To the Editor
The transient receptor potential vanilloid 1 (TRPV1) channel belongs to a family of proteins originally characterized in neuronal cells as a Ca2+-permeable, non-selective cation channels that sense physical and chemical stimuli such as noxious heat (>43°C), pH (<6.5 at 37°C), and vanilloid compounds like capsaicin (1). TRPV1 has been shown to be expressed and functional in adult human bronchial epithelial (HBE) cells, and to play a role in airway disease through increased mucus production (2), stimulation of cough reflex (3), airway smooth muscle contraction (4), and increased vascular permeability (5). Importantly, TRPV1 expression increases in airway epithelium from asthmatic as compared to non-asthmatic adult donors (6) and is upregulated by viral infections in neuroblastoma cells as well as primary and immortalized airway epithelial cells (7). However, age-dependent changes in TRPV1 expression and function have never been studied, nor it is known how this channel is affected by intrinsic and extrinsic factors predisposing to asthma in early life. Thus, we hypothesized that TRPV1 expression, localization, and function in HBE cells is age-dependent and is modulated by intrinsic asthma predisposition and intercurrent viral respiratory infections. To test this hypothesis, we first compared TRPV1 related Ca2+ changes with the specific agonist capsaicin in primary HBE from children and adults with and without asthma, and then repeated the same measurements after infection with respiratory syncytial virus (RSV).
In preliminary experiments, capsaicin concentrations ranging from 20 to 300 μM activated TRPV1 in a concentration-dependent fashion and with an EC50 of approximately 150 μM (Figure E1). TRPV1-dependent [Ca2+]i change was 5-fold larger in HBE from asthmatic children compared to that from age-matched non-asthmatic controls (p<0.001; Figure 1A). The capsaicin effect in non-asthmatic adults was approximately twice that measured in non-asthmatic children (p=0.07; Figure 1B), but increased only 2.5-fold in asthmatic adults (p<0.001). The average increment in TRPV1-mediated Ca2+ influx was twice as large in HBE from asthmatic children than from asthmatic adults (5.9 ± 1.0 vs. 2.8 ± 0.4 units; p<0.05). We then examined the effect of RSV infection on TRPV1 function in HBE derived from airways of children. We found that TRPV1 dependent Ca2+ influx more than doubled in infected cells from both non-asthmatic (p<0.01;) and asthmatic (p<0.001) children (Figure 1C). Remarkably, TRPV1-dependent [Ca2+]i in RSV-infected cells from asthmatic children was about 10-fold above its baseline value. In contrast, RSV infection had no significant effect on adult cells, independently of their asthma status (Figure 1D). These [Ca2+]i changes roughly paralleled the efficiency of RSV replication in different HBE cells, where a heavier viral load was seen in the bronchial epithelium of asthmatic children (Figure E2). To confirm that TRPV1 is indeed the predominant calcium channel responsible for the increased [Ca2+]i induced by capsaicin, TRPV1 gene expression was silenced with a specific siRNA. This strategy achieved approximately 70% reduction in TRPV1 mRNA transcripts as measured by qPCR (Figure E3), and importantly abolished the effect of RSV infection on capsaicin-induced Ca2+ influx in HBE from both asthmatic children (p<0.001; Figure 2) and non-asthmatic controls (p<0.01).
Figure 1. TRPV1-mediated Ca2+ influx in HBE.
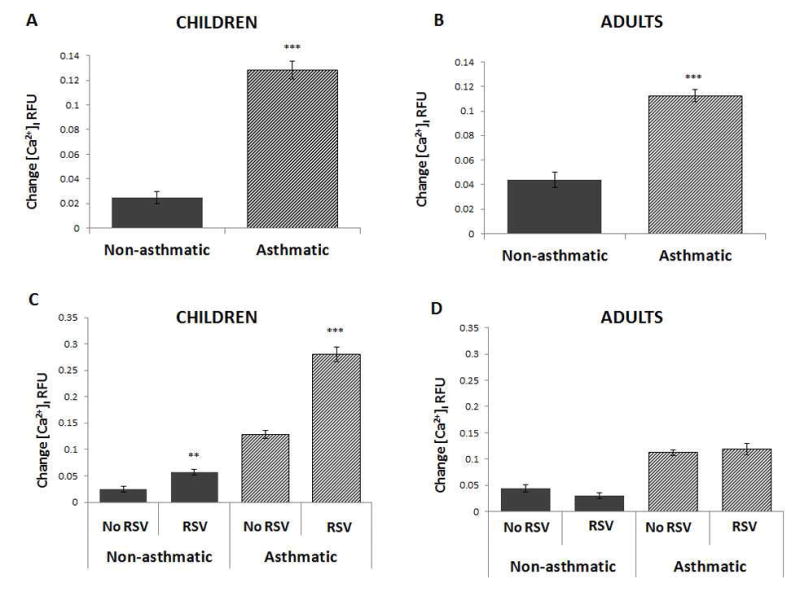
A. [Ca2+]i change induced by the specific TRPV1 agonist capsaicin (150 μM) in primary HBE was significantly larger in asthmatic vs. non-asthmatic children. B. Difference in TRPV1-mediated [Ca2+]i between asthmatic vs. non-asthmatic adults was approximately 50% than in children. C. Capsaicin-induced [Ca2+]i more than doubled in RSV-infected HBE (MOI of 1 for 24 h) from both non-asthmatic and asthmatic children. D. RSV infection had no significant effect on capsaicin-induced [Ca2+]i in adult HBE, independently from asthma status. All experiments were repeated ≥2 times in quadruplicate. Data are expressed as mean ± SEM. **P<0.01, ***P<0.001 compared to non-infected controls.
Figure 2.
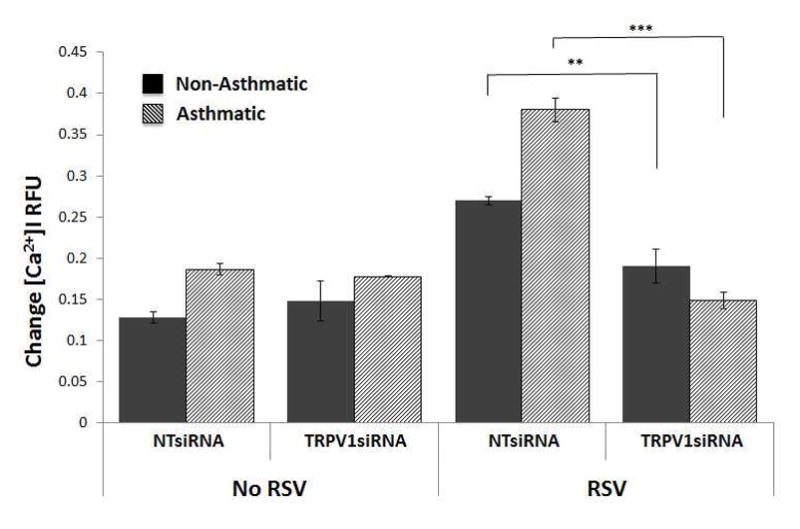
TRPV1 expression was silenced with a specific small interfering RNA (TRPV1siRNA). This strategy achieved approximately 70% reduction in TRPV1 mRNA transcripts as measured by qPCR and abolished the effect of RSV infection on capsaicin-induced [Ca2+]i in HBE from both asthmatic children and non-asthmatic controls. All experiments were repeated ≥2 times in quadruplicate. Data are expressed as mean ± SEM. **P<0.01, ***P<0.001 compared to controls treated with non-targeting siRNA (NTsiRNA).
Our results demonstrate for the first time significant differences in TRPV1-dependent Ca2+ flux in the airway epithelium that is dependent on age and asthma status. Specifically, the Ca2+ flux generated by stimulation of this channel is significantly greater in the bronchial epithelium of asthmatic vs. non-asthmatic children. This difference can also be measured in adults, albeit its average magnitude is approximately half than in children. More importantly, Ca2+ inflow in response to TRPV1 stimulation doubles when children’s epithelium is infected by RSV – the most common lower respiratory tract pathogen in infants and young children. The combined effect of this infection in children’s asthmatic cells can increase [Ca2+]i by 10-fold. In contrast, RSV has no effect on TRPV1 function in adult HBE regardless of the asthma status of the donor. RSV effect on TRPV1-mediated Ca2+ inflow correlates well with viral load, and therefore might result, at least in part, from more efficient entry and/or replication of RSV in the bronchial epithelium during early life.
Our data suggest that its role in the pathophysiology of RSV-related infant bronchiolitis and childhood asthma may be more important than in adult asthma. Also, our findings provide a suitable mechanism that may contribute to the different clinical manifestations of RSV infection in young children, in whom it frequently causes severe wheezing and cough poorly responsive to therapy with β-agonists and steroids, compared to the mild upper airway symptoms typical of this infection in adult life. The larger increment in Ca2+ permeability observed in HBE cells from asthmatic children is likely due at least in part to upregulation of TRPV1 rather than other Ca2+ channels, as shown by the finding that the increase in Ca2+ permeability in response to capsaicin was reduced significantly after silencing the TRPV1 gene with a targeted siRNA. It is possible that other respiratory viruses might affect TRPV1 differently from RSV. In particular, human rhinovirus (HRV) becomes the more common respiratory pathogen after the first years of life and is predominant in adults; however, a head to head comparison between RSV and different strains of HRV is quite complex and will require a separate study.
Although we have convincingly shown that TRPV1 is the channel responsible for changes in [Ca2+]i, there are some limitations to these studies. We realize that other calcium channels may contribute to the RSV mediated increase in [Ca2+]i. Of note, TRPA1 is activated upon rise in intracellular calcium (8); therefore, activation of TRPV1 with consequent increase in [Ca2+]i may in turn activate TRPA1 leading to a greater rise in [Ca2+]i. We also recognize that these studies were performed in vitro, which may not fully recapitulate what occurs in vivo. Finally, studies using primary bronchial epithelial cells from deceased donors are intrinsically limited by the availability of donor cells, especially in the pediatric age group. Additionally, history of atopic status and lung function is frequently not available, as well as comorbidities and cause of death. Thus, it is possible that the age effect could be explained by other factors and future studies will need to be done using cells from identified samples from patients with documented asthma status and severity.
In conclusion, the results of this study show for the first time that lower airway epithelium from asthmatic children displays elevated basal TRPV1 activity compared to non-asthmatic controls. We have also shown that RSV infection of epithelial cells from asthmatic children – but not from adults - leads to an increase in overall TRPV1 activation. Pharmacological inhibition of TRPV1 may lead in the future to strategies that might reduce the impact of RSV infections in both asthmatic and non-asthmatic children.
Methods
Cells
Primary human bronchial epithelial cells derived from non-asthmatic or asthmatic Caucasian, Black, or Hispanic donors of both sexes were purchased from Lonza (Walkersville, MD). The age of pediatric donors ranged from 2 to 6 years. We chose to study this subgroup because several epidemiologic studies have shown increased risk of post-RSV wheezing by age 6, with subsequent marked decrease with age and resolution before adolescence. Cells were maintained at 37°C in 5% CO2 using proprietary growth media and supplements provided by the vendor. The purity of cell cultures was assessed by immunocytochemistry for epithelial markers (e-cadherin; absence of fibroblast marker vimentin), visual morphology through passaging, and ability to differentiate into stratified epithelium by ciliation or goblet cells. Experiments were repeated using cells from different donors throughout the study to control for host genetics and environment (n = 3 non-asthmatic adults; 3 asthmatic adults; 4 non-asthmatic children; 3 asthmatic children). All experiments were performed on cells at passage number 2 to 5.
Virus
In order to easily verify active viral replication in our cells, we used a recombinant RSV A2 strain expressing the red fluorescent protein (RFP) gene (rrRSV), which was kindly provided by Dr. Mark Peeples (Nationwide Children’s Hospital, Columbus, OH) and Dr. Peter Collins (National Institutes of Health, Bethesda, MD) and was propagated using HEp-2 cells grown at 37°C/5%CO2 in Eagle’s minimum essential medium (MEM) supplemented with 10% fetal bovine serum (FBS) and 1% each of Glutamax, penicillin/streptomycin, and 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES). Cells at approximately 50% confluence were inoculated with 3 ml per dish of virus stock diluted 1/10 with heat-inactivated FBS. After incubation for 2 h at 37°C, the inoculum was removed and replaced with 25 ml of fresh medium. The medium was replaced again 2 days later, and the virus was harvested after 1 to 2 additional days of incubation, at which point all cells appeared bright red when viewed under a fluorescent microscope. To harvest the virus, infected cells were scraped from the plate, separated with a pipette, mixed at medium speed, and pelleted by centrifugation at 1,200 g for 5 min. The supernatant was collected and cell debris removed by centrifugation at 9,500 g for 20 min in a centrifuge refrigerated at 4°C. One-ml aliquots of the supernatant were snap-frozen in liquid nitrogen and stored at −70°C until use. The final titer was determined with a modified plaque-forming unit assay.
To measure viral load in infected HBE, total RNA was isolated using the RNeasy kit (Qiagen, Valencia, CA) and 25 ng per reaction was used for quantitative PCR (qPCR) analysis using the iTaq Universal Probes One-Step Kit (BioRad, Hercules, CA) and the CFX Connect real-time cycler (BioRad). Fluorescein amidite (FAM)-labeled RSV primer/probe set designed to detect the nucleocapsid gene and the positive control template were purchased from PrimerDesign (Chandler’s Ford, United Kingdom). Ten-fold serial dilutions of cRNA transcripts corresponding to copy numbers 101 to 106 were used to build the standard curve. Cq values were plotted on the standard curve and copy number was calculated automatically with the CFX Manager software (BioRad). Each sample was tested in triplicate, and the arithmetic mean of the measurements was used as the sample copy number. Samples were considered negative if Cq values exceeded 38 cycles.
Measurement of intracellular calcium
The calcium response to TRPV1 channel stimulation with capsaicin was measured using the Fluorogenic Imaging Plate Reader (FLIPR) assay (Molecular Devices, Sunnyvale, CA) in 80–90% confluent HBE grown in black-walled, clear-bottom Costar 96-well plates. Cells were loaded with Calcium-6 dye for 3 h and incubated at 37°C/5%CO2, according to manufacturer’s instructions. Plates were read in a Flexstation-3 reader, and fluorescence was measured over 3 min at 7-s intervals upon addition of ionomycin (0.5–1.0 μM in dimethyl sulfoxide; DMSO, Sigma-Aldrich, St. Louis, MO), capsaicin (150 μM in DMSO; Sigma-Aldrich), or vehicle. The ratio of maximum-minimum fluorescence/ionomycin fluorescence was used to calculate changes in intracellular Ca2+. For selected experiments measuring release of Ca2+ from intracellular stores, HBE were incubated overnight with or without RSV, then loaded with Calcium-6 dye in Hanks Balanced Salt Solution (HBSS) containing HEPES either with or without Ca2+ for 3 h. All samples were measured in quadruplicate and experiments were repeated a minimum of 3 times.
Gene silencing
Exponentially growing bronchial epithelial cells were transfected with SMARTpool ON-TARGET plus (GE Dharmacon, Lafayette, CO) small interfering (si)RNA directed against TRPV1 (50 nM) using Lipofectamine®2000 (Life Technologies, Carlsbad, CA), or ON-TARGET plus non-targeting pool siRNAs (50 nM). After 6 h transfection, the medium was replaced with fresh culture medium. Cells were harvested 48 h later and analyzed for knockdown of mRNA expression by qPCR.
Statistical analysis
All data are expressed as mean ± SEM. Multiple comparisons were performed by ANOVA followed by post-hoc analysis with the Dunnett’s test using the software GraphPad Prism version 5.0 (La Jolla, CA). P values less than 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
This article is dedicated to the memory of Dr. Mark E. Lauer, whose diligence and passion for science is left imprinted on all those lucky enough to have met and worked with him. We are indebted to Dr. Mark Peeples (Nationwide Children’s Hospital Research Institute, Columbus, OH) and Dr. Peter Collins (National Institutes of Health, Bethesda, MD) for providing the original batch of the RFP-expressing RSV virus.
Funding: These experiments were funded in part by grants from the U.S. National Institutes of Health: NHLBI RO1 HL-61007 to Dr. Piedimonte; NIAID K08 AI112781 to Dr. Rezaee; NHLBI RO1 HL119792 to Dr. Olman; and NHLBI K08 HL13380 to Dr. Scheraga. Funding was also provided by the Cystic Fibrosis Foundation Grant PIEDIM16G0 to Dr. Piedimonte.
Abbreviations
- TRPV1
Transient receptor potential vanilloid 1
- RSV
Respiratory syncytial virus
- HBE
Human bronchial epithelial
- LRTI
Lower respiratory tract infection
- siRNA
Small interfering RNA
- [Ca2+]i
Intracellular Ca2+ concentration
Footnotes
Disclosure of potential conflict of interest: The Authors have disclosed no potential conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–24. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 2.Yu H, Li Q, Zhou X, Kolosov VP, Perelman JM. Transient receptor potential vanilloid 1 receptors mediate acid-induced mucin secretion via Ca2+ influx in human airway epithelial cells. J Biochem Mol Toxicol. 2012;26:179–86. doi: 10.1002/jbt.20413. [DOI] [PubMed] [Google Scholar]
- 3.Groneberg DA, Niimi A, Dinh QT, Cosio B, Hew M, Fischer A, et al. Increased expression of transient receptor potential vanilloid-1 in airway nerves of chronic cough. Am J Respir Crit Care Med. 2004;170:1276–80. doi: 10.1164/rccm.200402-174OC. [DOI] [PubMed] [Google Scholar]
- 4.Lin RL, Hayes D, Jr, Lee LY. Bronchoconstriction induced by hyperventilation with humidified hot air: role of TRPV1-expressing airway afferents. J Appl Physiol. 2009;106:1917–24. doi: 10.1152/japplphysiol.00065.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu DE, Easton AS, Fraser PA. TRPV1 activation results in disruption of the blood-brain barrier in the rat. Br J Pharmacol. 2005;146:576–84. doi: 10.1038/sj.bjp.0706350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGarvey LP, Butler CA, Stokesberry S, Polley L, McQuaid S, Abdullah H, et al. Increased expression of bronchial epithelial transient receptor potential vanilloid 1 channels in patients with severe asthma. J Allergy Clin Immunol. 2014;133:704–12. doi: 10.1016/j.jaci.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 7.Omar S, Clarke R, Abdullah H, Brady C, Corry J, Winter H, et al. Respiratory virus infection up-regulates TRPV1, TRPA1 and ASICS3 receptors on airway cells. PLoS One. 2017;12:e0171681. doi: 10.1371/journal.pone.0171681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cavanaugh EJ, Simkin D, Kim D. Activation of transient receptor potential A1 channels by mustard oil, tetrahydrocannabinol and Ca2+ reveals different functional channel states. Neuroscience. 2008;154(4):1467–76. doi: 10.1016/j.neuroscience.2008.04.048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


