor J‐E (2007). A human hepatocellular in vitro model to investigate steatosis. Chem Biol Interact 165: 106–116. [DOI] [PubMed] [Google Scholar]
- Gomes AP, Duarte FV, Nunes P, Hubbard BP, Teodoro JS, Varela AT et al (2012). Berberine protects against high fat diet‐induced dysfunction in muscle mitochondria by inducing SIRT1‐dependent mitochondrial biogenesis. Biochim Biophys Acta 1822: 185–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Q, Hu Z, Zhang F, Cui A, Chen X, Jiang H et al (2016). Fibroblast growth factor 21 improves hepatic insulin sensitivity by inhibiting mammalian target of rapamycin complex 1 in mice. Hepatology 64: 425–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grozinger CM, Chao ED, Blackwell HE, Moazed D, Schreiber SL (2001). Identification of a class of small molecule inhibitors of the sirtuin family of NAD‐dependent deacetylases by phenotypic screening. J Biol Chem 276: 38837–38843. [DOI] [PubMed] [Google Scholar]
- Haigis MC, Guarente LP (2006). Mammalian sirtuins – emerging roles in physiology, aging, and calorie restriction. Genes Dev 20: 2913–2921. [DOI] [PubMed] [Google Scholar]
- Hardie DG (2011). Sensing of energy and nutrients by AMP‐activated protein kinase. Am J Clin Nutr 93: 16. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V et al (2007). Endocrine regulation of the fasting response by PPARα‐mediated induction of fibroblast growth factor 21. Cell Metab 5: 415–425. [DOI] [PubMed] [Google Scholar]
- Jun Y, Hanjie Z, Jianping Y (2008). Traditional Chinese medicine in treatment of metabolic syndrome. Endocrine, Metabolic & Immune Disorders‐Drug Targets 8: 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur J, Debnath J (2015). Autophagy at the crossroads of catabolism and anabolism. Nat Rev Mol Cell Biol 16: 461–472. [DOI] [PubMed] [Google Scholar]
- Kharitonenkov A, Shiyanova TL, Koester A, Ford AM, Micanovic R, Galbreath EJ et al (2005). FGF‐21 as a novel metabolic regulator. J Clin Invest 115: 1627–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong W, Wei J, Abidi P, Lin M, Inaba S, Li C et al (2004). Berberine is a novel cholesterol‐lowering drug working through a unique mechanism distinct from statins. Nat Med 10: 1344–1351. [DOI] [PubMed] [Google Scholar]
- Lee IH, Cao L, Mostoslavsky R, Lombard DB, Liu J, Bruns NE et al (2008). A role for the NAD‐dependent deacetylase Sirt1 in the regulation of autophagy. Proc Natl Acad Sci 105: 3374–3379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JM, Wagner M, Xiao R, Kim KH, Feng D, Lazar MA et al (2014). Nutrient‐sensing nuclear receptors coordinate autophagy. Nature 516: 112–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y et al (2006). Berberine, a natural plant product, activates AMP‐activated protein kinase with beneficial metabolic effects in diabetic and insulin‐resistant states. Diabetes 55: 2256–2264. [DOI] [PubMed] [Google Scholar]
- Levine B, Kroemer G (2008). Autophagy in the pathogenesis of disease. Cell 132: 27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M‐H, Zhang Y‐J, Yu Y‐H, Yang S‐H, Iqbal J, Mi Q‐Y et al (2014a). Berberine improves pressure overload‐induced cardiac hypertrophy and dysfunction through enhanced autophagy. Eur J Pharmacol 728: 67–76. [DOI] [PubMed] [Google Scholar]
- Li Y, Wong K, Giles A, Jiang J, Lee JW, Adams AC et al (2014b). Hepatic SIRT1 attenuates hepatic steatosis and controls energy balance in mice by inducing fibroblast growth factor 21. Gastroenterology 146: 539–549.e537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xu S, Giles A, Nakamura K, Lee JW, Hou X et al (2011a). Hepatic overexpression of SIRT1 in mice attenuates endoplasmic reticulum stress and insulin resistance in the liver. FASEB J 25: 1664–1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xu S, Jiang B, Cohen RA, Zang M (2013). Activation of sterol regulatory element binding protein and NLRP3 inflammasome in atherosclerotic lesion development in diabetic pigs. PLoS ONE 8 e67532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B et al (2011b). AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet‐induced insulin‐resistant mice. Cell Metab 13: 376–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Sheng M, Weng Y, Xu R, Lu N, Du H et al (2017). Berberine protects against ischemia/reperfusion injury after orthotopic liver transplantation via activating Sirt1/FoxO3α induced autophagy. Biochem Biophys Res Commun 483: 885–891. [DOI] [PubMed] [Google Scholar]
- Liu J‐F, Ma Y, Wang Y, Du Z‐Y, Shen J‐K, Peng H‐L (2011). Reduction of lipid accumulation in HepG2 cells by luteolin is associated with activation of AMPK and mitigation of oxidative stress. Phytother Res 25: 588–596. [DOI] [PubMed] [Google Scholar]
- Liu K, Czaja MJ (2013). Regulation of lipid stores and metabolism by lipophagy. Cell Death Differ 20: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michan S, Sinclair D (2007). Sirtuins in mammals: insights into their biological function. Biochem J 404: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008). Autophagy fights disease through cellular self‐digestion. Nature 451: 1069–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou X, Lee MR, Huang X, Messina‐Graham S, Broxmeyer HE (2014). SIRT1 positively regulates autophagy and mitochondria function in embryonic stem cells under oxidative stress. Stem Cells 32: 1183–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen Bryn M, Ding X, Morgan Donald A, Coate Katie C, Bookout Angie L, Rahmouni K et al (2014). FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab 20: 670–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peck B, Chen C‐Y, Ho K‐K, Di Fruscia P, Myatt SS, Coombes RC et al (2010). SIRT inhibitors induce cell death and p53 acetylation through targeting both SIRT1 and SIRT2. Mol Cancer Ther 9: 844–855. [DOI] [PubMed] [Google Scholar]
- Purushotham A, Schug TT, Xu Q, Surapureddi S, Guo X, Li X (2009). Hepatocyte‐specific deletion of SIRT1 alters fatty acid metabolism and results in hepatic steatosis and inflammation. Cell Metab 9: 327–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin J, Zhou J, Dai X, Zhou H, Pan X, Wang X et al (2016). Short‐term starvation attenuates liver ischemia–reperfusion injury (IRI) by Sirt1‐autophagy signaling in mice. American Journal of Translational Research 8: 3364–3375. [PMC free article] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P (2005). Nutrient control of glucose homeostasis through a complex of PGC‐1α and SIRT1. Nature 434: 113–118. [DOI] [PubMed] [Google Scholar]
- Settembre C, Ballabio A (2014). Lysosome: regulator of lipid degradation pathways. Trends Cell Biol 24: 743–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M et al (2009). Autophagy regulates lipid metabolism. Nature 458: 1131–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh JM, Jonker JW, Ahmadian M, Goetz R, Lackey D, Osborn O et al (2014). Endocrinization of FGF1 produces a neomorphic and potent insulin sensitizer. Nature 513: 436–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner N, Li JY, Gosby A, To SW , Cheng Z, Miyoshi H et al (2008). Berberine and its more biologically available derivative, dihydroberberine, inhibit mitochondrial respiratory complex I: a mechanism for the action of berberine to activate AMP‐activated protein kinase and improve insulin action. Diabetes 57: 1414–1418. [DOI] [PubMed] [Google Scholar]
- Wang N, Feng Y, Zhu M, Tsang C‐M, Man K, Tong Y et al (2010). Berberine induces autophagic cell death and mitochondrial apoptosis in liver cancer cells: the cellular mechanism. J Cell Biochem 111: 1426–1436. [DOI] [PubMed] [Google Scholar]
- Wobser H, Dorn C, Weiss TS, Amann T, Bollheimer C, Buttner R et al (2009). Lipid accumulation in hepatocytes induces fibrogenic activation of hepatic stellate cells. Cell Res 19: 996–1005. [DOI] [PubMed] [Google Scholar]
- Xia X, Yan J, Shen Y, Tang K, Yin J, Zhang Y et al (2011). Berberine improves glucose metabolism in diabetic rats by inhibition of hepatic gluconeogenesis. PLoS ONE 6 e16556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Lloyd DJ, Hale C, Stanislaus S, Chen M, Sivits G et al (2009). Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet‐induced obese mice. Diabetes 58: 250–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H‐M, Xia M‐F, Wang Y, Chang X‐X, Yao X‐Z, Rao S‐X et al (2015). Efficacy of berberine in patients with non‐alcoholic fatty liver disease. PLoS ONE 10 e0134172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Li P, Fu S, Calay ES, Hotamisligil GS (2010). Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metab 11: 467–478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R, Zhang Z‐q, Wang B, Jiang H‐x, Cheng L, Shen L‐m (2014). Berberine‐induced apoptotic and autophagic death of HepG2 cells requires AMPK activation. Cancer Cell Int 14: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X, Wang J, Tang X, Li Y, Xia P, Gao X (2015). Berberine ameliorates nonalcoholic fatty liver disease by a global modulation of hepatic mRNA and lncRNA expression profiles. J Transl Med 13: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zhang H, Li B, Meng X, Wang J, Zhang Y et al (2014). Berberine activates thermogenesis in white and brown adipose tissue. Nat Commun 5: 5493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 The effects of hepatic SIRT1 deficiency on the liver weight or hepatic steatosis in HFHS diet‐fed or fasted mice, respectively. (A) The liver weight of the mice was measured. Wild type (WT) and SIRT1 LKO male mice at sixteen‐week‐old were fed on a HFHS diet for 12 weeks, and then treated with berberine (5 mg·kg−1·day−1) or vehicle (PBS) by intraperitoneal injection once‐daily for five weeks. The data are presented as the mean ± SEM, n = 6. (B‐C) The beneficial effect of berberine on improving hepatic steatosis is compromised by hepatic specific deletion of SIRT1 in fasted mice. Male SIRT1 LKO or WT mice were treated with berberine (5 mg·kg−1·day−1) or vehicle (PBS) by intraperitoneal injection once‐daily for four weeks, and then subjected to fasting for 24 hours (Fasted). Hepatic steatosis was assessed by Oil Red O staining (B) and quantification of Oil Red O stained areas (C). The data are presented as the mean ± SEM, n = 5, *P < 0.05, versus WT mice treated with vehicle.
Figure S2 Effects of berberine on physical activity in mice. Male C57BL/6 mice were treated with berberine (5 mg·kg−1·day−1) or vehicle (PBS) by intraperitoneal injection once‐daily for four weeks. The physical activity were measured by comprehensive metabolic monitoring in mice over a 24‐hour period with food and over a 24‐hour fast, and normalized to lean body mass. The bar graph of physical activity is expressed as total counts measured by summing X and Y beam breaks over a 12‐hour block of light and dark phases in the fed and fasted conditions. The data are presented as the mean ± SEM, n = 6.
Table S1 Quantitative RT‐PCR primers.


